Abstract
Background
Intoxication with Amanita phalloides is associated with high morbidity and mortality. Treatment therapies include general support, toxin elimination, pharmacotherapy with agents such as the hepatoprotective agent silibinin, and, in extreme states, liver transplantation. Despite these therapeutic interventions, mortality remains relatively high.
Case Reports
We present reports of 2 patients with severe hepatic failure following intoxication with Amanita phalloides. Both patients were admitted to the intensive care unit; 1 patient suffered from hepatic failure solely, and the second patient experienced severe 5-organ failure. In addition to conventional intensive care treatment, both patients were treated additively with classical homeopathy. The 2 patients survived without any residual pathological sequelae.
Conclusion
Based on the 2 cases, including 1 extreme situation, we suggest that adjunctive homeopathic treatment has a role in the treatment of acute Amanita phalloides–induced toxicity following mushroom poisoning. Additional studies may clarify a more precise dosing regimen, standardization, and better acceptance of homeopathic medicine in the intensive care setting.
Keywords: Amanitins, homeopathy, toxins–biological, toxophallin–Amanita phalloides
INTRODUCTION
Intoxication by Amanita phalloides is associated with high morbidity and mortality. Treatment therapies include general support, toxin elimination, pharmacotherapy with agents such as the hepatoprotective agent silibinin,1 and, in extreme cases, liver transplantation.2 Despite these therapeutic interventions, mortality remains relatively high.3-6 We report 2 cases of Amanita phalloides intoxication in which additive homeopathy was used in treatment. Both patients exhibited severe hepatic failure; 1 patient suffered additionally from multiorgan failure, including the heart, lungs, kidneys, and brain. In this patient, arsenicum album (arsenic trioxide) was used initially. When liver failure was diagnosed, phosphorus was administered to both patients. Both patients survived without any residual pathological sequelae.
CASE REPORT 1 (PZ, VY)
An otherwise healthy 20-year-old female ate wild mushrooms (day 1). Twelve hours later, she began experiencing repetitive, painful vomiting and abdominal burning. She developed profuse, painful diarrhea with blood and mucus that occurred in excess of 20 times per day.
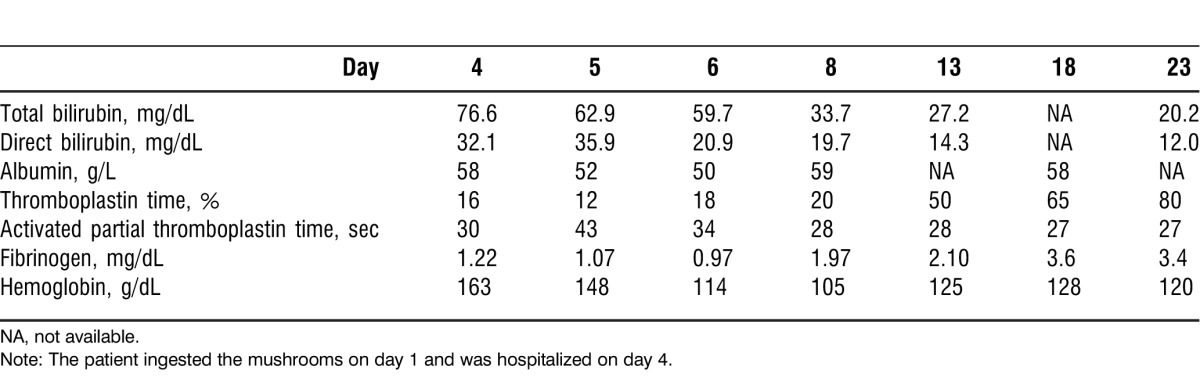
The patient was hospitalized in Shumen, Bulgaria, 72 hours after the onset of the poisoning (day 4). When she was admitted to the hospital, her diagnosis included (1) acute-stage Amanita phalloides poisoning, (2) severe gastroenterocolic choleriform syndrome, (3) progressive hepatic dysfunction, and (4) coagulopathy caused by impaired synthesis of blood coagulating factors. Laboratory tests indicated a necrotic hepatic cytolysis with an aspartate aminotransferase (AST) level of 7,612 U/L, alanine aminotransferase (ALT) of 6,204 U/L, and thromboplastin time (TT) of 16%. Within 24 hours (day 5), these laboratory parameters changed to AST of 11,308 U/L, ALT of 14,102 U/L, and TT of 12%, respectively (Figure and Table 1).
Figure.
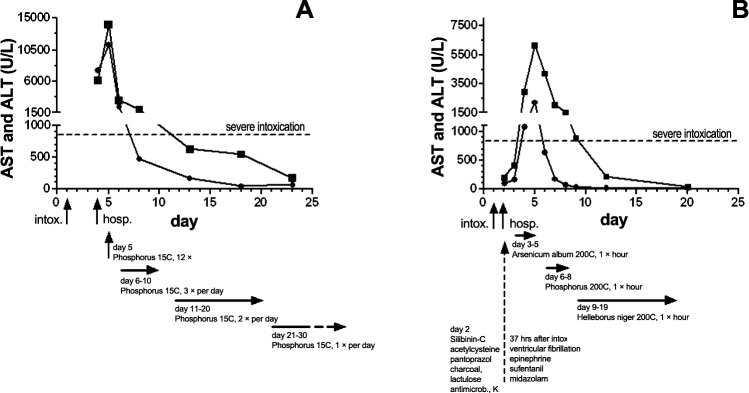
The time course of aspartate aminotransferase (AST •) and alanine aminotransferase (ALT ▪) levels after Amanita phalloides ingestion in patient 1 (A) and patient 2 (B). The ALT is considered minor if ≤85 U/L, moderate if ≥85 U/L, and severe if ≥850 U/L.3
Hosp, hospitalization; intox, intoxication (ingestion of the mushrooms).
Note: The ordinates indicate different scales.
Table 1.
Laboratory Results of Patient 1
The patient presented with significant hypotension on admission to the hospital. Treatment was instituted with 2 courses of carbohemoperfusion; however, no significant beneficial effect resulted. A hemorrhagic syndrome started on day 6 (2 days after hospitalization) with bleeding from her gums, vagina, abdomen, and intestines. Serous effusions into the abdominal cavity and pleura were observed.
The late hospitalization, the late stage of poisoning, the acuteness of her course, and her multiorgan lesions encouraged a complementary therapeutic approach. Phosphorus 15C was added to the therapy on day 5 (second day of hospitalization) at a dosage of 5 globules 6 times daily every 2 hours.
On day 6, 1 day after the administration of phosphorus, objective and laboratory data demonstrated an abortive evolution of the Amanita phalloides–induced poisoning. The patient's vomiting, diarrhea, and hemorrhages disappeared.
Ultrasonography on day 10 revealed a diffuse parenchymal hepatic disorder with an enlarged liver, mild ascites, and pleural effusions on both sides. The vena portae and vena lienalis were not dilated; her spleen was normal size; and her gallbladder, gall ducts, pancreas, and kidneys were normal. Ultrasonography on day 17 showed that the liver had regained its normal size with slight hyperechogenicity. Administration of phosphorus 15C was reduced to 3 times daily for 5 days (days 6 to 10), further reduced to 2 times daily for 10 days (days 11 to 20), and then to once daily for another 10 days (days 21 to 30). Phosphorus 15C was given to the patient once every other day for another month, and then once weekly for another 2 months. The entire treatment lasted approximately 4 months.
The patient was discharged from the hospital on day 23 without any subjective or objective complaints. Her AST and ALT levels returned to normal ranges within 2 weeks of discharge.
Three months later, follow-up demonstrated complete clinical recovery through normal laboratory tests and liver ultrasonography. The follow-ups were repeated every 3 months for 1.5 years without evidence of postnecrotic cirrhosis or any other clinical pathological signs.
CASE REPORT 2 (MF, BW, KT, KZL, WB, ADK)
A 69-year-old female, 162 cm tall and weighing 70 kg, experienced intoxication with Amanita phalloides. The patient had a significant medical history of longstanding hypertension, a remote myocardial infarction, and moderately decreased renal function that had been detected 27 years earlier (serum creatinine 1.5 mg/100 mL, blood urea nitrogen 40 mg/100 mL).
The patient, along with 3 other people, ate a lunch consisting of young baked mushrooms (day 1). Following a latency of 8-9 hours, the patient experienced massive nonhemorrhagic vomiting and diarrhea accompanied by severe abdominal pain. The next day (day 2) at 7:30 am, she was admitted to the local hospital. Laboratory examination showed leukocytosis (17 × 109/L) and a slight elevation of transaminases (AST of 98 U/L, ALT of 195 U/L) (Figure). Mushrooms left over from the meal were identified as Amanita phalloides by the Department of Botany and Biodiversity Research at the University of Vienna in Austria.
Silibinin-C (Legalon) was administered intravenously as a single bolus at a dosage of 5 mg/kg and subsequently given as an infusion at a dosage of 24 mg/kg/24 h.
Infusion with silibinin-C was continued and the patient received acetylcysteine (Fluimucil), pantoprazole (Pantoloc), charcoal (Carbomix), and lactulose concentrates (Laevolac). In addition, the patient received intravenous antimicrobial therapy and potassium infusions.
On day 3, approximately 37 hours after mushroom ingestion, the patient experienced ventricular fibrillation and was converted to sinus rhythm after 2 defibrillations. The patient was started on epinephrine (Suprarenin) with a maximal dosage of 0.42 μg/kg/min. After defibrillation, the patient was comatose and had to be intubated. The patient was mechanically ventilated and received sufentanil (Sufenta) and midazolam (Dormicum). The arterial pH deteriorated to 7.16; therefore, sodium bicarbonate was administered. Lactate increased to 17.6 mmol/L (Table 2). The transaminases increased: AST to 167 U/L and ALT to 413 U/L (Figure).
Table 2.
Laboratory Results of Patient 2
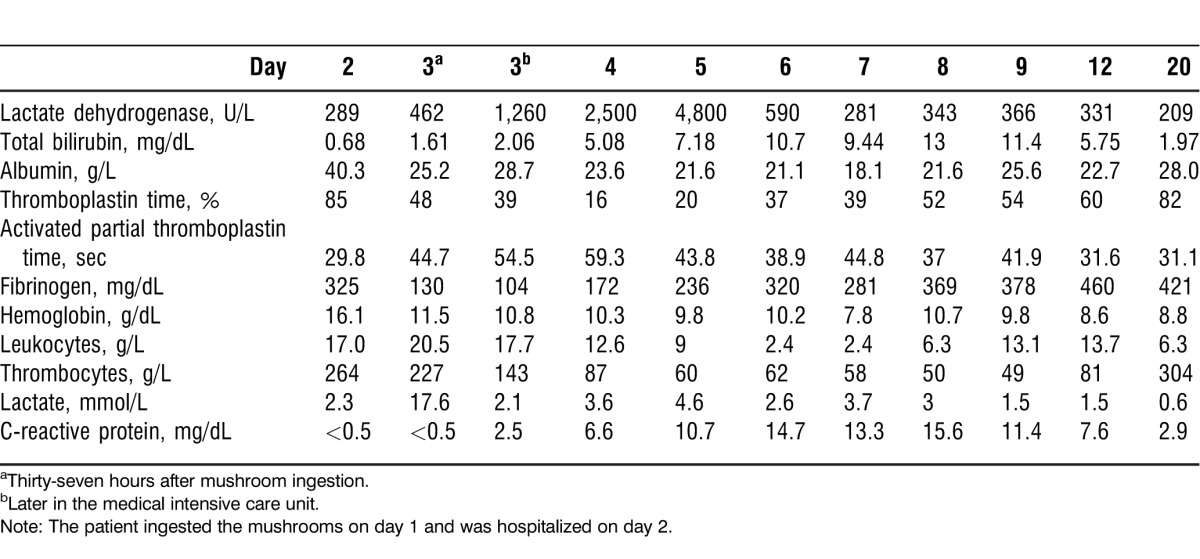
Late on day 3, the patient was transferred to the medical intensive care unit of the Medical University of Vienna, Austria. The patient developed acute renal failure, and continuous venovenous pump-driven hemofiltration was started. Because the patient had hyperglycemia, insulin was administered intravenously. Because of the patient's tachycardia, epinephrine was replaced by norepinephrine. During the rest of day 3, the patient was stable; however, transaminases increased dramatically (AST to 450 U/L, ALT to 1,070 U/L), while TT dropped significantly to 39%.
A liver transplant was discussed, but the coordinator for liver transplants declined the patient, citing age and multiorgan failure. The patient's family was informed of her extremely poor prognosis.3,7
Categories of symptoms, known as rubrics, were utilized to determine the appropriate homeopathic treatment.8 The search rubrics included rectum, diarrhea, eating, after, aggravates; generalities, intoxication, after, fish, from poisonous; generalities, intoxication, after, mushrooms, from poisonous; abdomen, inflammation, peritonitis, enteritis, liver; generalities, pulse, weak; kidney, suppression of urine; generalities, inflammation, general.
This rubric indicated the homeopathic remedies agaricus muscarius, atropinum, belladonna, camphora, and pyrogenium, but additional symptoms are typical indications for these treatments. For a treatment of agaricus muscarius, the phase of intoxication is accompanied by great mental excitement, incoherent talking, and shifts between immoderate gaiety and melancholy. Physical strength is increased, including the ability to lift heavy loads. These symptoms were absent in the patient. With atropinum, similar to belladonna, sudden aggravation is typical. Also for a belladonna indication, the patient commonly has severe pain and a red coloration in the head. For camphora treatments, patients typically experience icy coldness of the whole body, including the nose, extremities, and breath. Pyrogenium is known for its action in sepsis as well as for treating offensive discharges. With pyrogenium, patients experience soreness that becomes better with motion. Rapid decubitus of septic origin is often observed in patients needing pyrogenium.
Potentized arsenicum album was administered to the patient although this treatment was not named in the rubric intoxication, after, mushrooms, from poisonous.8 Arsenicum album was indicated to mitigate inflammation of the liver. Arsenicum album has been reported as a remedy following intoxications and allergic reaction.9
Beginning on day 3, 5 globules of arsenicum album 200C were administered every hour over a period of 2 days. The globules were administered alongside the orally placed endotracheal tube so they were dissolved either under the tongue or behind the lips. No mouth hygiene was performed from 15 minutes before to 15 minutes after administration of the globules.
On day 4, the patient's hemodynamics and respiratory status stabilized. Transaminases continued to increase (AST of 1,090 U/L, ALT of 2,950 U/L), TT fell to 16%, and lactate dehydrogenase (LDH) increased to 2,500 U/L (Figure and Table 2).
On day 5, the highest transaminases levels were found (AST of 2,240 U/L, ALT of 6,170 U/L), LDH was 4,800 U/L, and TT was 20%. Bilirubin increased to a concentration of 7.18 mg/dL, indicating a significant liver disease process. Using the rubrics abdomen, liver and region of, ailments of; abdomen, fatty degeneration of liver; abdomen, atrophy, liver; and skin, discoloration, yellow, jaundice, icterus, the patient's homeopathic treatment was switched to phosphorus, a known remedy for liver dystrophy.9 Phosphorus develops the picture of catabolism; in regular, larger doses, it causes yellow atrophy of the liver and subacute hepatitis with intense jaundice.8 Phosphorus is characterized by sudden onset of symptoms. Five globules of phosphorus 200C were administered hourly over a period of 3 days, and arsenicum album was canceled. On day 6, the patient's transaminases began to decrease.
On day 7, the patient still needed norepinephrine at 0.45 μg/kg/min, and the respiratory setting was fraction of inspired oxygen (FiO2) 0.35 with bilevel intermittent positive airway pressure (BIPAP). The patient demonstrated leucopenia (2.4 g/L) accompanied by no level of consciousness. Antimicrobial therapy with netilmicin (Certomycin) and amoxicillin clavulanic acid (Augmentin) was started. Because of her anemia, the patient received 2 units of packed red blood cells.
On day 8, norepinephrine was reduced to 0.06 μg/kg/min, transaminases continued to fall (AST of 66 U/L, ALT of 1,530 U/L), and C-reactive protein (CRP) and bilirubin reached their highest values of 15.6 mg/dL and 13 mg/dL, respectively. Although the patient showed no reaction to pain stimulus, she spontaneously opened her eyes and reacted to verbal stimuli after administration of 1 mg flumazenil (Anexate). Neurologically, the patient showed signs of encephalopathy; cranial computed tomography scan was negative. Because the liver showed significant improvement and because of the relevant deteriorated cerebral situation, phosphorus was canceled. To treat the patient's stupor, 5 globules of Helleborus niger 200C were administered once daily for a period of 12 days.
On day 9, norepinephrine was maintained at 0.06 μg/kg/min, and the patient was weaned from BIPAP to assisted spontaneous breathing. Thrombocytes reached a trough level of 49 g/L. Hemofiltration was stopped. Norepinephrine was stopped in the morning of day 12; CRP decreased to 7.6 mg/dL; and AST, bilirubin, and TT all returned to normal values (Figure and Table 2).
On day 16, the patient was sedated for tracheotomy because of the prolonged weaning process. During the next few days, the patient breathed spontaneously with continuous flow. A profuse milky-yellowish secretion, consistent with Pseudomonas aeruginosa, was treated with antimicrobial therapy. On day 20, the patient was hemodynamically and respiratorily stable, was sitting upright in bed, was fully conscious, and was able to eat without help. Therapy with Helleborus niger was stopped.
The course of illness continued to show improvement. On day 20, liver parameters were normal (AST of 10 U/L, ALT of 32 U/L, and LDH of 209 U/L). On day 27, the patient was transferred to the general ward and was discharged from the hospital on day 43 without any organic or neurologic sequelae. Two of the 3 other patients who consumed the poisonous mushroom meal and who underwent conventional treatment without additive homeopathy died.
DISCUSSION
These 2 case reports describe severe intoxication from Amanita phalloides. The literature describes intoxications with elevations of ALT of more than 850 U/L3 and decreases of TT below 10%4 as severe. In addition, as was present with patient 2, 5-organ failure is associated with high mortality.10 Survival of patient 2 was especially unexpected by the intensivists and the transplant coordinator because of her extremely disturbed liver parameters and her cardiac, pulmonary, renal, and cerebral failure.
Amanita phalloides
The cap of Amanita phalloides, also called the “death cap,” is 4-16 cm wide. Its color is white, with light green/yellow to olive green and olive brown pigments. The cap is often adorned with 1 to several patches of thin white veil tissues. The cap can easily be parted from the stalk; the stalk itself is white to pallid and up to 16 cm long. The base features a large rounded bulb with a volva, which is the remaining part of the universal veil. The bulb and the volva are often buried in the soil. Amanita phalloides can be found under oak and beech trees from July to November in the northern hemisphere.
Amanita phalloides contains 2 main types of toxins, amatoxins and phallotoxins. Amatoxins are thermostable bicyclic octapeptides; dried mushrooms contain approximately 5 mg of toxin per gram of mushroom. Phallotoxins are heptapeptides. Amatoxins are 10-20 times more potent than phallotoxins and are considered to play a critical role in the induction of liver injury. The prototype of the amanitins is α-amanitin, which inhibits ribonucleic acid (RNA) polymerase II and RNA polymerase III. Thus, α-amanitin blocks RNA synthesis and protein synthesis, most prominently in the liver and kidney. Phallotoxins are not taken up by the cells, but they bind to actin at the cell membrane, resulting in cell injury and cell degeneration. Signs of intoxication may not be evident for 6-36 hours. Death in cases of severe intoxication may occur 15 hours after ingestion, presumably from liver dystrophy caused by the poisonous effects of amanitins.
Amanita phalloides Symptomatology
Symptomatology is triphasic. Following an asymptomatic latency of 8-24 hours, severe diarrhea, bloody vomiting, abdominal pains, and nausea occur. Then symptoms decline while the liver deteriorates dramatically (transaminases increase and coagulation factors decrease). Three to 5 days later, multiorgan failure with heart and renal decompensation and acute liver dystrophy may occur.
Amanita phalloides Diagnostics
Generally, botanical classification by experts confirms the type of mushroom. Amanita toxin may be confirmed by radioimmunoassay in urine and in serum. Because symptoms are initially latent, may imitate common influenza and other gastrointestinal viruses, and may show improvement in most patients beginning 2 or 3 days after ingestion, an early diagnosis and treatment may be prevented. Elevated transaminases and serum bilirubin levels are the first and most reliable signs of disturbance of liver function and should be observed carefully.
Amanita phalloides Immediate Therapy
Because a specific antidote for amatoxins is not available, therapy is solely supportive. For removal of toxins, gastrointestinal lavage may be performed with administration of charcoal or other absorbing perfusions. Removal of toxins is often impossible because patients are admitted in a relatively late phase of intoxication. Therefore, rapidly established hemodialysis and simultaneous forced diuresis are initiated. Carbohemoperfusions should be considered if the following criteria are fulfilled: fewer than 48 hours have passed since ingestion, the patient shows biochemical evidence of toxicity, a potentially lethal dosage was ingested, and serum enzymes have increased. Because toxins may be absorbed via the surface of the skin, the patient should be washed carefully.
Amanita phalloides Pharmacologic Therapy
Pharmacologically, penicillin G and silibinin are used. Penicillin G, administered intravenously at a dosage of 1,000,000 IU/kg/d, provides some protection against hepatic toxicity. Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (Legalon), is administered intravenously at a dosage of 20-50 mg/kg/d. Future research should investigate whether a direct hepatoprotective effect exists by blocking penetration of amanitin into hepatocytes and thereby the direct competition with amatoxin against transmembraneous transport. In the presence of disseminated intravascular coagulopathy and coagulation disorder, heparin, glutamat-pyruvat-transaminase, and antithrombin III are to be administered.
Amanita phalloides Advanced Therapy
Orthotopic liver transplantation should be considered if hepatic encephalopathy, significant disturbance of coagulation factors, and a rise in liver enzymes are feared. The main indications for liver transplantations are initially lowered TT and factor V concentrations (<10%) together with an inadequate response to substitution. Of all mushroom-related poisonings in North America, 90% of mortality is caused by intoxication with Amanita phalloides.11 In a review of 205 cases of Amanita phalloides intoxication between 1971 and 1980, patient age and the latency period between mushroom ingestion and first clinical symptoms were of prognostic significance, and lethality was 22.4%.4 Regarding age, children younger than 10 years had a death rate of 51.3%, while patients older than 10 years had a death rate of only 16.5%. In fatal cases, the average latency period was 10.3 hours; it was 12.6 hours for the surviving patients. Other factors, including country, sex, year, and time of hospitalization, did not affect lethality. Faybik et al described 6 cases treated with extracorporeal albumin dialysis.6 One patient with multiorgan failure died, while the surviving patients did not exhibit multiorgan failure.
TT was also prognostically relevant in the Floersheim et al study.4 The majority of patients with TT values below 10% died, while patients with minimal values of 40% or more survived. Serum transaminases had a lower correlation with survival rate, and creatinine showed no correlation to survival rate. Patients received an average of 8 therapeutic measures, but up to 20 could be administered to the same patient. Of the 30 recorded treatments, 8 involved general support, 7 were toxin eliminations, 14 were pharmacotherapy interventions, and 1 case did not detail therapeutic intervention. A multiple regression analysis was performed, taking into account age, latency period, and the effects of all the other measures. Higher survival rate was identified independently with penicillin and hyperbaric oxygenation. Increased survival was associated with penicillin and silibinin compared to penicillin alone.
The study showed that 20 therapeutic measures were neither positive nor negative in terms of clinical outcome. Exchange transfusion, thiocytic acid, sulfamethoxazole, plasma expanders, hemodialysis, treatment of hemorrhagic diathesis, and tris-hydroxymethyl aminomethane/sodium bicarbonate for acidosis were administered more often to patients who did not survive.4
Our case reports suggest that homeopathy may have a positive influence on a patient experiencing severe Amanita phalloides–induced intoxication. Interestingly, we independently and at different times used the same remedy (phosphorus) for acute liver disorder in our 2 patients. Patient 2 was treated first with arsenicum album because of severe initial multiorgan failure. However, when hepatic disorder became prominent, phosphorus was used similarly to its use for patient 1. In both cases, the patients recovered despite an extremely critical state.
Homeopathic Considerations
Although arsenicum album is not typically identified as an important remedy in mushroom poisonings, it is well known in homeopathy as an important remedy for intoxications and allergies. A review of the literature indicates that arsenicum album has also been used with benefit in cases of chronic hepatic disorders.12 Phosphorus is well accepted as a therapeutic treatment for many pathological states involving the liver. These 2 homeopathic remedies have a wide application in the treatment of acute and chronic hepatic disorders. From a clinical toxicology perspective, acute Amanita phalloides poisoning and acute phosphorus intoxication have a similar clinical picture and evolution. Both of our patients experienced acute hepatic necrosis with excessively elevated levels of liver enzymes. A fatal outcome with hepatic insufficiency and coagulopathy was expected of both patients.
Helleborus niger, which was administered to patient 2, produces a condition of sensorial depression. It is a homoeopathic remedy for patients in low states of vitality and with serious disease. Boericke9 describes the indications for Helleborus niger in his Homoeopathic Materia Medica: the speech of the patient is slow; the memory is weakened; thinking is impaired, dull, and sluggish; and the patient appears thoughtless and stares vacantly. The patient also shows indifference and apathy towards pleasure and is slow to answer. Therefore, Helleborus was chosen because of patient 2's decreased state of consciousness and low state of vitality.
Because groundwater arsenic contamination places millions of individuals at risk, several studies have examined the effects of arsenic contamination.13,14 Belon et al13 evaluated whether a potentized homeopathic remedy demonstrating ameliorating potentials in individuals living in high-risk arsenic-contaminated areas while drinking arsenic-free water can ameliorate arsenic toxicity. The majority of the subjects showed improvement in general health and appetite, suggesting this remedy in remote arsenic-contaminated areas for large populations at risk for groundwater arsenic intoxication as an interim health-support measure. Arsenicum album is one of the major homeopathic remedies used in several types of intoxication.
Kundu studied the salvage of mice intoxicated by AsO3 through homeopathic preparations of arsenicum album.15 Administration of arsenicum album, in a dose of 30C, resulted in prevention of liver damage or enhanced repair as indicated by serum enzymatic markers.15 Arsenicum album might have similar positive effects in humans. The mechanism of the underlying beneficial effect of complementary treatment with arsenicum album and phosphorus are not known. The effect appears to depend on interactions within the whole (human) organism. Preliminary data using a hepatocellular carcinoma cell line as a model did not reveal a protective or beneficial action of phosphorus 6C-30C, arsenicum album 6C-30C, or Prunus laurocerasus 6C-30C upon α-amanitin (personal communication with Stark F, Frass M, and Bursch W, 2010). We did not find analogous information on epidemiological and experimental studies of phosphorus in relevant databases. Basic experiments may not reflect the reaction of the whole human organism that could potentially include several organ systems.
CONCLUSION
Our case reports suggest that homeopathic therapy can be beneficial to patients suffering from acute Amanita phalloides–induced toxicity following mushroom poisoning. Both patients exhibited severe hepatic failure; one patient was suffering in addition from multiorgan failure that involved the heart, the lungs, the kidneys, and the brain. In this patient, arsenicum album was used initially. When liver failure was diagnosed, phosphorus was administered to both patients. Both patients survived without any residual pathological sequelae. We suggest that a beneficial effect of homeopathic treatment might be considered and further observations and studies might help support these case report findings. When clinically advisable, combining homeopathic medicine with conventional treatment is beneficial, and these case reports represent successful management utilizing both types of medical treatment simultaneously. Additional studies may provide a more precise dosing regimen, standardization, and better acceptance of homeopathic medicine in the intensive care setting.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Lionte C, Sorodoc L, Simionescu V. Successful treatment of an adult with Amanita phalloides-induced fulminant liver failure with molecular adsorbent recirculating system (MARS) Rom J Gastroenterol. 2005 Sep;14(3):267–271. [PubMed] [Google Scholar]
- 2.Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning—from diarrhea to liver transplantation. Am J Gastroenterol. 2001 Nov;96(11):3195–3198. doi: 10.1111/j.1572-0241.2001.05283.x. [DOI] [PubMed] [Google Scholar]
- 3.Persson HE, Sjöberg GK, Haines JA, Pronczuk de Garbino J. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213. doi: 10.3109/15563659809028940. [DOI] [PubMed] [Google Scholar]
- 4.Floersheim GL, Weber O, Tschumi P, Ulbrich M. Clinical death-cap (Amanita phalloides) poisoning: prognostic factors and therapeutic measures. Analysis of 205 cases [In German] Schweiz Med Wochenschr. 1982 Aug 21;112(34):1164–1177. [PubMed] [Google Scholar]
- 5.Bresinsky A, Besl H. Giftpilze. Ein Handbuch für Ärzte, Apotheker und Biologen. 1st ed. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH;; 1985. [Google Scholar]
- 6.Faybik P, Hetz H, Baker A, et al. Extracorporeal albumin dialysis in patients with Amanita phalloides poisoning. Liver Int. 2003;23(Suppl 3):28–33. doi: 10.1034/j.1478-3231.23.s.3.8.x. [DOI] [PubMed] [Google Scholar]
- 7.Kotwica M, Czerczak S. Acute poisonings registered since 1970: trends and characteristics. Analysis of the files collected in the National Poison Information Centre, Łódź, Poland. Int J Occup Med Environ Health. 2007;20(1):38–43. doi: 10.2478/v10001-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 8.Zandvoort R. Complete Repertory [computer program] San Rafael, CA: Kent Homeopathic Associates Inc; 2000. [Google Scholar]
- 9.Boericke W. Pocket Manual of Homoeopathic Materia Medica. 8th ed. San Francisco, CA: Boericke & Runyan Co; 1922. [Google Scholar]
- 10.Vincent JL. Prevention and therapy of multiple organ failure. World J Surg. 1996 May;20(4):465–470. doi: 10.1007/s002689900073. [DOI] [PubMed] [Google Scholar]
- 11.Paydas S, Kocak R, Erturk F, Erken E, Zaksu HS, Gurcay A. Poisoning due to amatoxin-containing Lepiota species. Br J Clin Pract. 1990 Nov;44(11):450–453. [PubMed] [Google Scholar]
- 12.Guermonprez M. Matiere Medicale Homeopathique. Lyon, France: Editions Boiron;; 2000. [Google Scholar]
- 13.Belon P, Banerjee A, Karmakar SR, et al. Homeopathic remedy for arsenic toxicity? Evidence-based findings from a randomized placebo-controlled double blind human trial. Sci Total Environ. 2007 Oct 1;384((1-3)):141–150. doi: 10.1016/j.scitotenv.2007.06.001. Epub 2007 Jul 12. [DOI] [PubMed] [Google Scholar]
- 14.Khuda-Bukhsh AR, Pathak S, Guha B, et al. Can homeopathic arsenic remedy combat arsenic poisoning in humans exposed to groundwater arsenic contamination?: a preliminary report on first human trial. Evid Based Complement Alternat Med. 2005 Dec;2(4):537–548. doi: 10.1093/ecam/neh124. Epub 2005 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundu SN, Mitra K, Khuda Bukhsh AR. Efficacy of a potentized homoeopathic drug (Arsenicum-album-30) in reducing cytotoxic effects produced by arsenic trioxide in mice: III. Enzymatic changes and recovery of tissue damage in liver. Complement Ther Med. 2000;8:76–81. doi: 10.1054/ctim.2000.0367. [DOI] [PubMed] [Google Scholar]


