Abstract
Background
Cytomegalovirus (CMV) is a double-stranded DNA virus and a member of the Herpesviridae family. It is a lytic virus that causes a cytopathic effect in vitro and in vivo. CMV affects nearly all humans, with a 60%-100% seroprevalence worldwide, and in the immunocompetent it typically manifests as a mild and self-limiting mononucleosis syndrome. In immunocompromised patients, CMV has the ability to cause significant inflammation in many different organ systems.
Case Report
We report an unusual case of hepatitis and pancreatitis secondary to CMV in an immunocompetent patient. A 29-year-old male was admitted with elevated lipase, elevated aminotransferases, and epigastric pain after an acute viral prodrome. An initial CMV DNA polymerase chain reaction workup was positive, indicating acute or reactivated infection. Liver histology was consistent with CMV infection. Magnetic resonance cholangiopancreatography, endoscopic ultrasound, and intraoperative cholangiogram were not indicative of other etiologies. CMV viremia was successfully cleared with ganciclovir, and the patient experienced clinical and biochemical improvements.
Conclusion
Pancreatitis and hepatitis secondary to CMV are rare but should be considered in the workup of immunocompetent patients, especially in the presence of a recent viral-like illness.
Keywords: Cytomegalovirus, hepatitis, immunocompetence, pancreatitis
INTRODUCTION
Cytomegalovirus (CMV) is a common viral pathogen in humans. It is a lytic virus that causes a cytopathic effect in vitro and in vivo. Seroprevalence for CMV worldwide ranges from 60%-100% but the severity of illness varies.1 Primary CMV may be asymptomatic or may cause a mild and self-limiting mononucleosis-like syndrome.2-4 The self-limiting course of CMV infection typically includes fever, malaise, splenomegaly, mild hepatomegaly, small increases in serum transaminase activity, and variable elevation of serum alkaline phosphatase.4,5 CMV infection can cause severe hepatitis, meningitis, encephalitis, myelitis, Guillain-Barré syndrome, colitis, uveitis, retinitis, and pneumonitis in the immunocompromised.1,6 Some rare case reports describe severe CMV infection in immunocompetent adults. We report CMV hepatitis and pancreatitis in a young immunocompetent male.
CASE REPORT
A previously healthy 29-year-old male with no significant past medical history was admitted to the hospital for treatment of acute pancreatitis. Two weeks prior to admission, the patient had developed a febrile syndrome with severe myalgias and arthralgias. These symptoms persisted for a week. The patient then developed burning epigastric pain and was found to be Helicobacter pylori positive. He was started on clarithromycin, amoxicillin, pantoprazole, and sucralfate. Over the following 4 days, he defervesced but the pain progressed and radiated to his back. An abdominal computed tomography (CT) scan showed mild splenomegaly and did not show walled-off necrosis or pancreatic inflammation. Upon admission, the patient's lipase level was 398 U/L. The patient was hospitalized for nonalcoholic acute pancreatitis.
A review of systems revealed the patient exhibited chills, diaphoresis, night sweats, fatigue, nausea, nonbloody emesis, anorexia, orthostasis, and 5-pound weight loss. He had a temperature of 98.8°F, blood pressure of 133/83 mmHg, heart rate of 103 beats per minute, and respiratory rate of 18 breaths per minute, and his oxygen saturation was 100% on room air. Physical examination was negative for cervical lymphadenopathy or pharyngeal exudates. He was tender in the right upper quadrant and had a positive Murphy sign.
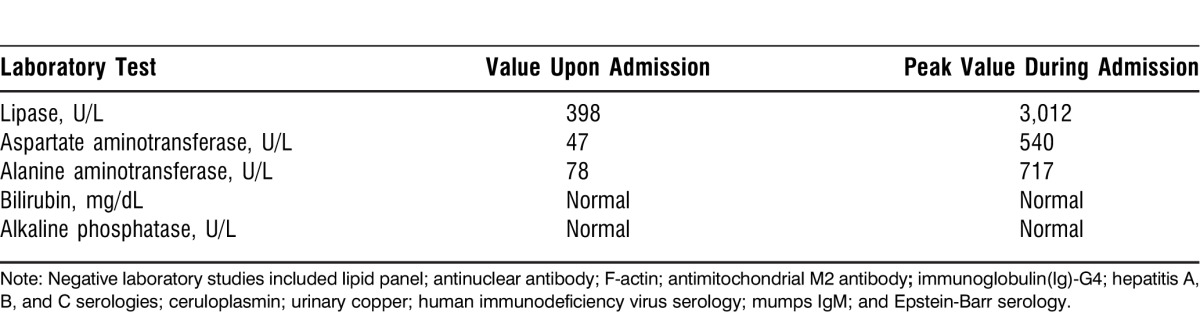
Upon admission, the patient's lipase level was 398 U/L and his alanine aminotransferase (ALT) test was 78 U/L. His total bilirubin, aspartate aminotransferase (AST) test, and alkaline phosphatase were normal. The patient was treated with supportive measures for pancreatitis, and treatment for Helicobacter pylori was discontinued. Over the subsequent 2 weeks, the patient's lipase peaked at 3,012 U/L and his AST and ALT peaked at 540 U/L and 717 U/L, respectively (Table 1). His alkaline phosphatase and bilirubin levels remained normal. The patient's triglycerides level was normal. The patient underwent several tests and his antinuclear antibody, F-actin, antimitochondrial M2 antibody, and immunoglobulin (Ig)-G4 were negative. Hepatitis A, B, and C serologies were negative. The patient's ceruloplasmin, urinary copper, and ferritin were normal. His human immunodeficiency virus (HIV) serology was negative. Mumps IgG showed immunity with a negative IgM. Epstein-Barr virus (EBV) serology showed evidence of past exposure but no active infection. Notably, the patient's CMV IgM test was positive at 209 U/mL, IgG test was positive at 4.8 U/mL, and CMV DNA polymerase chain reaction (PCR) was 1,000 copies (log 3.0).
Table 1.
Laboratory Investigations
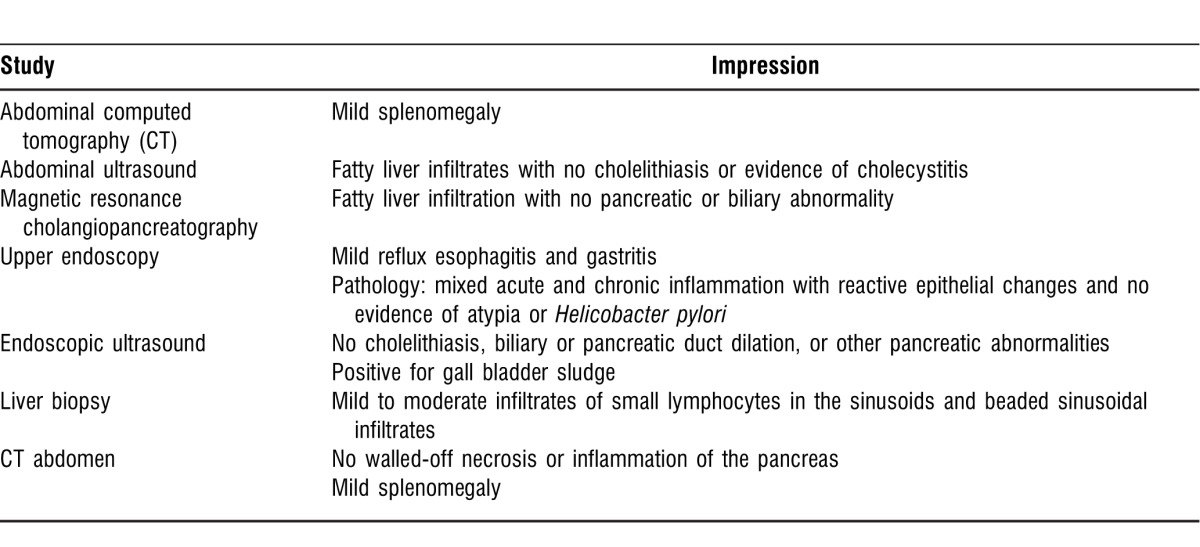
An abdominal ultrasound on admission showed fatty liver infiltrates. Two days after admission, a magnetic resonance cholangiopancreatography revealed fatty liver infiltration without choledocholithiasis. An upper endoscopy showed mild reflux esophagitis and gastritis, and histopathology revealed mixed acute and chronic inflammation with reactive epithelial changes and no evidence of atypia. An endoscopic ultrasound was negative for biliary system dilation and/or choledocholithiasis (Table 2).
Table 2.
Imaging and Procedures in Chronological Order
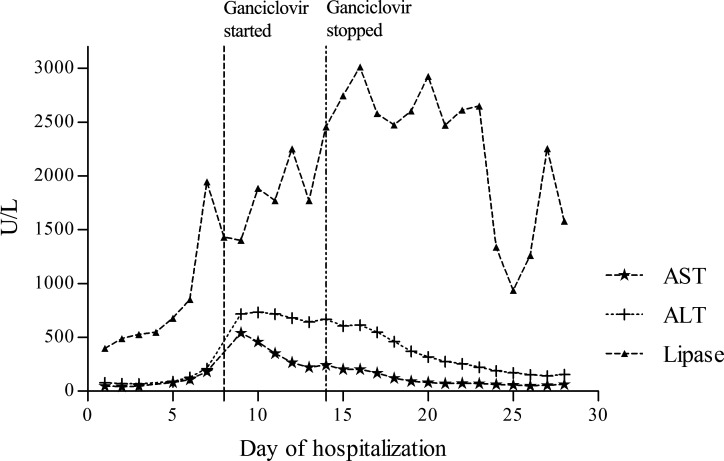
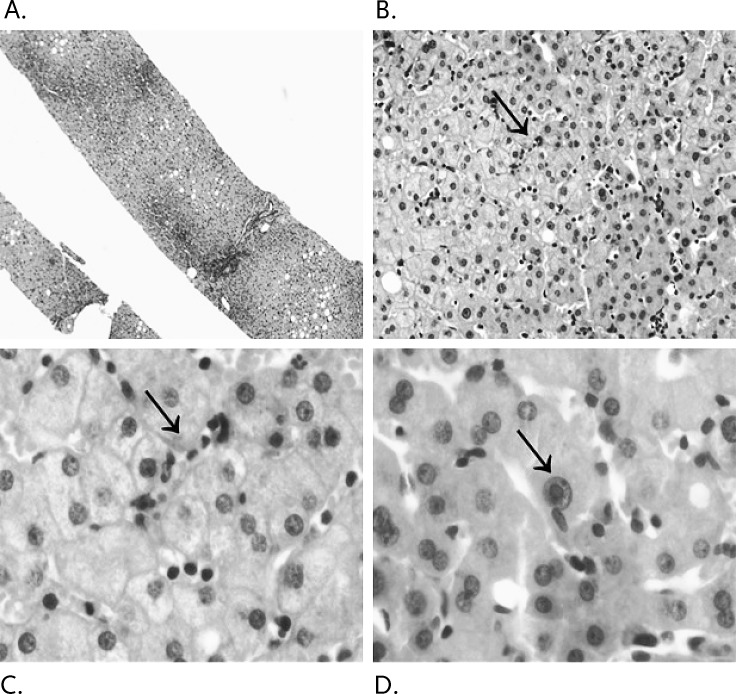
Ganciclovir was initiated on day 8 of his hospitalization at a dose of 5 mg/kg intravenously every 12 hours and discontinued on day 14 with a notable downward trend in the patient's lipase and transaminases. Following this course, his symptoms abated and a repeat CMV PCR was negative (Figure 1). A CT-guided liver biopsy showed mild to moderate infiltrate of small lymphocytes in the sinusoids and a beaded sinusoidal infiltrate characteristic of CMV infection (Figure 2).
Figure 1.
Biochemical profile throughout hospitalization. The graph includes the patient's aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lipase levels.
Figure 2.
Liver histology demonstrating the patient's (A) core liver biopsy, (B) a 20 × magnification view of sinusoidal lymphocytes, (C) a 100 × magnification view of sinusoidal beading, and (D) the viral inclusion body at 100 × magnification.
DISCUSSION
CMV is a DNA virus and a member of the herpesvirus group.4,6,7 CMV's prevalence is high, but its course is usually self-limiting in immunocompetent patients. Our case highlighted a young immunocompetent male who presented with a protracted febrile illness, splenomegaly, lymphocytosis, acute pancreatitis, hepatitis, and CMV viremia. Although severe manifestations of CMV have been reported in immunocompetent adults, CMV in this population remains rare. We found only 3 reports of CMV pancreatitis, regardless of immunologic status. Two cases were reported in HIV-positive patients, while the third was reported in an immunocompetent male.7-9 The immunocompetent patient presented with jaundice and liver dysfunction. He underwent endoscopic retrograde cholangiography that revealed a focal, irregular biliary stricture suspicious for biliary malignancy. A pancreatoduodenectomy was performed. Histology showed frequent CMV inclusion bodies in the ductular and acinar cells of the pancreas, as well as in the epithelial and desquamated cells of the common bile duct. Interestingly, serum IgG suggested past exposure to CMV, but IgM was negative. PCR procedures did not detect CMV DNA, ruling out systemic disease. The patient was not treated with antiviral agents because symptoms of CMV infection resolved after surgery.7
Our patient presented with hepatitis and interstitial pancreatitis, supported by the prolonged persistence of severe pain and the lipase peak.10 His liver biopsy results were consistent with CMV, confirming organ involvement (Figure 2). We recognize the shortcoming of not obtaining definitive proof of CMV pancreatitis via histology. Clinical improvement was rapid following ganciclovir treatment, and histology would not have changed management. We attempted to compensate for this shortcoming by providing workups for other viral, structural, vasculitic, and autoimmune causes of pancreatitis. These evaluations were negative.
Drugs remain another potential confounder, as the patient received treatment for H. pylori infection prior to admission. This therapy was considered a potential culprit in the etiology of pancreatitis. The H. pylori medications were discontinued on admission, and concordant improvement in the transaminases and lipase did not occur until the patient was treated with ganciclovir. The presence of definite CMV hepatitis, as well as systemic viremia, also pointed toward CMV as a probable cause for the patient's pancreatitis.
CMV hepatitis is typically mild and self-limiting, although cases of severe hepatitis have included fatal hepatic necrosis.5,11 In our patient, other causes of hepatitis (including hepatitis A, B, and C; EBV; autoimmune disease; and infiltrative diseases, such as copper overload and hemochromatosis) were excluded. Extensive imaging was also completed, and imaging results were not consistent with a locoregional inflammatory process to explain simultaneous pancreas involvement.
Because of its potential toxicity, ganciclovir is typically reserved for severe CMV infections because of potential complications that include myelosuppression, central nervous system disorders, hepatotoxicity, and teratogenesis.1
CONCLUSION
CMV has been reported to cause disease in immunocompetent patients. Pancreatitis and hepatitis secondary to CMV are rare but should be considered in the workup of immunocompetent patients, especially in the presence of a recent viral-like illness. Treatment of CMV with ganciclovir is typically reserved for severe cases of CMV infection because of potential undesirable side effects, but this treatment may need further investigation as this case demonstrates. The patient exhibited improvement in his clinical symptoms and liver and pancreatic enzymes after initiation of antivirals, and he was eventually cleared of CMV viremia. Early recognition and treatment of CMV hepatitis and pancreatitis could lead to improved outcomes and may spare the patient unnecessary diagnostic or invasive interventions.
Footnotes
*: These authors have contributed to the manuscript equally and share first authorship.
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008 Mar 27;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serna-Higuera C, González-García M, Milicua JM, Muñoz V. Acute cholestatic hepatitis by cytomegalovirus in an immunocompetent patient resolved with ganciclovir. J Clin Gastroenterol. 1999 Oct;29(3):276–277. doi: 10.1097/00004836-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Tzavella K, Zantidis A, Economou I, et al. Portal hypertension caused by acute cytomegalovirus infection with liver involvement in an immunocompetent patient. Scand J Infect Dis. 2007;39(2):177–178. doi: 10.1080/00365540600794410. [DOI] [PubMed] [Google Scholar]
- 4.Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003 Dec 15;37(12):1603–1606. doi: 10.1086/379711. Epub 2003 Nov 18. [DOI] [PubMed] [Google Scholar]
- 5.Shusterman NH, Frauenhoffer C, Kinsey MD. Fatal massive hepatic necrosis in cytomegalovirus mononucleosis. Ann Intern Med. 1978 Jun;88(6):810–812. doi: 10.7326/0003-4819-88-6-810. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Feng J, Qi Y, Dou XG. An immunocompetent adult patient with hepatitis and guillain-barré syndrome after cytomegalovirus infection. Virol J. 2011 Mar 4;8:95. doi: 10.1186/1743-422X-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oku T, Maeda M, Waga E, et al. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol. 2005 Oct;40(10):987–992. doi: 10.1007/s00535-005-1683-z. [DOI] [PubMed] [Google Scholar]
- 8.Cella JP, Gupta S. Diagnosis of cytomegalovirus pancreatitis in AIDS by endoscopic retrograde cholangiopancreaticography. N Engl J Med. 1992 Jan 16;326(3):204. doi: 10.1056/NEJM199201163260316. Erratum in: N Engl J Med. 1992 Mar 19;326(12):844. [DOI] [PubMed] [Google Scholar]
- 9.Joe L, Ansher AF, Gordin FM. Severe pancreatitis in an AIDS patient in association with cytomegalovirus infection. South Med J. 1989 Nov;82(11):1444–1445. doi: 10.1097/00007611-198911000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Banks PA, Freeman ML. Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006 Oct;101(10):2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 11.Lamb SG, Stern H. Cytomegalovirus mononucleosis with jaundice as presenting sign. Lancet. 1966 Nov 5;2(7471):1003–1006. doi: 10.1016/s0140-6736(66)92929-1. [DOI] [PubMed] [Google Scholar]