Abstract
Background
Studies examining the influence of maternal age and birth order on birthweight have not effectively disentangled the relative contributions of each factor to birthweight, especially as they may differ by race.
Methods
A population-based, cross-sectional study of North Carolina births from 1999 to 2003 was performed. Analysis was restricted to 510 288 singleton births from 28 to 42 weeks’ gestation with no congenital anomalies. Multivariable linear regression was used to model maternal age and birth order on birthweight, adjusting for infant sex, education, marital status, tobacco use and race.
Results
Mean birthweight was lower for non-Hispanic black individuals (NHB, 3166 g) compared with non-Hispanic white individuals (NHW, 3409 g) and Hispanic individuals (3348 g). Controlling for covariates, birthweight increased with maternal age until the early 30s. Race-specific modelling showed that the upper extremes of maternal age had a significant depressive effect on birthweight for NHW and NHB (35+ years, p<0.001), but only age less than 25 years was a significant contributor to lower birthweights for Hispanic individuals, p<0.0001. Among all racial subgroups, birth order had a greater influence on birthweight than maternal age, with the largest incremental increase from first to second births. Among NHB, birth order accounted for a smaller increment in birthweight than for NHW and Hispanic women.
Conclusion
Birth order exerts a greater influence on birthweight than maternal age, with signficantly different effects across racial subgroups.
One of the most persistent health disparities in America is the difference in birth outcomes between African-American and white women.1–3 In 2007, compared with non-Hispanic white (NHW), non-Hispanic black (NHB) babies were twice as likely to be low birthweight (LBW) (13.8% vs 7.2%) and almost three times as likely to be very LBW (3.2% vs 1.2%).4 The incidence of LBW is 13.8% for NHB, compared with 7.2% and 6.9% in NHW and Hispanic babies, respectively. The relative differences have remained fairly constant over the past few decades, narrowing only slightly due to an increase in white multifetal gestations.5
Maternal age and birth order, or parity, are important determinants of fetal growth and therefore birthweight. Advancing maternal age is associated with an increasing incidence of pre-existing and pregnancy-related medical complications, such as hypertension and diabetes, as well as genetic and congenital malformations in the offspring.6–9 Increasing parity has been associated with increasing pre-pregnancy maternal weight, which has direct effects on maternal health and possibly birthweight.10,11 Furthermore, increasing parity implies having children in the home already, which could increase social and financial stress for families.12,13
Early studies of birthweight from the 1950s to the 1970s demonstrated varied approaches, often assessed the contribution of maternal age and birth order separately, and had differing analytical results.14–19 Assessing birth order only, Camilleri and Cremona14 and James17 found that birthweight increased with parity. Including both maternal age and birth order, Gebre-Medhin et al,15 Gibson and McKeown16 and O’Sullivan et al19 found that birthweight increased with advancing age and birth order but the authors did not examine the relative contributions separately. Murphy and Mulcahy’s18 Irish study found birthweight increased with maternal age to 30–34 years and with parity through the fourth child. In a newer study, Seidman et al,20 using the Jerusalem Perinatal Study, controlled for maternal age in multivariable analysis and found that birthweight increased with parity. In an analysis of women having at least 10 deliveries, Juntunen et al21 found that parity was an independent determinant of birthweight even until the tenth delivery. More recently, Wilcox et al22 examined 3457 British term births with no maternal complications and detected a 138 g increase in the mean crude birthweight from first to second pregnancy. Although they provide a good foundation, these studies are not necessarily applicable to our current understanding of maternal age, parity and birthweight as they were all conducted 10–60 years ago or outside of the USA.
While it is generally accepted that women have larger babies as they get older and as they have more children, it is unclear how this phenomenon manifests across racial and ethnic subgroups. The ‘weathering hypothesis’, proposed by Geronimus,23 posits that the cumulative and interactive adverse effects of social inequality compound with age, leading to birth outcome disparities through young and middle adulthood. Investigations of weathering have shown that adverse outcomes increase with advancing maternal age at a particularly steep rate among NHB compared with NHW.23–25 If this relationship is robust, we would expect that birthweight among NHB may increase at a slower rate or even decrease with advancing maternal age and parity, as black women ‘weather’ compared with NHW.
Weathering hypothesis analyses have varied from limitation to first births, separation of first and second births, and the inclusion of maternal medical conditions.23–25 These analyses typically focus on the rates of LBW and prematurity rather than birthweight across the entire gestational age spectrum.23–27 Analyses of birthweight-by-gestation distributions have shown that NHB infants are generally smaller than NHW for a given gestational age.28–30 However, such analyses were descriptive in nature and failed to adjust for significant differences among the three groups (eg, maternal age, parity, education and medical complications). In addition, given the rapidly growing Hispanic population in the USA, and the observation that Hispanic rates of LBW and preterm birth are comparable to NHW, only recently has attention been paid to the evaluation of birthweight by gestation for Hispanic individuals.31–33
Given the historic increase in birthweight internationally, combined with the distinctly different racial composition and associated health disparities in the USA, re-examination of the influences of maternal age and birth order with special attention to racial differences is warranted.33 We thus sought to determine how advancing maternal age and birth order individually and jointly influence birthweight and whether differential effects occur across racial subgroups.
METHODS
The North Carolina detailed birth record database contains extensive information on all documented live births in the State of North Carolina, including maternal age, birthweight, gestational age, plurality, maternal medical complications, congenital anomalies, tobacco and alcohol use, number of living children, number of children born alive and now dead and maternal and paternal demographic characteristics. Access to the data, as well as methods for receiving, storing, linking and analysing data and presenting results related to this study, were all governed by a research protocol (#1081) approved by Duke University’s institutional review board.
From 1999 to 2003, 579 594 live births occurred in North Carolina. In order to isolate the relative contributions of advancing maternal age and birth order on birthweight, we restricted our analysis to singleton births between 28 and 42 weeks’ gestational age. Maternal age was limited to 15–44 years and birth order was limited to the first to fourth births. Births with congenital anomalies were excluded. Self-reported maternal race and ethnicity were used to establish three distinct subgroups: NHW, NHB and Hispanic women. The following numbers of births were excluded from the dataset: 18 561 multifetal gestations, 4708 births less than 28 or over 42 weeks’ gestation, 1769 births to mothers under 15 or over 44 years of age, 16 508 births with birth order greater than four, 5287 births with congenital anomalies and 20 828 mothers of other racial subgroups. Among the 511 933 births meeting all inclusion criteria, 1645 observations with missing data for at least one covariate (infant sex, maternal education and maternal marital status, maternal smoking status) or birthweight less than 400 g were omitted from the analyses. Among the 510 288 remaining births, 326 761 were NHW, 122 351 were NHB and 61 176 were Hispanic. Twelve per cent of the Hispanic women in our study population were US born.
Multivariable linear regression modelling of birthweight as explained by maternal age and birth order was implemented on 510 288 births. Models were adjusted for infant sex, maternal education, maternal race, marital status, tobacco use and interaction terms for maternal age and birth order. Maternal age 25–29 years, first births, male infant sex, completed high school education, NHW and married served as the reference groups for all analyses. All analyses were conducted using SAS version 9.1.3 employing an α value of 0.01.
RESULTS
Table 1 describes maternal demographic characteristics and birth outcomes. The majority of subjects were NHW (64%) followed by NHB (24%) and Hispanic (12%). NHB and Hispanic women were more likely to be younger, have lower educational achievement and be unmarried than NHW women. The birth order distribution is roughly equivalent across all three racial/ethnic groups, although a greater portion of births to NHW women are first and second births and a smaller portion are third and fourth births compared with NHB and Hispanic women. Mean birthweight was less for NHB (3166 g) and Hispanic (3348 g) compared with NHW (3409 g) babies (joint comparison p<0.0001).
Table 1.
Demographic characteristics by racial group
| All N=510 288 % |
NHW N=326 761 % |
NHB N=122 351 % |
Hispanic N=61 176 % |
|
|---|---|---|---|---|
| Maternal age (years) | ||||
| 15–19 | 12.69 | 9.36 | 19.94 | 16.02 |
| 20–24 | 28.26 | 23.82 | 35.41 | 37.67 |
| 25–29 | 27.04 | 28.40 | 22.95 | 27.91 |
| 30–34 | 21.67 | 25.98 | 14.17 | 13.64 |
| 35–39 | 8.88 | 10.72 | 6.34 | 4.10 |
| 40–44 | 1.47 | 1.72 | 1.20 | 0.66 |
| Maternal education (completed) | ||||
| Middle school | 5.97 | 1.68 | 1.60 | 37.59 |
| Some high school | 16.03 | 12.02 | 21.60 | 26.26 |
| High school | 30.58 | 28.84 | 39.32 | 22.38 |
| Some college | 23.39 | 24.40 | 24.05 | 8.38 |
| College | 25.03 | 33.05 | 13.43 | 5.39 |
| Unmarried | 33.91 | 19.52 | 66.21 | 46.17 |
| Birth order | ||||
| First | 43.33 | 44.95 | 40.33 | 40.66 |
| Second | 35.07 | 35.99 | 33.54 | 33.18 |
| Third | 16.10 | 14.74 | 18.62 | 18.36 |
| Fourth | 5.50 | 4.31 | 7.50 | 7.80 |
| Infant sex male | 51.07 | 51.23 | 50.64 | 51.10 |
| Gestational age in weeks (mean, SD) | 38.82 (1.83) | 38.86 (1.75) | 38.59 (2.07) | 39.03 (1.71) |
| Birthweight in grams (mean, SD) | 3343.63 (557.97) | 3409.15 (547.47) | 3166.25 (568.67) | 3348.39 (513.20) |
All values are expressed as percentages unless otherwise specified.
NHB, non-Hispanic black; NHW, non-Hispanic white.
Detailed results of mean birthweight by maternal age and birth order are presented in table 2. Reading down the columns, birthweight for first-born infants increases with maternal age from 15 to 19 years to 25–29 years. It remains stable until 35–39 years when birthweight appears to decrease to the 40–44 year range. For second, third and fourth-born infants, birthweight increases with maternal age from 15 to 19 years to 30–34 years. Reading across the rows of table 2, birthweight increases appreciably from first to second births, with a relatively small incremental increase in the younger maternal age categories.
Table 2.
Mean birthweight in grams by maternal age and birth order
| Parity | ||||||||
|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | |||||
| Maternal age (years) |
Sample size |
Mean BW (g) |
Sample size |
Mean BW (g) |
Sample size |
Mean BW (g) |
Sample size |
Mean BW (g) |
| 15–19 | 51285 | 3194 | 11658 | 3206 | 1652 | 3173 | 175 | 3124 |
| 20–24 | 67314 | 3287 | 51743 | 3317 | 20008 | 3268 | 5127 | 3213 |
| 25–29 | 53697 | 3356 | 50382 | 3424 | 25071 | 3268 | 8811 | 3331 |
| 30–34 | 35488 | 3355 | 44714 | 3468 | 22336 | 3458 | 8025 | 3421 |
| 35–39 | 11401 | 3295 | 17781 | 3429 | 11242 | 3451 | 4870 | 3427 |
| 40–44 | 1931 | 3235 | 2671 | 3372 | 1866 | 3361 | 1040 | 3414 |
BW, birthweight.
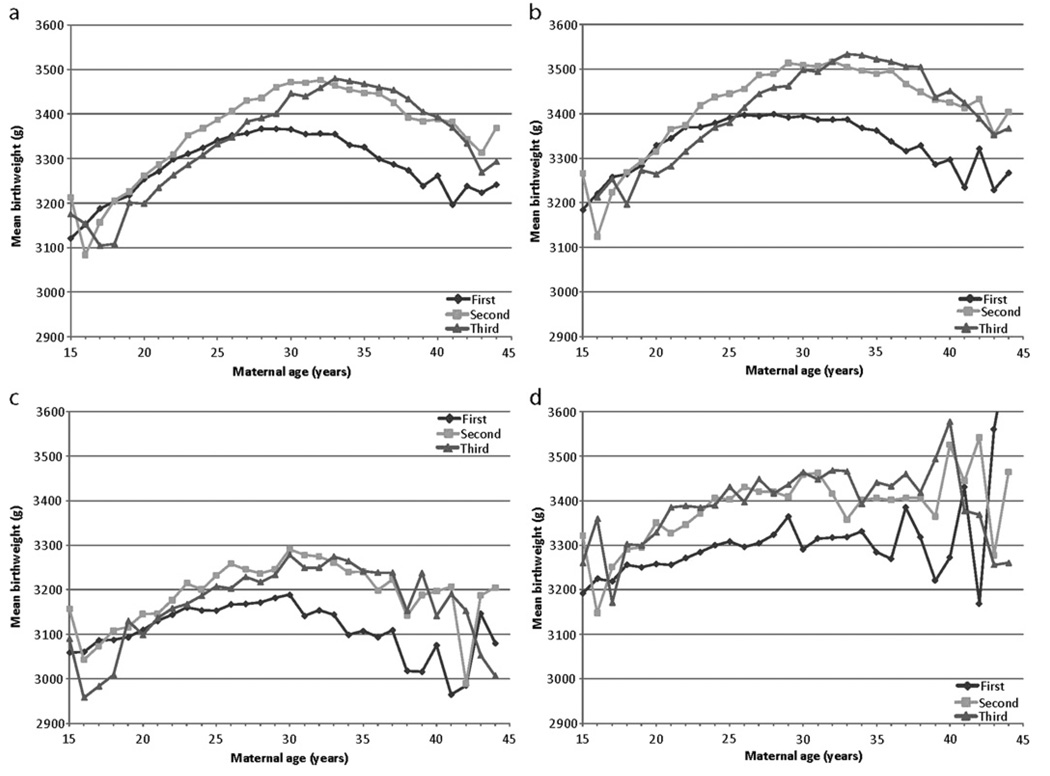
Figure 1A also graphically depicts mean birthweight by maternal age and birth order. Fourth births were excluded from the graph because of significant instability in the curve due to small individual cell sample sizes. Examination of the three curves clearly demonstrates that first births are generally smaller than both second and third births. The mean birthweight for first births is less than second births for all maternal ages greater than 18 years, with the fluctuation before 18 years of age likely to be due to the smaller number of second births in this category. In addition, the mean birthweight is less for first births than for third births for maternal age greater than 26 years. In contrast, the second and third births curves cross several times. Third births lie below second births at younger maternal ages (<33 years) and above at older ages (33–40 years), with greater variability after age 40 years.
Figure 1.
Mean birthweight versus maternal age by parity for (A) all racial subgroups, (B) non-Hispanic (NH) white individuals only, (C) non-Hispanic black individuals only and (D) Hispanic individuals only.
Figures 1B–D further stratify maternal age, birth order and mean birthweight by racial subgroup. There are notable differences and similarities attributable to racial subgroups. For example, when comparing first and second births, the curves appear to diverge at age 19 years for Hispanic women and age 23 years for NHW and NHB women. This indicates that both the magnitude and the timing of the effect of maternal age on birthweight may occur disparately by race. However, the generalisation that second and third-born babies are usually ‘heavier’ than first-born babies holds true for all three race groups.
Whereas figure 1 is informative, it fails to control for standard and well-documented covariates such as the sex of the infant, maternal education, maternal race, marital status and tobacco use. Table 3 presents the results of multivariable regression models of birthweight for the full sample and for racial subgroups, controlling for covariates. Parameter estimates can be interpreted as gram changes in birthweight. All covariates were significant predictors in the model. Male sex accounted for a 116 g increment in birthweight (p<0.0001). Unmarried mothers, on average, had offspring that were 43 g smaller (p<0.0001). Maternal education categories followed the expected dose–response pattern; ie, lower educational attainment was associated with lower birthweight (all p<0.0001). Tobacco use accounted for the largest decrement in birthweight of 226 g (p<0.001). Compared with the NHW referent group, Hispanic ethnicity and NHB race corresponded to 48 and 224 g decrements in birthweight, respectively (both p<0.0001).
Table 3.
Linear regression models of birthweight
| Parameter | Overall Model | Model NHWhite only | Model NHBlack only | Model Hispanic only | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Pr > |t| | Estimate | Pr > |t| | Estimate | Pr > |t| | Estimate | Pr > |t| | |
| Intercept | 3320.0 | <0.0001 | 3316.92 | <0.0001 | 3104.29 | <0.0001 | 3287.44 | <0.0001 |
| Male | 116.20 | <0.0001 | 120.70 | <0.0001 | 112.20 | <0.0001 | 100.15 | <0.0001 |
| Maternal age | ||||||||
| 15–19 | −1.15 | 0.7590 | 17.33 | 0.0004 | 1.46 | 0.8628 | −59.61 | <0.0001 |
| 20–24 | 20.13 | <0.0001 | 35.02 | <0.0001 | 15.95 | 0.0459 | −37.20 | <0.0001 |
| 30–34 | −21.85 | <0.0001 | −19.59 | <0.0001 | −34.25 | 0.0014 | −12.90 | 0.3734 |
| 35–39 | −66.47 | <0.0001 | −61.54 | <0.0001 | −99.8 | <0.0001 | −31.09 | 0.2151 |
| 40–44 | −114.32 | <0.0001 | −117.20 | <0.0001 | −129.40 | <0.0001 | 17.81 | 0.7777 |
| Birth order | ||||||||
| Second | 115.19 | <0.0001 | 119.96 | <0.0001 | 100.56 | <0.0001 | 103.40 | <0.0001 |
| Third | 114.03 | <0.0001 | 117.49 | <0.0001 | 94.34 | <0.0001 | 118.31 | <0.0001 |
| Fourth | 114.53 | <0.0001 | 102.79 | <0.0001 | 85.34 | <0.0001 | 160.18 | <0.0001 |
| Maternal education | ||||||||
| Middle school | −44.54 | <0.0001 | −86.28 | <0.0001 | −24.39 | 0.0632 | −33.17 | <0.0001 |
| Some high school | −39.37 | <0.0001 | −57.01 | <0.0001 | −31.31 | <0.0001 | −15.42 | 0.0097 |
| Some college | 26.27 | <0.0001 | 25.95 | <0.0001 | 31.75 | <0.0001 | 2.11 | 0.8000 |
| College | 62.80 | <0.0001 | 60.95 | <0.0001 | 79.14 | <0.0001 | 10.74 | 0.2986 |
| Not married | −43.32 | <0.0001 | −42.97 | <0.0001 | −54.95 | <0.0001 | −29.08 | <0.0001 |
| Tobacco Use | −225.96 | <0.0001 | −238.36 | <0.0001 | −166.17 | <0.0001 | −135.79 | <0.0001 |
| Maternal race | ||||||||
| Non-Hispanic | −223.96 | <0.0001 | ||||||
| black | ||||||||
| Hispanic | −47.89 | <0.0001 | ||||||
| Age * Birth
order interaction |
||||||||
| 15–19 * second | −79.72 | <0.0001 | −86.99 | <0.0001 | −65.73 | <0.0001 | −59.25 | 0.0003 |
| 15–19 * third | −79.78 | <0.0001 | −94.83 | <0.0001 | −64.30 | 0.0027 | −62.80 | 0.0601 |
| 15–19 * fourth | −94.18 | 0.0212 | −157.90 | 0.0710 | −85.57 | 0.1049 | 44.73 | 0.7018 |
| 20–24 * second | −48.33 | <0.0001 | −53.85 | <0.0001 | −42.46 | <0.0001 | −8.79 | 0.4748 |
| 20–24 * third | −57.19 | <0.0001 | −79.05 | <0.0001 | −37.96 | 0.0017 | 0.14 | 0.9925 |
| 20–24 * fourth | −73.65 | <0.0001 | −88.65 | <0.0001 | −45.14 | 0.0073 | −57.03 | 0.0155 |
| 30–34 * second | 16.39 | 0.0011 | 11.06 | 0.0522 | 35.66 | 0.0101 | 18.79 | 0.3120 |
| 30–34 * third | 45.79 | <0.0001 | 7.44 | <0.0001 | 55.44 | 0.0003 | 35.71 | 0.0621 |
| 30–34 * fourth | 50.53 | <0.0001 | 63.76 | <0.0001 | 58.22 | 0.0024 | 5.51 | 0.8095 |
| 35–39 * second | 25.35 | 0.0004 | 22.47 | 0.0054 | 39.92 | 0.0359 | 5.78 | 0.8570 |
| 35–39 * third | 68.00 | <0.0001 | 66.88 | <0.0001 | 83.46 | <0.0001 | 35.97 | 0.2596 |
| 35–39 * fourth | 73.58 | <0.0001 | 84.72 | <0.0001 | 98.12 | <0.0001 | 14.19 | 0.6828 |
| 40–44 * second | 28.61 | 0.0789 | 25.78 | 0.1582 | 34.39 | 0.3938 | 22.04 | 0.7798 |
| 40–44 * third | 45.56 | 0.0105 | 48.75 | 0.0174 | 33.15 | 0.4287 | 4.68 | 0.9530 |
| 40–44 * fourth | 108.86 | <0.0001 | 148.71 | <0.0001 | 95.32 | 0.0546 | −136.61 | 0.0880 |
By definition, race was not included as a covariate in the race-specific models.
Grey highlighting denotes statistical significance using α=0.01.
Reference groups=female sex, maternal age 25–29 years, married, non-smoker, first birth order, high school completed, non-Hispanic (NH) white race, interaction term 25–29 years.
First birth overall and each individual age category.
Infants of mothers aged 15–19 years had weights indistinguishable from those of the 25–29 year reference group. Mothers in the 20–24 year age group had larger infants than 25–29-year-old mothers (p<0.0001). Mothers aged 30–34, 35–39 and 40–44 years had infants with significantly lower birthweights of 22, 66 and 114 g, respectively (both p<0.0001). This adjusted analysis confirms the descriptive results in figure 1: birthweight increases with maternal age, but only until the early-30s.
Second, third, and fourth births tended to produce significantly heavier infants weighing 115, 114 and 115 g more than first-born infants, respectively (all p<0.0001). Also, parity had a larger impact on birthweight compared with maternal age, as noted by the overall larger parameter estimates for birth order than maternal age categories.
The interaction term that crosses maternal age with birth order documents the dual influence of these two variables on birthweight. For example, for women in the 15–19 years age category, second birth order has an adverse effect on birthweight (p<0.0001), suggesting that the positive influence on birthweight of second birth order is mitigated by the negative influence of having a second child at such a young age (~80 g reduction). Whereas the interaction with third birth order is the same (~80 g reduction), fourth birth order for mothers aged 15–19 years was also negative but was not statistically significant. This is most likely due to the small number of births in this category (n=175).
For women in the 20–24 years age group, the interaction of age and any birth order category was associated with a significantly adverse effect on birthweight. In contrast, for mothers in the 30–34 year age group, the interaction of age and any birth order was associated with a statistically significant increase in birthweight. This suggests that beyond the maternal age and birth order influences in isolation, the combination of being 30–34 years old and being parous leads to estimated increases in birthweight of 16, 46 and 51 g, for second, third and fourth births, respectively. While the sizes of these coefficients are small, the interactive effects remain important. Similar patterns are seen for the 35–39 and 40–44 years age groups.
In order to examine how maternal age and birth order affect birthweight differentially across racial/ethnic lines, similar models of birthweight were constructed separately for NHW, NHB and Hispanic women, as shown in table 3. As in the full model, covariates of infant sex, maternal education, marital status and tobacco use were statistically significant in all three models. Whereas the parameter estimates cannot be directly contrasted across the models, the magnitude, relationship to other covariates and achievement of statistical significance within each individual model can be compared. For NHW mothers, age followed a pattern similar to the full model except for the 15–19 year age category, which had a positive and significant impact on birthweight. Whereas maternal age less than 30 years was not significant for NHB women, this was not the case for Hispanic women. The younger maternal age categories, 15–19 and 20–24 years, had a significant and negative effect on infant birthweight, whereas the older age categories were not statistically different from the 25–29-year-old referent group.
Parity was associated with an increase in birthweight among all three racial subgroups, with the largest incremental increase between first and second births (120, 101 and 103 g for NHW, NHB and Hispanic women, respectively). Note that parity has a smaller effect for NHB than for the other groups. Low maternal educational attainment and not being married have a larger depressive effect on birthweight among infants born to NHW women. High educational attainment has the largest positive effect on birthweight among infants born to NHB women.
The interaction terms of maternal age and birth order provide some interesting results. Having a second, third, or fourth child at maternal age 20–24 years magnified the negative effect on birthweight for infants born to NHW and NHB women, but not so for those born to Hispanic women. The interaction terms for Hispanic women were not significant for women aged 30–44 years. However, for NHW and NHB women, an increment to birthweight for women having second, third and fourth births while aged 30–34 years was observed for both groups. A similar pattern was observed for women aged 35–39 years.
DISCUSSION
Using a large population-level cohort of births from New Carolina, we examined the joint effects of maternal age and birth order on birthweight and how these effects vary across racial subpopulations. By restricting our analysis to singleton births without congenital anomalies, we were able to focus on the relative contribution of maternal age and parity in comparison with previous studies and also control for important maternal demographic and behavioural characteristics.
Graphical representation of mean birthweight by maternal age and parity demonstrates interesting relationships among the different curves. These key curve crossover points (26 years for first to third and 32 years for second to third) represent time points after which the interaction of maternal age and parity has a positive effect on birthweight. For example, women having a third birth in their early 30s compared with their early 20s are more likely to have adequate spacing between births. (The older women are also more likely to have achieved a more stable financial status.) As a result, both the maternal age component and parity component exert a positive influence on birthweight, with the not-so-surprising result that the joint influence is synergistic.
Higher parity at younger maternal ages, particularly 15–19 year olds having their second or third birth, appears to have adverse effects on birthweight. Blankson et al34 found that adolescents with an adverse outcome such as preterm birth or fetal growth restriction during their first pregnancy had a significantly increased risk of recurrence in their second pregnancy. This is especially troubling given that 30–50% of primiparous adolescents will have a second birth within 12–24 months.35 In addition, pregnant adolescents often have additional risk factors that can affect birth outcomes, for example inadequate prenatal care, tobacco and substance use, single parenthood and ongoing reproductive development and maturation.36
While controlling for parity, we determined that birthweight increases with maternal age up to the early 30s but then tends to level off. The largest effect of parity on birthweight comes at the transition from first to second births when, on average, a 115 g incremental increase in birthweight was seen. The increases in birthweight from first to third or fourth births are statistically significant, but the incremental change with each subsequent birth is negligible (−1 and 0 g for third and fourth births, respectively). Therefore, both higher parity and advancing maternal age tend to increase birthweight, with the former effect larger than the latter.
Both the graphical displays and multivariable linear regression modelling results demonstrate that first births are clearly distinct from second, third and fourth births, regardless of racial subgroup. Other than at the extremes of age, first-born infants are generally smaller than infants of higher birth order, for reasons that are unclear. Studies of pregnancy outcomes often restrict analyses to first births, thus limiting the ability to evaluate any interactive effect of parity and maternal age. Our results underscore the importance of including and specifically addressing the contribution of parity in investigations of pregnancy outcomes.
The contributions of maternal age and birth order in our study sample notably differed by race. Parity, but not maternal age, strongly affected birthweight for Hispanic women, whereas both maternal age and birth order appreciably influenced infant birthweight for NHW and NHB women. The weathering hypothesis of Geronimus23 originally focused on the accumulation of negative effects associated with advancing maternal age only. The contribution of higher parity on birth outcomes and birthweight could be an additionally important aspect of 'weathering'. In their examination of the contribution of maternal age to racial disparities in birthweight, Rauh and colleagues24 analysed first and second births separately. They demonstrated that low birthweight did increase with advancing age at a steeper rate for NHB than for NHW; however, this was mostly explained by the increasing rates among first births only, whereas the rates of low birthweight remained relatively constant across maternal ages for both races. Although the mean birthweight did increase with both maternal age and parity for all race groups, our findings show that the increase in birthweight among NHB women is not as steep as the incremental increase for the other racial subgroups. Consistent with the ‘weathering’ hypothesis, it is thus plausible that the cumulative impact of social and economic adversity faced by NHB women has a collective effect on birthweight.
Because our analysis utilised a cross-sectional dataset, we are not able to address how maternal age and parity affect birthweight within an individual mother. Furthermore, modelling of mean birthweight may diminish or obscure the importance of births with weights at the extremes, either very small or very large. Whereas differences in the prevalence of LBW by race are well known, less is known about the incidence of large-for-gestational age (LGA) or macrosomia by race.4 We did examine the contribution of race to LGA (results not shown) and found that both Hispanic and NHB women had a lower risk compared with NHW women. Diabetes, a known contributor to LGA, was actually highest among NHB women and lowest among Hispanic women in our study population. Further research is warranted to determine why NHW women are at higher risk for delivering macrosomic infants.
Although we included mothers with medical conditions such as hypertension, diabetes and anaemia in our study population, we chose not to adjust for these as covariates in our analysis. Maternal medical conditions probably function as intermediate effects or outcomes of advancing maternal age and parity on birthweight, particularly given the varying prevalence of disease by race. Furthermore, maternal medical complications may not be reported accurately in birth certificate registries. An additional constraint of using birth record data for analysis is the lack of detailed socioeconomic measures such as employment status or individual/household income, making it difficult for us to examine the social patterning of maternal age. While we were limited to using maternal education and marital status as measures of social status, both factors were significant contributors to infant birthweight regardless of maternal racial group. Overall, our investigation provides an important insight into the significant racial disparities in pregnancy outcomes in the USA and identifies the need for additional analyses across the entire distribution of births.4
What is already known on this subject.
One of the most persistent and unexplained health disparities in the USA is the difference in birth outcomes. For example, LBW occurs among 13.8% of NHB births compared with 7.2% and 6.9% among NHW and Hispanic births, respectively. Maternal age and birth order are important determinants of birthweight. Birthweight has been shown to increase with advancing maternal age and birth order. However, studies thus far have not effectively disentangled the relative contributions of each factor to birthweight, especially as they may differ by race.
What this study adds.
We utilised a population-level birth cohort in the USA to examine how advancing maternal age and birth order individually and jointly influence birthweight and whether differential effects occur across racial subgroups. Birthweight increased with maternal age until the early 30s. In race-specific modelling, maternal age 35 years or greater had a significant depressive effect on birthweight for NHW and NHB, but only age less than 25 years was a significant contributor to LBW for Hispanic women. Overall, birth order had a greater influence on birthweight than maternal age, with the largest incremental increase from first to second births. However, birth order accounted for a smaller increment in birthweight for NHB than for NHW and Hispanic women. Our investigation provides a valuable insight into the significant racial disparities in birth outcomes in the USA and the importance of including and specifically addressing the contribution of birth order in future research.
Acknowledgments
Funding This research was supported by funding from the National Institutes of Health (5P2O-RR020782-O3) and the Environmental Protection Agency (RD-83329301-0).
Footnotes
This work was presented at the 41st Annual Society for Epidemiologic Research Meeting, Chicago, Illinois, 24–27 June 2008.
Competing interests None declared.
Ethics approval This study was conducted with the approval of the Duke’s Institutional Review Board #1081.
Provenance and peer review Not commissioned; not externally peer reviewed.
REFERENCES
- 1.Johnston RB, Williams MA, Hogue CJ, et al. Overview: new perspectives on the stubborn challenge of preterm birth. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):3–6. doi: 10.1046/j.1365-3016.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh GK, Yu SM. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. Am J Public Health. 1995;85:957–964. doi: 10.2105/ajph.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise PH, Kotelchuck M, Wilson ML, et al. Racial and socioeconomic disparities in childhood mortality in Boston. N Engl J Med. 1985;313:360–366. doi: 10.1056/NEJM198508083130605. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2007. Natl Vital Stat Rep. 2009;57 [PubMed] [Google Scholar]
- 5.Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981–98. Paediatr Perinat Epidemiol. 2002;16:8–15. doi: 10.1046/j.1365-3016.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 6.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58:282–285. [PubMed] [Google Scholar]
- 7.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727–733. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 8.Joseph KS, Allen AC, Dodds L, et al. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105:1410–1418. doi: 10.1097/01.AOG.0000163256.83313.36. [DOI] [PubMed] [Google Scholar]
- 9.van Katwijk C, Peeters LL. Clinical aspects of pregnancy after the age of 35 years: a review of the literature. Hum Reprod Update. 1998;4:185–194. doi: 10.1093/humupd/4.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Sobal J, Frongillo EA, et al. Parity and body weight in the United States: differences by race and size of place of residence. Obes Res. 2005;13:1263–1269. doi: 10.1038/oby.2005.150. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe WS, Sobal J, Olson CM, et al. Parity-associated weight gain and its modification by sociodemographic and behavioral factors: a prospective analysis in US women. Int J Obes Relat Metab Disord. 1997;21:802–810. doi: 10.1038/sj.ijo.0800478. [DOI] [PubMed] [Google Scholar]
- 12.Bell CB, Johnson JE, McGillicuddy-Delisi AV, et al. Normative stress and young families: adaptation and development. Family Relations, Family Stress, Copying and Adaptation. 1980;29:453–458. [Google Scholar]
- 13.Oumlstberg M, Hagekull B. A structural modeling approach to the understanding of parenting stress. J Clin Child Adolesc Psychol. 2000;29:615–625. doi: 10.1207/S15374424JCCP2904_13. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri AP, Cremona V. The effect of parity on birthweight. J Obstet Gynaecol Br Commonw. 1970;77:145–147. doi: 10.1111/j.1471-0528.1970.tb03493.x. [DOI] [PubMed] [Google Scholar]
- 15.Gebre-Medhin M, Gurovsky S, Bondestam L. Association of maternal age and parity with birth weight, sex ratio, stillbirths and multiple births. J Trop Pediatr Environ Child Health. 1976;22:99–102. doi: 10.1093/tropej/22.3.99. [DOI] [PubMed] [Google Scholar]
- 16.Gibson JR, McKeown T. Observations on all births (23,970) in Birmingham, 1947. V. Birth weight related to economic circumstances of parents. Br J Soc Med. 1951;5:259–264. doi: 10.1136/jech.5.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James WH. Birth weight and birth order. Ann Hum Genet. 1969;32:411–412. doi: 10.1111/j.1469-1809.1969.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JF, Mulcahy R. The effect of age, parity, and cigarette smoking on baby weight. Am J Obstet Gynecol. 1971;111:22–25. doi: 10.1016/0002-9378(71)90920-3. [DOI] [PubMed] [Google Scholar]
- 19.O’Sullivan JB, Gellis SS, Tenney BO. Aspects of birth weight and its influencing variables. Am J Obstet Gynecol. 1965;92:1023–1029. doi: 10.1016/0002-9378(65)90739-8. [DOI] [PubMed] [Google Scholar]
- 20.Seidman DS, Ever-Hadani P, Stevenson DK, et al. Birth order and birth weight reexamined. Obstet Gynecol. 1988;72:158–162. [PubMed] [Google Scholar]
- 21.Juntunen KS, Laara EM, Kauppila AJ. Grand grand multiparity and birth weight. Obstet Gynecol. 1997;90:495–499. doi: 10.1016/s0029-7844(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox MA, Chang AM, Johnson IR. The effects of parity on birthweight using successive pregnancies. Acta Obstet Gynecol Scand. 1996;75:459–463. doi: 10.3109/00016349609033354. [DOI] [PubMed] [Google Scholar]
- 23.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42:589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 24.Rauh VA, Andrews HF, Garfinkel RS. The contribution of maternal age to racial disparities in birthweight: a multilevel perspective. Am J Public Health. 2001;91:1815–1824. doi: 10.2105/ajph.91.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Buka SL, Brennan RT, et al. Diverging associations of maternal age with low birthweight for black and white mothers. Int J Epidemiol. 2003;32:83–90. doi: 10.1093/ije/dyg008. [DOI] [PubMed] [Google Scholar]
- 26.Grady SC. Racial disparities in low birthweight and the contribution of residential segregation: a multilevel analysis. Soc Sci Med. 2006;63:3013–3029. doi: 10.1016/j.socscimed.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Lane SD, Teran S, Morrow CB, et al. Racial and ethnic disparity in low birth weight in Syracuse, New York. Am J Prev Med. 2003;24:128–132. doi: 10.1016/s0749-3797(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26:260–267. doi: 10.1053/sper.2002.34772. [DOI] [PubMed] [Google Scholar]
- 29.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data 2006. Natl Vital Stat Rep. 2009;57 [PubMed] [Google Scholar]
- 30.Zhang J, Bowes WA. Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstet Gynecol. 1995;86:200–208. doi: 10.1016/0029-7844(95)00142-e. [DOI] [PubMed] [Google Scholar]
- 31.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 32.Ogunyemi D, Manigat-Wilson B, Bazargan M, et al. Birth weight for gestational age patterns by ethnicity, gender, and parity in an urban population. South Med J. 2007;100:615–616. doi: 10.1097/SMJ.0b013e318048798c. [DOI] [PubMed] [Google Scholar]
- 33.Overpeck MD, Hediger ML, Zhang J, et al. Birth weight for gestational age of Mexican American infants born in the United States. Obstet Gynecol. 1999;93:943–947. doi: 10.1016/s0029-7844(98)00553-5. [DOI] [PubMed] [Google Scholar]
- 34.Blankson ML, Cliver SP, Goldenberg RL, et al. Health behavior and outcomes in sequential pregnancies of black and white adolescents. JAMA. 1993;269:1401–1403. [PubMed] [Google Scholar]
- 35.Coard SI, Nitz K, Felice ME. Repeat pregnancy among urban adolescents: sociodemographic, family, and health factors. Adolescence. 2000;35:193–200. [PubMed] [Google Scholar]
- 36.Sangalang BB, Barth RP, Painter JS. First-birth outcomes and timing of second births: a statewide case management program for adolescent mothers. Health Soc Work. 2006;31:54–63. doi: 10.1093/hsw/31.1.54. [DOI] [PubMed] [Google Scholar]