To the Editor
Dedicator of cytokinesis 8 (DOCK8) deficiency is a primary combined immune deficiency characterized by profound T cell lymphopenia and impaired humoral antibody responses, increased serum IgE levels and eosinophilia 1, 2. DOCK8, encodes a guanine nucleotide exchange factor that coordinates the actin cytoskeleton response to mitogenic and chemokine signals through the reversible activation of small G proteins, including cell division cycle 42 (CDC42) 3. Subjects with DOCK8 deficiency are particularly susceptible to viral infections, especially with herpes simplex (HSV), varicella zoster (VZV), human papillomavirus (HPV), molluscum contagiousum (MCV) and JC viruses. Overall, these infections represent a major cause of morbidity and at times mortality in DOCK8 deficiency. We here describe two patients with DOCK8 deficiency and severe, progressive oral herpes labialis infection refractory to therapy with acyclovir and its analogue valacyclovir, who were successfully treated with systemic interferon alpha-2b (IFN-α 2b). Importantly, analysis revealed profound deficiency of plasmacytoid dendritic cells (pDC), the major immune cell population involved in IFNα production, in DOCK8 deficient subjects.
The demographic and genetic findings of the two patients are detailed in Table E1, and the immunological findings in Table E2 at the online Repository www.jacionline.org. The first patient is a Turkish girl with a homozygous splice acceptor mutation in intron 16 of DOCK8 (c.1869 –1 G>C) who first presented at the age of five years with herpes labialis, confirmed by a positive Tzanck smear, at 5 years of age. Her infection initially resolved with oral acyclovir treatment but later recurred, leading to her placement on oral acyclovir prophylaxis. At the age of 6 ½, and despite prophylaxis, breakthrough disease recurred and progressively worsened with tissue destruction despite high acyclovir and later valacyclovir therapy (1500mg/m2 and 150 mg/kg/d, respectively) (Figure 1A, B). Pegilated IFN-α 2b was applied topically to little effect. Subcutaneous pegilated IFN-α 2b was then started on a weekly dose of 40 μg/m2, and the dose was increased stepwise over 4 months to 80 microgram/m2/wk. Clinical response was noted after reaching a dose of 60 microgram/m2/wk during the second month of therapy, and complete remission was achieved at 6 months of therapy (Figure 1C). The patient continues to be maintained on IFN-α 2b at 80 microgram/m2/wk with no recurrence of disease and no complications of therapy.
Figure 1.
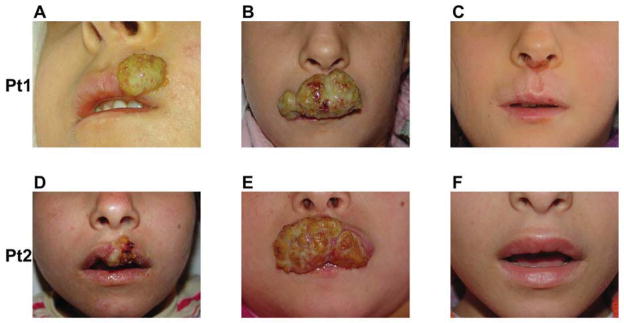
Representative pictures of the lesions in patient (Pt) 1 and 2 early in their infection (A, D), just before IFN-α 2b therapy (B, E), and 6 months into IFN-α 2b therapy (C, F), respectively.
The second case is a Turkish girl with a homozygous deletion in DOCK8, encompassing exon 26 through the 3′ end of the gene, who was hospitalized at the age of six years with a four-week history of purulent otitis media resistant to oral antibiotics, a left ear ulceration and oral vesicular lesions that were positive on Tzanck smears, consistent with herpes labialis. She was hospitalized with intravenous antibiotics and acyclovir. IVIG prophylaxis was restarted (0.5g/kg every 3 weeks). Oral herpes labialis persisted with increasing severity and tissue destruction despite acyclovir and valcyclovir therapy (Figure 1D, E). Subcutaneous pegilated IFN-α 2b was started at 40 μg/m2 and escalated up to 80 μg/m2 followed by weekly maintenance therapy, as detailed for the first patient, with complete and sustained remission of her lesions (Figure 1F).
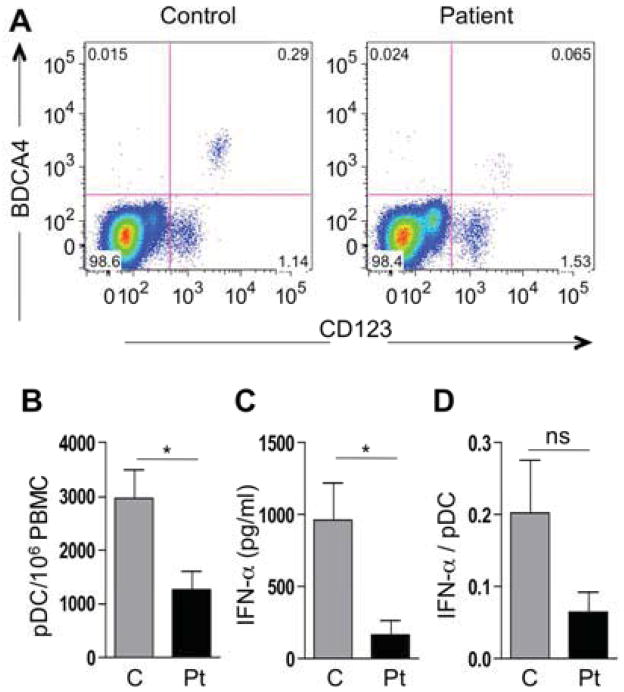
pDCs play a key role in the innate immune response to viral infections, including HSV, by virtue of their capacity to produce copious amounts of type I IFN upon activation 4. pDCs sense virus nucleoside-based products derived from DNA viruses including herpes through toll-like receptor 9 (TLR9) and the TLR adaptor MyD88, a pathway that is profoundly impaired in DOCK8 deficiency, leading us to examine the capacity of patient pDCs to produce IFN-α in response to unmethylated CpG dinucleotides 5. To that end, we first analyzed the population of pDCs present in the peripheral blood of a group of DOCK-deficient as compared to control subjects. Details of the clinical and genetic findings of the DOCK8-deficient subjects we tested are provided in Table E1 at the online Repository www.jacionline.org. Flow cytometric analysis of pDCs, identified by the markers CD123 and BDCA-4, revealed that their severe deficiency in the peripheral blood is a common attribute of DOCK8-deficient subjects (Figure 2A, B). We further examined the capacity of peripheral blood mononuclear cells (PBMC) to produce IFN-α in vitro in response to CpG-A DNA (ODN 2216) treatment, as measured by ELISA, (Mabtech AB). IFN-α production was profoundly depressed in patients compared to control subjects, consistent with the markedly decreased number of pDCs in the former group (Figure 2C). There was also decreased IFN-α production on per cell basis down to one third of control values that did not achieve significance (p=0.1), suggesting this poor responsiveness to viral DNA may potentially compound pDC deficiency as a cause of decreased IFN-α production in this disease (Figure 2D).
Figure 2.
A. Representative FACS analysis for BDCA-4+ CD123+ pDCs in the lymphocyte gate of PBMCs from a control and a DOCK8-deficient subject. B. Total number of pDCs/106 cells in controls (C, n=10) and patients (Pt, n= 9). C. IFN-α production in supernatants of CpG-stimulated PBMCs from controls (n=9) and patients (n=8). D. Production of IFN-α/pDC in controls and patients (n=7 each). *P<0.05.
The mechanisms by which DOCK8 deficiency compromises the pDC compartment are presently unclear, and may include aberrant development, peripheral sequestration and/or poor survival. DOCK8 regulates interstitial DC migration in response to chemotactic factors, suggesting that impaired mobilization of pDCs may also be a contributing mechanism 3.
IFN-α 2b therapy may act by inhibiting viral replication and also by activating effector lymphocytes, including NK and cytotoxic T cells 4. Its utility in DOCK8 deficiency may extend beyond HSV infections to include other viral pathogens. IFN-α has previously been employed in a child with Heck’s disease, associated with chronic oral and vulvar HPV infection, later documented to have DOCK8 deficiency due to deletion in exon 38 2, 6. IFN-α 2b therapy markedly improved the papillomatosis.
Beside circulating pDC deficiency, other mechanisms have been invoked to explain the unusual susceptibility to viral infections in DOCK8 deficient subjects. These include poor CD8+ cytotoxic T cell memory responses, deficiency of natural killer T cells, impaired natural killer cell function and defective DOCK8 and MyD88-dependent Toll-like receptor signaling 5, 7–9. These defects may synergistically interact to precipitate severe, persistent viral infections in DOCK8-deficient patients.
In summary, deficiency of circulating pDCs and its attendant decreased IFN-α production are previously undescribed attributes of DOCK8 deficiency that likely contribute to the heightened susceptibility to severe viral infections, especially those affecting the mucocutaneous compartment. Systemic IFN-α therapy may be beneficial in those DOCK8-deficient subjects who prove unresponsive to conventional antiviral chemotherapies.
Supplementary Material
Acknowledgments
The work was supported by the National Institutes of Health (5R01AI065617, 1R21AI087627 to T.A.C. and 1R01AI100315 to R.S.G.), and by a grant from the Scientific and Technological Research Council of Turkey (1059B191300622 to S.K.).
Abbreviations
- CDC42
Cell division cycle 42
- DOCK8
Dedicator of cytokines 8
- HPV
Human papilloma virus
- HSV
Herpes simplex virus
- IFN-α 2b
Interferon alpha-2b
- MCV
Molluscum contagiousum virus
- pDC
Plasmacytoid dendritic cell
- TLR9
Toll-like receptors 9
- VZV
Varicella zoster virus
- PBMC
Peripheral blood mononuclear cells
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302. e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–61. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–20. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozarmagan G, Didem Yazganoglu K, Agacfidan A. Hyper-IgE syndrome with widespread premalign oral papillomas treated with interferon alpha2b. Acta Derm Venereol. 2005;85:433–5. doi: 10.1080/00015550510030041. [DOI] [PubMed] [Google Scholar]
- 7.Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, et al. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122:2052–61. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–8. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.