Abstract
Dental tissues provide an alternate source of stem cells compared with bone marrow and have a similar potency as that of bone marrow derived mesenchymal stem cells. It has been established there are six types of dental stem cells: Dental pulp stem cells, stem cells from human exfoliated deciduous teeth, stem cells from apical papilla, periodontal ligament stem cells, dental follicle progenitor cells, oral periosteum stem cells and recently gingival connective tissue stem cells. Most of the dental tissues have a common developmental pathway; thus, it is relevant to understand whether stem cells derived from these closely related tissues are programmed differently. The present review analyzes whether stem cells form dental tissues depict distinct characteristics by gaining insight into differences in their immunophenotype. In addition, to explore the possibility of establishing a unique phenotypic fingerprint of these stem cells by identifying the unique markers that can be used to isolate these stem cells. This, in future will help in developing better techniques and markers for identification and utilization of these stem cells for regenerative therapy.
Keywords: Dental papilla, dental pulp, dental sac, mesenchymal stem cells, periodontal ligament, stem cells
INTRODUCTION
Mesenchymal stem cells (MSCs) were first identified in aspirates of adult bone marrow. They developed clonogenic clusters of adherent fibroblastic colony-forming units with the potential to undergo extensive proliferation in vitro and to differentiate into different stromal cell lineages.[1] Since then, bone marrow was the most utilized source of MSCs; however, there was a need to isolate MSCs from accessible tissues with less surgical trauma. In recent years, stem cells from dental tissues have provided that alternate source of MSCs with characterization of stem cells within the dental pulp stem cells (DPSCs),[2] periodontal ligament stem cells (PDLSCs),[3,4,5] stem cells from human exfoliated deciduous teeth (SHED),[6] dental follicle progenitor cells (DFPCs),[7] stem cells from apical papilla (SCAP),[8] oral periosteum stem cells (OPSCs)[9] and recently from gingival connective tissue (GING SCs) [Figure 1].[10] Dental stem cells (SCs) can differentiate into odontoblasts, adipocytes, neuronal-like cells, glial cells, osteoblasts, chondrocytes, melanocytes, myotubes and endothelial cells.[2,4,5,8] Possible applications of these cells in various fields of medicine makes them good candidates for future research as a new, powerful tool for therapy.
Figure 1.
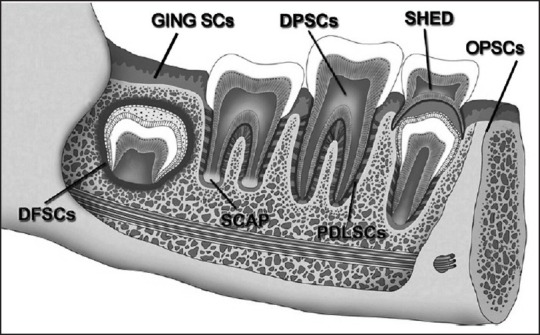
Depicting the various sources of stem cells within the dental tissues. The dental pulp dental pulp stem cells, periodontal ligament stem cells, stem cells from exfoliated deciduous teeth, dental follicle progenitor cells, stem cells from apical papilla, oral periosteum stem cells and gingival connective tissue stem cells
Various experiments have compared the characteristics of dental stem cells. Differences have been noted between DPSCs and bone marrow mesenchymal stem cells (BMMSCs), wherein DPSCs were shown to share a similar pattern of protein expression with BMMSCs in vitro.[11] Comparison of the in vitro phenotypic characteristics of DPSCs and SCAP has shown that SCAP represent an early stem/progenitor cells with superior cell source for tissue regeneration.[12] Stem cells from human dental pulp, dental follicle (DF) and root apical papilla from wisdom teeth have been analyzed for the expression of transcription factors (Octamer 4 [Oct-4], Nanog and Sox-2) and cell surface markers cluster of differentiation (CD) 44, CD90 and CD105.[13] It was observed that there was a high expression of transcription factors in SCAP and osteogenic differentiation was less in DPSCs compared with SCAP. The PDLSCs have been observed to express the antigens CD90, CD29, CD44, CD166, CD105 and CD13 that are identified as stromal precursors of the bone marrow.[14,15] Despite lots of encouraging data on dental stem cell and the vast quantity of experiments focused on them, several open questions have remained about their biology; of the important ones is their immunophenotype. Over the recent years, a variety of phenotypic markers including adhesion molecule, lineage antigens, growth factor receptors, cytokine/chemokine receptors, immune-related proteins etc., on MSCs from different origins, have been investigated.[16,17] Conflicting results emphasize the need for gathering more information to complete our understanding of dental stem cells phenotype. There have been no systematic comparisons of the phenotypic characteristics in terms of putative stem cell markers expressed by the dental stem cells. The present review compares the phenotypes of dental stem cell populations by analyzing differences in expression of various cell-surface markers used to identify putative MSCs.[2] In addition, to gain insight into the unique “phenotypic fingerprint” of these stem cells by ascertaining whether these stem cells depict distinct immunophenotype.
METHODS TO ESTABLISH PHENOTYPES OF DENTAL STEM CELLS
Phenotypically, dental stem cells express a range of surface markers (including CD49a/CD29, CD44, STRO-1, CD90, CD105, CD106, CD146, CD140b, CD166 and CD27). This suggests a common link between different cell types because most of these markers are expressed by all MSCs.[2,18,19,20] Immunophenotyping is a technique used to study the protein expressed by cells. This technique is commonly used in basic science research and laboratory diagnostic purposes. This can be carried out on tissue section (fresh or fixed tissue), cell suspension, etc. The tests used to detect immunophenotypes are:[21]
Fluorescent microscopy – identifies stem cell surface markers using the fluorescent tags [Figure 2] that are attached to a cell receptor on the stem cell. Each specific stem cell marker can be identified on the cell surface by viewing under the fluorescent microscope by their fluorescence in a particular wavelength of light. An e.g., is the expression of pluripotent markers identified on DPSCs and PDLSCs such as Vimentin, Oct-4 and Nanog[22] [Figure 3].
Fluorescent activated cell sorting (FACS) — a laser beam is used to separate cell fractions from the larger population, using differential light absorbing or fluorescing properties of certain stem cell populations. It has been observed that expression of classical markers such as CD34, CD45 and CD133 differ between virtually same stem and progenitor cells which are endothelial or MSCs, when they were obtained from different tissues.[20,21] This finding raises questions whether phenotypic differences are due to the source or is only caused by different isolation and experimental conditions. FACS facilitates identification of a variety of markers. The method of detection is depicted[23,24,25] [Figure 4].
Figure 2.
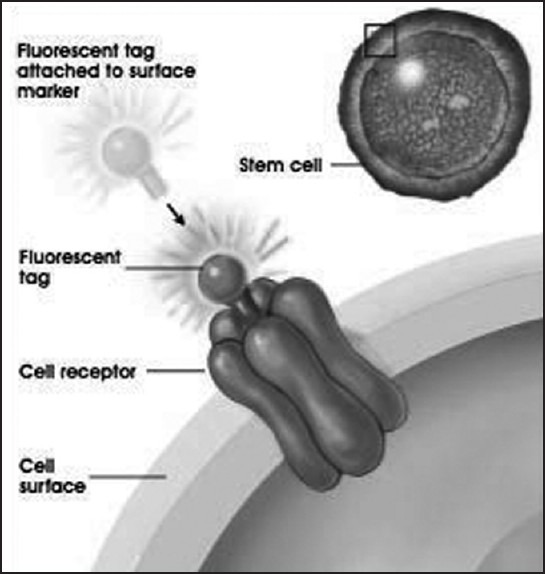
Identifying stem cell surface marker by indirect immunoflourescence by tagging with fluorescent antibodies
Figure 3.
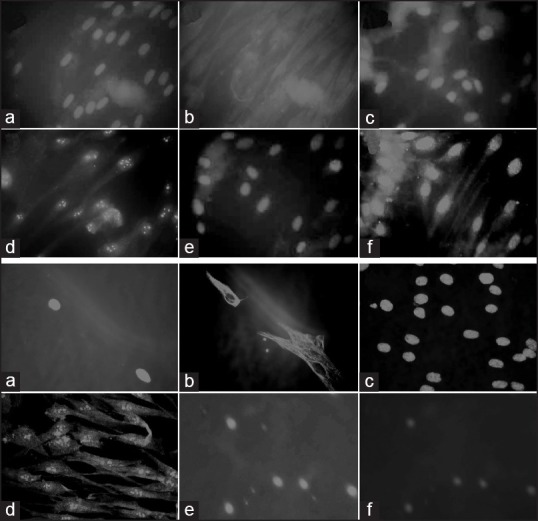
Identifying pluripotent markers by fluorescent microscopy in dental pulp and periodontal ligament. (a) Depicting DPSCs expression of Vimentin (a and b), Octamer 4 (Oct-4) (c and d) and Nanog (e and f). Blue fluorescence depicts the nuclei of the cells and the markers are identified by green fluorescence. (b) Depicting periodontal ligament stem cells expression of Vimentin (a and b), Oct-4 (c and d) and Nanog. (e and f) The antibodies are fluorescence isothiocyanate conjugated. The images are original ×40
Figure 4.
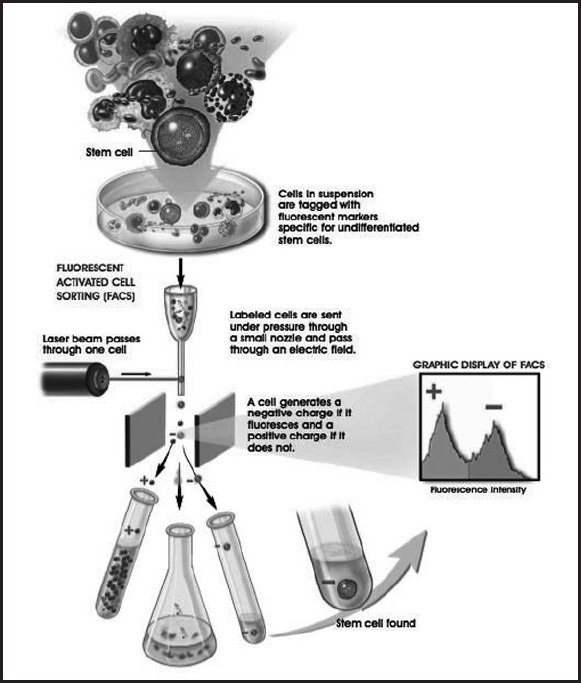
Fluorescent activated cell sorting methodology wherein a cell suspension tagged with fluorescent markers is passed under pressure through a nozzle and through an electric field. A laser beam then sorts the cells according to the + or – charge. A negative charge is emitted by a cell, which is fluorescent and positive if the cell is not fluorescent
Dental stem cells usually cannot be distinguished by a single stem cell marker, because their expression partially overlaps between lineages;[26] however, the specificity of surface phenotype suggests that the stem cell is a distinct functional unit.[23,24,25,27,28,29]
STEM CELLS MARKERS AND THEIR SIGNIFICANCE
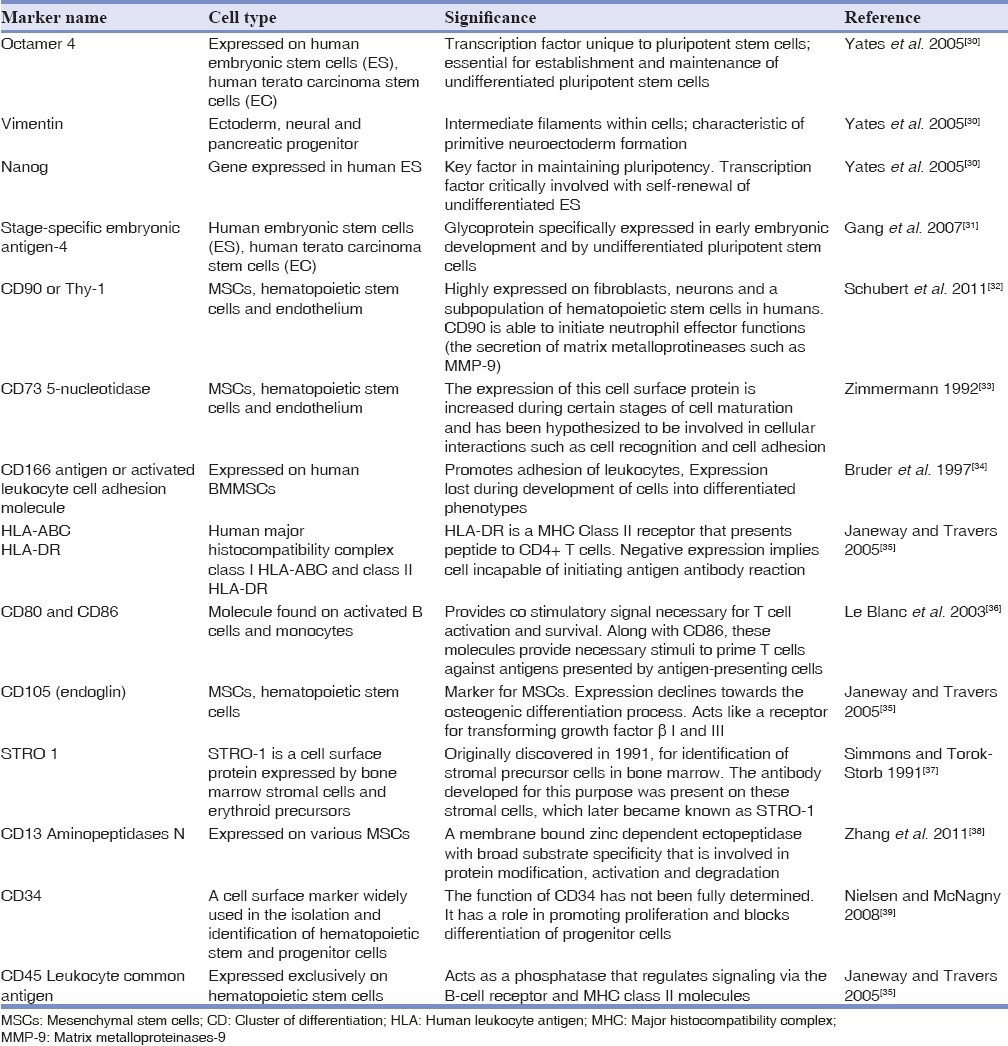
A few relevant surface and intracellular stem cell markers are described in Table 1.[30,31,32,33,34,35,36,37,38,39] These markers presence or absence can help to establish phenotypes of MSCs.
Table 1.
A summary of the panel of cell surface markers used to characterize MSCs
CHARCTERISTICS OF DENTAL STEM CELLS POST NATAL DPSCS
The DPSCs represent less than 1% of the total cell population present in dental pulp.[40] The presence of stem cells in dental pulp was proposed by Fitzgerald et al.[41] However, the identification and isolation of DPSCs in the adult dental pulp was first reported by Gronthos et al. in 2000[2] after which they identified stromal stem cells in the bone marrow[42] and found that DPSCs have similar characteristics as that of BMMSCs. They extensively studied the stem cell properties of human DPSCs and observed that DPSCs are also capable of differentiation into adipocytes and neural — like cells.[43] Heterogeneous populations of DPSCs exist in the dental pulp, which is capable of multilineage differentiation.[44] It is believed that these cells reside in the various regions inside the dental pulp. In adult dental tissue, the stem cell niches are usually quiescent and become activated only after injury.[45] Various labeling studies have shown that notch signaling plays a vital role in DPSCs differentiation and proliferation.[46,47] It has been observed that DPSCs produced bone instead of dentin when these cells were implanted into subcutaneous sites in immunocompromised mice with hydroxyapatite tricalcium phosphate powder as their carrier.[48] DPSCs thus belong to a novel population of post-natal somatic stem cells.
Immunophenotype of DPSCs
Although there is no specific marker for identifying DPSCs, it has been noted that they express a range of mesenchymal and bone marrow stem cell markers such as STRO-1, CD146 and CD31.[43] They also express embryonic stem cell markers such as Oct-4, Nanog and mesenchymal marker Vimentin.[22] Recently, SSEA4, an embryonic stem cell marker has been shown to be useful in identifying DPSCs and it was observed that 46% of the DPSCs were SSEA-4 positive.[49] It has been observed that the there are two populations of DPSCs, one of neural crest origin and other of mesenchymal origin.[45] Both populations express the markers STRO-1 and CD31.
SHED
It was first isolated by Miura et al. in 2003[6] from dental pulp of exfoliated deciduous teeth and observed to be highly proliferative and clonogenic. The isolation technique was similar to that used for DPSCs. Although, there were some differences compared with DPSCs.
-
a)
SHED formed sphere-like cell-cluster formation, which could be separated and grown as individual fibroblast – like cells.
-
b)
Higher proliferation rate than DPSCs and high number of colony forming cells.
SHED were thought to represent a more immature multipotent stem cells than DPSCs.
Immunophenotype of SHED
These cells express the cell surface molecules STRO-1 and CD146, two early MSC markers also known to be expressed by BMMSCs and DPSCs. These STRO-1 and CD146 positive cells are located around blood vessels suggesting that SHED possibly originate from a perivascular environment.[6] It has also been observed that SHED express embryonic stem cells markers oct4, nanog, stage specific embryonic antigens (SSEA-3, SSEA-4).[50,51] Owing to their higher proliferation rate and their osteogenic potential they are considered a more immature form than DPSCs.
SCAP
During tooth development, the dental papilla evolves into the dental pulp and contributes to the development of the root. The apical part of the dental papilla is loosely attached to the developing root and is separated from the differentiated pulp tissue by a cell rich zone. It contains less blood vessels and cellular components than the pulp tissue and separating rich zone.[8,12] In 2006, Sonoyama et al. isolated a new population of dental stem cells and called them SCAP.[52]
Immunophenotype of SCAP
SCAP are clonogenic fibroblast-like cells, but have a higher proliferation rate than DPSCs.[52] As other dental stem cells, SCAP express the early mesenchymal surface markers, STRO-1 and CD146. However, SCAP also express CD24, which could be a unique marker for this cell population as it is not detected on DPSCs or BMMSCs.[8] The expression of CD24 by SCAP is down regulated in response to osteogenic stimulation. However, the biological significance of this finding needs to be investigated. SCAP show a two- to threefold higher proliferation rate than do DPSCs and are more committed to osteo/dentinogenicity. SCAP exhibit a heterogeneous nature by showing:
Co-expression of STRO-1 with a variety of osteo/dentinogenic markers and
A low percentage of STRO-1 – positive cells while a high percentage of cells positive with osteo/dentinogenic markers in cultures.
PDLSCs
The periodontal ligament (PDL) is a specialized connective tissue derived from the DF and originates from neural crest cells.[53,54] Seo et al. identified stem cells in human PDL and found that PDLSCs implanted into nude mice generated cementum/PDL-like structures that resemble the native PDL as a thin layer of cementum that interfaced with dense collagen fibers, similar to Sharpey's fibers. These cells can also differentiate into adipocytes, odontoblasts, myotubes, NFM-positive neuron-like cells, glial fibrillary acidic protein-positive astrocyte-like cells and CNPase-positive oligodendrocyte-like cells.[4,55,56]
Immunophenotype of PDLSCs
PDLSCs and DPSCs both showed similar characteristics as compared with BMMSCs. All cell types were strongly positive for CD44, CD90 (cell surface markers associated with stromal cells), CD105 and CD166 (cell surface markers associated with stromal cells and endothelial cells), but negative for CD40, CD80 and CD86 (cell surface markers of hematopoietic cells).[57] PDLSCs and DPSCs expressed HLA-ABC (MHC class I antigen) similar to BMMSCs, while HLA-DR (MHC class II antigen) expression was not detected in these cell populations.[22] PDLSCs express a variety of stromal cell markers like CD90, CD29, CD44, CD105, CD166 and CD13.[14] PDLSCs also express STRO-1 and CD146 and scleraxis (tendon specific transcription factor). Scleraxis is found to be highly expressed in PDLSCs than in DPSCs and BMMSCs. This finding supports the fact that periodontal ligament has similar structural characteristics as that of a tendon and can withstand mechanical stress during physiological activity.[3] The possibility of obtaining stem cells from cryopreserved adult human PDL[4] indicates that samples from tissue banks could be viable for future applications.
DFPCs
In 2005, Morsczeck et al. isolated stem cells from the DF of the human impacted third molar, using the same methodology of DPSCs isolation and culture. DFPCs are localized in the DF, a mesenchymal tissue that surrounds the tooth germ and can be easily isolated after wisdom tooth extraction.[7,58] The DF is a loose connective tissue of an ectomesenchymal origin and it is present as a sac surrounding the unerupted tooth.[59] During tooth development, it has been found that DF plays a significant role in the eruption process by controlling the osteoclastogenesis and osteogenesis needed for eruption.[60,61] It is also believed that DF differentiates into the periodontium as the tooth is erupting and becomes visible in the oral cavity.[62] However, these cells are available only from patients during wisdom tooth eruption, usually between 15 years and 28 years of age.[63]
Immunophenotype of DFPCs
The cells are observed to be fibroblast-like and expressed putative stem cell markers such as Nestin, Notch-1 and STRO-1.[7,58,64] Compared with bone marrow-derived stem cells, DFPCs express higher amounts of IFF-2 transcripts. In addition, these cells have also been found to be positive for Vimentin, a typical marker for mesenchymal cells.[58] DFPCs were also able to differentiate and express cementoblast markers (cementum attachment protein and cementum protein-23) after being induced with enamel matrix derivatives, or bone morphogenetic protein-1 (BMP-1) and BMP-7.[64]
GING SCs
The presence of stem cells in gingival connective tissue was investigated by Mitrano et al..[10] Consequently, the identification of MSCs in human gingiva was carried out and they were characterized phenotypically and functionally. The gingiva derived MSCs also had the potential to differentiate into osteocytes, chondrocytes and adipocytes.
Immunophenotype of GING SCs
The immunophenotype characterization was evaluated from culture at the third through fifth passages. A positive immunostaining was consistently obtained for gingival MSCs including CD90, CD105, CD73, CD44 and CD13. They were weakly positive or negative for typical hematopoietic markers such as CD45, CD34, CD54 and CD38.[10]
OPSCs
OPSCs were first isolated by characteristic surface markers in 2005.[65] Their osteogenic properties were compared with stem cells of different origin such as bone marrow and alveolar process. The percentage of mineralization observed with OPSCs at 12 weeks was equal to 58.2% versus 26.9% of BMMSCs and 41.1% of those in origin from the alveolar process.[66] The mesengenic multipotency of cryopreserved OPSCs was evaluated for chondrogenesis, osteogenesis and adipogenesis. They were found to differentiate into mesodermal lineages and maintained growth until passage 15.[67]
Immunophenotype of OPSCs
Using FACS, it was observed that OPSCs expressed CD 9, CD90, CD105 and CD166.[66]
ASCERTAINING THE PHENOTYPIC FINGERPRINT OF DENTAL STEM CELLS
The distinct phenotypes of dental stem cells can be ascertained by analyzing differences in expression of various cell-surface markers used to identify putative MSCs.[2] The present review attempts to gain insight into their “phenotypic fingerprint” by analyzing whether these stem cells depict distinct immunophenotypes. The in vitro phenotypes of various dental stem cell populations observed until date compared to BMMSCs is summarized in Table 2.
Table 2.
Comparison of in vitro phenotypes of dental stem cells to BMMSCs
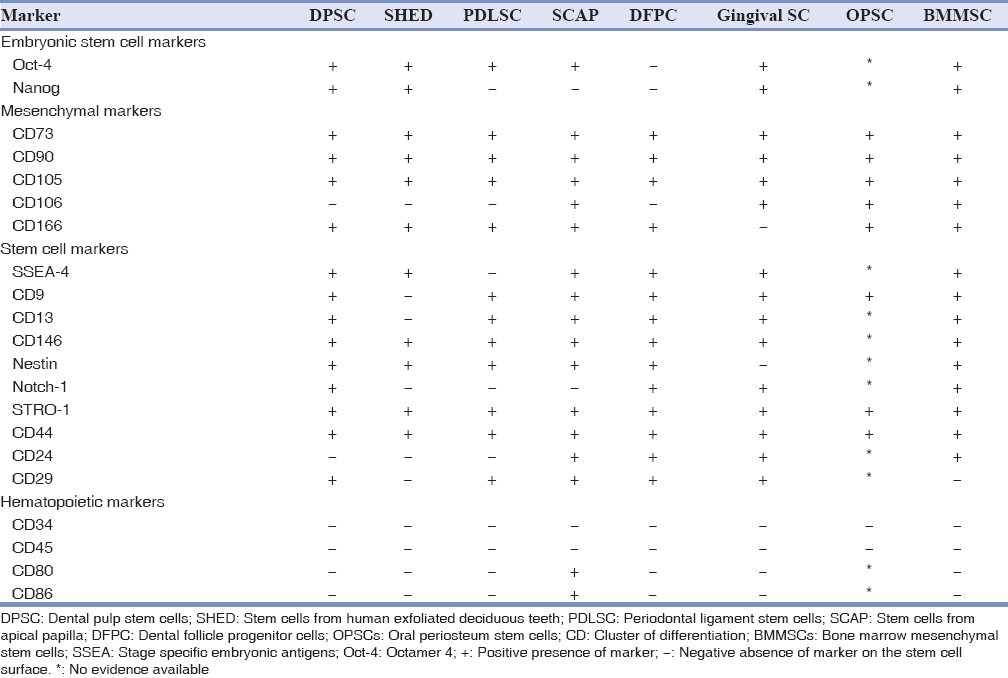
It can be observed that PDLSCs have a unique phenotype compared to DPSCs [Table 2]. The DPSCs are positive for SSEA4 and Notch-1 embryonic stem cell markers and PDLSCs are negative for it. This suggests that DPSCs are a more primitive population of stem cells.
CD29 on the other hand is negative in DPSCs and positive in PDLSCs. CD106, which is negative in both DPSCs and PDLSCs is positive in OPSCs. Nanog, which is a marker for pluripotency is absent in PDLSCs, SCAP and DFPCs suggesting they are multipotent stem cells.
Understanding that different stem cells express distinct phenotypes may have further implications in understanding the factors that regulate the formation of mineralized matrices and other associated connective tissues. Different levels of expression of certain stem cell markers will help in understanding the multi lineage differentiation potential of these dental stem cells and which population can be utilized for regeneration of specific tissues such as bone, periodontal ligament, cementum and even neurogenic tissues. For e.g., CD9, a protein highly expressed in cells that differentiate into osteogenic precursors, is expressed more in OPSCs[66] and PDLSCs[22] compared to other dental stem cells. The difference in expression of CD9 suggests that these stem cells are more likely to differentiate into osteogenic precursors. This knowledge in a clinical scenario will lead to novel tissue engineering strategies in future utilizing stem cells. A tissue engineering approach using stem cells for bone augmentation is depicted, which can be carried out as a chair side method or using bio engineered matrices. [Figure 5].[68]
Figure 5.
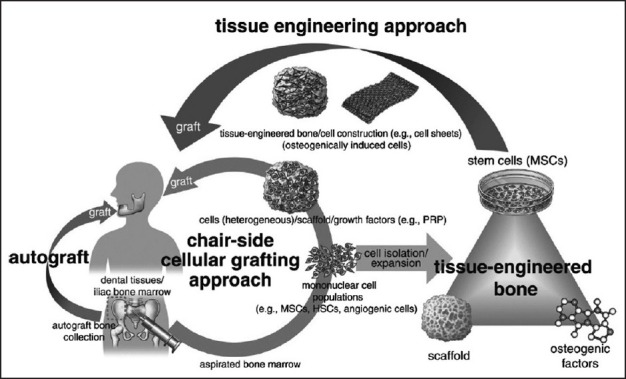
Depicting current clinical approaches to stem-cell-based bone augmentation. The chair-side cellular grafting approach (orange arrow) uses patient-derived freshly processed bone marrow (mononuclear cell population), which contains mesenchymal stem/stromal cells, hematopoietic stem cells and angiogenic cells, mixed with a scaffold and growth factors, like platelet-rich plasma as a grafting material
CONCLUSION
The craniofacial/dental complex is continuously remodeling and subject to wear and tear.[55] This lends credence to the fact there are many populations of stem cells within this complex located in unique niches and it has been observed there is more than one population of stem cells even within the periodontal ligament.[22] This suggests the heterogeneous nature of stem cells in the dental complex.
The present review provides an insight into the understanding of how MSCs from related tissue could depict distinct characteristics. Understanding that different stem cells express distinct phenotypes may have further implications in understanding the factors that regulate the formation of mineralized matrices and other associated connective tissues. Different levels of expression of certain stem cell markers will help in understanding the multi lineage differentiation potential of these dental stem cells and which population can be utilized for regeneration of specific tissues such as bone, periodontal ligament, cementum and even neurogenic tissues.
However, definitive experiments like in situ hybridization of specific markers and further clonal studies need to be performed to highlight the heterogeneous nature of stem cells from dental tissues. However, identifying the unique phenotypic fingerprints of dental stem cells provides streamlined regenerative therapies with predictable outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 2.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 4.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–12. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 5.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodontal Res. 2006;41:547–53. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 6.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicconetti A, Sacchetti B, Bartoli A, Michienzi S, Corsi A, Funari A, et al. Human maxillary tuberosity and jaw periosteum as sources of osteoprogenitor cells for tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:618.e1–12. doi: 10.1016/j.tripleo.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Mitrano TI, Grob MS, Carrión F, Nova-Lamperti E, Luz PA, Fierro FS, et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–25. doi: 10.1902/jop.2010.090566. [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–9. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JH, Park BW, Byun JH, Kang EJ, Rho GJ, Shin SH, et al. Isolation and characterization of human dental tissue-derived stem cells in the impacted wisdom teeth: Comparison of dental follicle, dental pulp, and root apical papilla-derived cells. J Korean Assoc Oral Maxillofac Surg. 2010;36:186–96. [Google Scholar]
- 14.Trubiani O, Di Primio R, Traini T, Pizzicannella J, Scarano A, Piattelli A, et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–21. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- 15.Lindroos B, Mäenpää K, Ylikomi T, Oja H, Suuronen R, Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329–35. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 17.Ten Cate AR. Tencate. Tencate's Oral Histology, Development, Structure and Function. 8th ed. St. Louis: Mosby Elsevier; 2008. Development of the tooth and its supporting tissues; pp. 79–107. [Google Scholar]
- 18.Gronthos S, Simmons PJ. The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood. 1995;85:929–40. [PubMed] [Google Scholar]
- 19.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–6. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 20.Lin NH, Gronthos S, Bartold PM. Stem cells and future periodontal regeneration. Periodontol 2000. 2009;51:239–51. doi: 10.1111/j.1600-0757.2009.00303.x. [DOI] [PubMed] [Google Scholar]
- 21.Siggins RW, Zhang P, Welsh D, Lecapitaine NJ, Nelson S. Stem cells, phenotypic inversion, and differentiation. Int J Clin Exp Med. 2008;1:2–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Ponnaiyan D, Bhat KM, Bhat GS. Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. Int J Immunopathol Pharmacol. 2012;25:127–34. doi: 10.1177/039463201202500115. [DOI] [PubMed] [Google Scholar]
- 23.Alhadlaq A, Mao JJ. Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev. 2004;13:436–48. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 24.Bonner WA, Hulett HR, Sweet RG, Herzenberg LA. Fluorescence activated cell sorting. Rev Sci Instrum. 1972;43:404–9. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- 25.Herzenberg LA, De Rosa SC. Monoclonal antibodies and the FACS: Complementary tools for immunobiology and medicine. Immunol Today. 2000;21:383–90. doi: 10.1016/s0167-5699(00)01678-9. [DOI] [PubMed] [Google Scholar]
- 26.Giordano G, La Monaca G, Annibali S, Cicconetti A, Ottolenghi L. Stem cells from oral niches: A review. Ann Stomatol (Roma) 2011;2:3–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Yutoku M, Grossberg AL, Pressman D. A cell surface antigenic determinant present on mouse plasmacytes and only about half of mouse thymocytes. J Immunol. 1974;112:1774–81. [PubMed] [Google Scholar]
- 28.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–65. [PubMed] [Google Scholar]
- 29.Julius MH, Masuda T, Herzenberg LA. Demonstration that antigen-binding cells are precursors of antibody-producing cells after purification with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1972;69:1934–8. doi: 10.1073/pnas.69.7.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates A, Chambers I. The homeodomain protein Nanog and pluripotency in mouse embryonic stem cells. Biochem Soc Trans. 2005;33:1518–21. doi: 10.1042/BST0331518. [DOI] [PubMed] [Google Scholar]
- 31.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 32.Schubert K, Polte T, Bönisch U, Schader S, Holtappels R, Hildebrandt G, et al. Thy-1 (CD90) regulates the extravasation of leukocytes during inflammation. Eur J Immunol. 2011;41:645–56. doi: 10.1002/eji.201041117. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann H. 5’-Nucleotidase: Molecular structure and functional aspects. Biochem J. 1992;285:345–65. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruder SP, Horowitz MC, Mosca JD, Haynesworth SE. Monoclonal antibodies reactive with human osteogenic cell surface antigens. Bone. 1997;21:225–35. doi: 10.1016/s8756-3282(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA, Jr, Travers P. Antigen presentation to T lymphocytes. In: Janeway CA, Travers P, Walport M, Shlomchik M, editors. Immunobiology. 6th ed. New York: Garland Science; 2005. pp. 70–7. [Google Scholar]
- 36.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 37.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 38.Zhang X, Zhang J, Zhang L, Feng J, Xu Y, Yuan Y, et al. Design, synthesis and biological evaluation of novel 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives as aminopeptidase N/CD13 inhibitors. Bioorg Med Chem. 2011;19:6015–25. doi: 10.1016/j.bmc.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–92. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 40.Sloan AJ, Waddington RJ. Dental pulp stem cells: What, where, how? Int J Paediatr Dent. 2009;19:61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald M, Chiego DJ, Jr, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707–15. doi: 10.1016/0003-9969(90)90093-p. [DOI] [PubMed] [Google Scholar]
- 42.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 43.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 44.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waddington RJ, Youde SJ, Lee CP, Sloan AJ. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs. 2009;189:268–74. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- 46.He F, Yang Z, Tan Y, Yu N, Wang X, Yao N, et al. Effects of Notch ligand Delta1 on the proliferation and differentiation of human dental pulp stem cells in vitro. Arch Oral Biol. 2009;54:216–22. doi: 10.1016/j.archoralbio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Chang J, Sonoyama W, Shi S, Wang CY. Inhibition of human dental pulp stem cell differentiation by Notch signaling. J Dent Res. 2008;87:250–5. doi: 10.1177/154405910808700312. [DOI] [PubMed] [Google Scholar]
- 48.Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007;31:1191–7. doi: 10.1016/j.cellbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Kawanabe N, Murata S, Fukushima H, Ishihara Y, Yanagita T, Yanagita E, et al. Stage-specific embryonic antigen-4 identifies human dental pulp stem cells. Exp Cell Res. 2012;318:453–63. doi: 10.1016/j.yexcr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–16. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 51.Koyama N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501–6. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coura GS, Garcez RC, de Aguiar CB, Alvarez-Silva M, Magini RS, Trentin AG. Human periodontal ligament: A niche of neural crest stem cells. J Periodontal Res. 2008;43:531–6. doi: 10.1111/j.1600-0765.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 54.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–91. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 56.Techawattanawisal W, Nakahama K, Komaki M, Abe M, Takagi Y, Morita I. Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochem Biophys Res Commun. 2007;357:917–23. doi: 10.1016/j.bbrc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–76. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 58.Morsczeck C, Völlner F, Saugspier M, Brandl C, Reichert TE, Driemel O, et al. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig. 2010;14:433–40. doi: 10.1007/s00784-009-0310-4. [DOI] [PubMed] [Google Scholar]
- 59.Ten Cate AR. The development of the periodontium – A largely ectomesenchymally derived unit. Periodontol 2000. 1997;13:9–19. doi: 10.1111/j.1600-0757.1997.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 60.Cahill DR, Marks SC., Jr Tooth eruption: Evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 61.Wise GE, Frazier-Bowers S, D’Souza RN. Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med. 2002;13:323–34. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- 62.Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- 63.Morsczeck C, Schmalz G, Reichert TE, Völlner F, Galler K, Driemel O. Somatic stem cells for regenerative dentistry. Clin Oral Invest. 2008;12:113–8. doi: 10.1007/s00784-007-0170-8. [DOI] [PubMed] [Google Scholar]
- 64.Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–94. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 65.Lim SM, Choi YS, Shin HC, Lee CW, Kim DI. Isolation of human periosteum-derived progenitor cells using immunophenotypes for chondrogenesis. Biotechnol Lett. 2005;27:607–11. doi: 10.1007/s10529-005-3625-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhu SJ, Choi BH, Huh JY, Jung JH, Kim BY, Lee SH. A comparative qualitative histological analysis of tissue-engineered bone using bone marrow mesenchymal stem cells, alveolar bone cells, and periosteal cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:164–9. doi: 10.1016/j.tripleo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Choi YS, Noh SE, Lim SM, Lee CW, Kim CS, Im MW, et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett. 2008;30:593–601. doi: 10.1007/s10529-007-9584-2. [DOI] [PubMed] [Google Scholar]
- 68.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry – Part II: Clinical applications. J Prosthodont Res. 2012;56:229–48. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]


