Abstract
Background:
The aim of this study was to quantify the number of mast cells in focal reactive hyperplastic lesions of the oral cavity and to compare these two number of mast cells in normal gingival tissues and to correlate their presence with the state of connective tissue changes in reactive lesions and probably suggest a role for mast cells in these lesions.
Materials and Methods:
Patient records were retrieved during a 10 year period from 2001 to 2010. Data of all reactive hyperplasias namely focal fibrous hyperplasia, pyogenic granuloma (PG), peripheral ossifying fibroma (POF) and peripheral giant cell granuloma (PGCG) were reviewed and 10 cases seen in the gingiva were selected for each category and stained with 1% toluidine blue for mast cells. Statistical analysis was applied to see the significant differences between the groups and with the normal gingival tissue. One-way ANOVA-F and unpaired t-test was applied and significant differences were seen between the groups at 5% level of significance.
Results:
In this study, mast cell count was maximum in POF and fibrous hyperplasia (FH) followed by cases of PG and PGCG.
Conclusion:
The number of mast cells was more numerous in POF and FH suggesting that mast cell activation is a characteristic feature of chronic inflammation, a condition that may lead to fibrosis as a result of increased collagen synthesis by fibroblasts.
Keywords: Fibrous hyperplasia, mast cells, peripheral giant cell granuloma, pyogenic granuloma, peripheral ossifying fibroma, toluidine blue
INTRODUCTION
Oral mucosa is constantly subjected to external and internal stimuli and therefore manifests a spectrum of diseases that range from developmental, reactive and inflammatory to neoplastic.[1] Reactive hyperplastic lesions represent the most frequently encountered oral mucosal lesions in humans.[2] These lesions represent a reaction to some kind of irritation or low grade injury such as chewing, trapped food, calculus, fractured teeth and iatrogenic factors including overextended flanges of dentures and overhanging dental restorations.[3] Kfir et al. have specifically classified reactive hyperplastic lesions into pyogenic granuloma (PG), peripheral giant cell granuloma (PGCG), peripheral ossifying fibroma (POF) and fibrous hyperplasia (FH).[4] Despite the presence of chronic etiologic factor for all of these entities, there is variation for their histopathological characteristics. Mast cells have a critical role in the development of inflammation in the oral mucosa, both in early vaso-inductive events and in the transition from acute to chronic inflammation suggesting that mast cells may play a role in recruitment of inflammatory cells and angiogenesis.[5] The role of mast cells in connective tissue is still a matter of speculation and it has been suggested that these cells participate in cell regulation and in the control of the accumulation of connective tissue components. The present study was thus, undertaken to identify as well as quantify mast cells in oral reactive lesions and compare it with the average count of mast cells in normal oral mucosa. The study aimed at determining the average mast cell count in normal oral mucosa and to determine average count of mast cells in oral reactive lesions and to assess the role of these cells in the pathogenesis of the reactive lesions of the oral cavity.
MATERIALS AND METHODS
In this study, all existing records in the archives of oral pathology and microbiology, Subharti Dental College, Meerut were extracted between 2001 and 2010. Patient records were assessed to select those with the histopathological diagnosis of reactive hyperplastic lesions as classified by Kfir et al.[4] The cases for inclusion in this study were those categorized as FH, PG, POF and PGCG. Clinical data regarding age, gender, location of the lesions were obtained for each case from the patient records. After reviewing the histological slides, 10 cases seen in the gingiva were selected for each category and stained with 1% toluidine blue for mast cells. Because sulfated proteoglycans in secretory granules of mast cells have a metachromatic property that can be stained by toluidine blue, the solution used to stain mast cells contained toluidine blue, which was 0.2 g in 100 ml of distilled water and 2 ml of acetic acid.[6] The advantage of this study was the utilization of cheaper and more accessible staining method.[7] As controls, 10 clinically normal oral mucosa tissue specimens were obtained from those who had surgical removal of impacted tooth and stained for comparative analysis of mast cells with the reactive lesions [Figures 1–5]. Mast cells were counted in 10 high power fields (X400) where the highest number of mast cells was seen. The degranulated mast cells were not taken into count as these cells lose their characteristic features after degranulation and are difficult to be counted histologically. Data were analyzed statistically using One-way ANOVA-F and unpaired t-test and 5% level of significance was considered [Tables 1–4].
Figure 1.
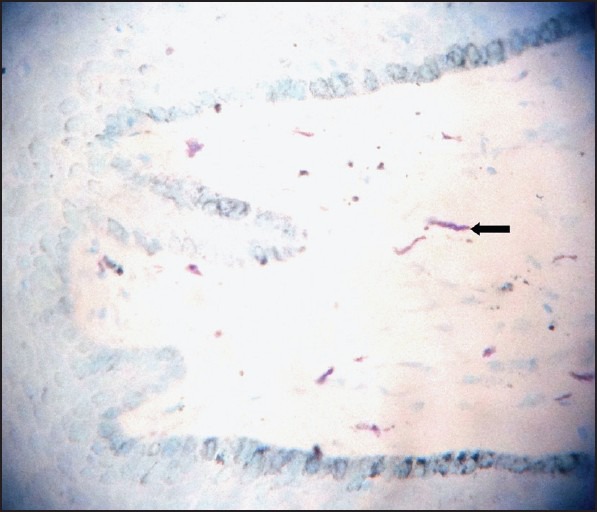
Normal gingival tissue stained with Toulidine blue (×100)
Figure 5.
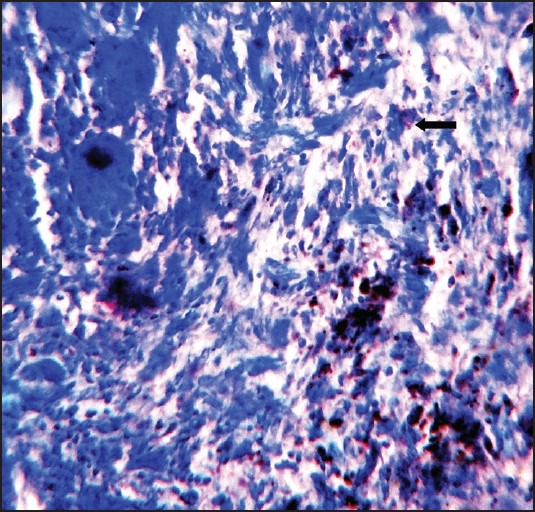
Peripheral giant cell granuloma showing minimal mast cells stained with Toulidine blue (×100)
Table 1.
Mean and SD of different mast cell counts
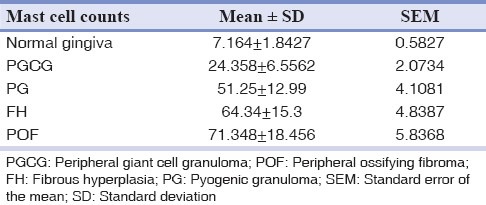
Table 4.
Comparisons of different mast cell counts with normal gingival using unpaired t-test
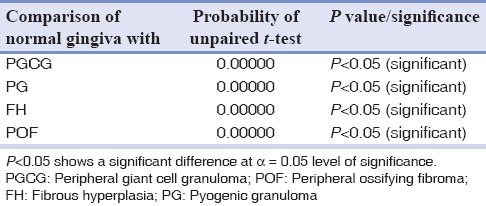
Figure 2.
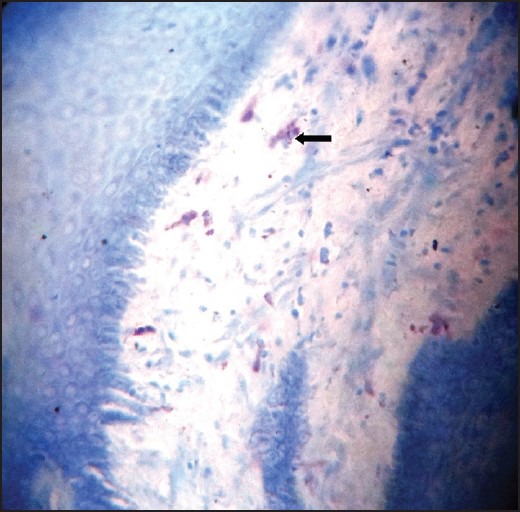
Fibrous hyperplasia showing spindle shaped mast cells stained with Toulidine
Figure 3.
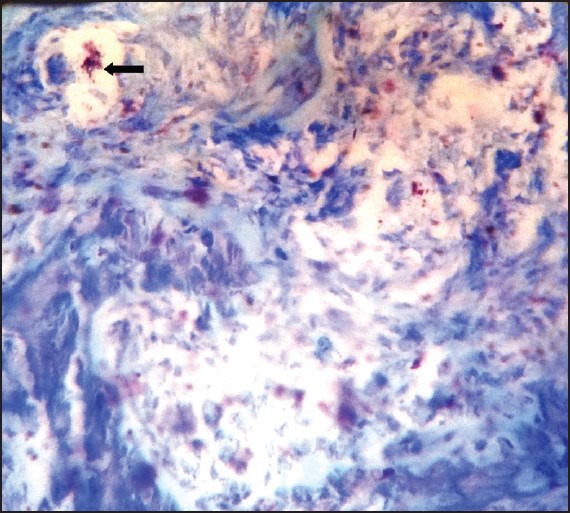
Pyogenic granuloma showing degranulated mast cells stained with Toulidine blue (×100)
Figure 4.
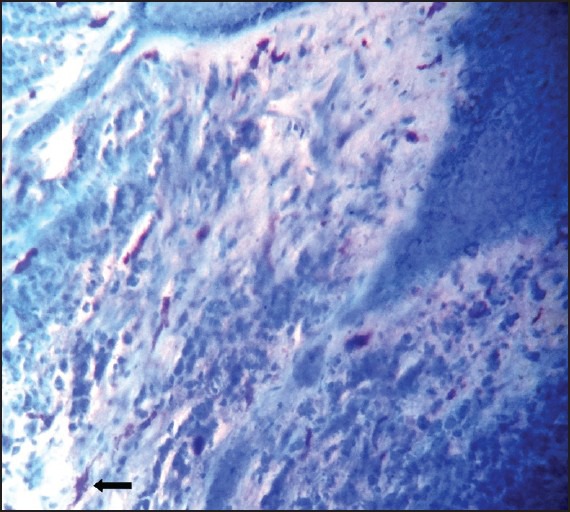
Peripheral ossifying fibroma showing numerous mast cells stained with Toulidine blue (×100)
Table 2.
One-way ANOVA-F table for significant difference among mast cell counts
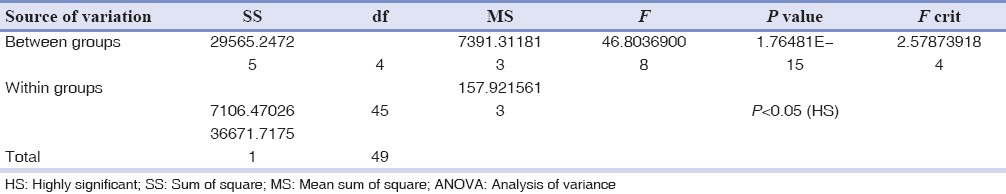
Table 3.
Karl-Pearson's correlation coefficients among mast cell counts
RESULTS
From a total of 1634 records evaluated during 10 year interval 209 of the lesions were reactive hyperplasia. This constituted 12.8% of the total biopsies accessed during the period. The most common lesion was found to be FH with 120 cases (57.4%), followed by 39 cases (18.7%) of PG, 37 cases (17.7%) of POF and 13 cases (6.22%) of PGCG. Of all patients examined 84 were males and 125 were females and the ratio was 1:1.5. The age of patients ranged from 7 years to 82 years with a mean age of 31.56 years. The mean age of patients with focal FH, PG, peripheral cemento-ossifying fibroma and PGCG was 36.56, 28.04, 32.49 and 29.16 years respectively. No statistical significant difference in mean age was observed between the two genders. Gingiva was the most common site with 171 cases (81.8%) followed by buccal mucosa with 17 cases (8.1%), lip with 7 cases (3.35%), palate with 6 cases (2.9%), tongue with 5 cases (2.4%) and alveolar mucosa with 3 cases (1.43%). In microscopic sections stained with toluidine blue, mast cells were most often located in lamina propria, particularly around blood vessels and appeared as purple and granular mononuclear cells in all the study groups. The mean total number of mast cells was 64.34 ± 15.3 in FH, 51.24 ± 12.99 in PG, 71.34 ± 18.456 in POF 24.35 ± 6.5562 in PGCG and 7.164 ± 1.8427 in normal oral gingival mucosa specimens. One way ANOVA-F and unpaired t-test revealed a significant difference in mast cell count between the groups at 5% level of significance [Tables 1–4].
DISCUSSION
Mast cells are immune cells that are found in all connective tissue and mucosal environments and in the peripheral and central nervous system.[8] They arise from a multipotent CD34 precursor in bone marrow and circulate in peripheral blood as a granular, monocytic appearing cells.[9] They range from 5 μm to 15 μm in diameter as in histologic sections they often appear ovoid, tad pole/spindle shaped cells. The most significant features of mast cells is their metachromatically staining secretory cytoplasmic granules that vary in size from 0.2 to 0.5 μ diameter.[10]
They are characterized by the surface expression of the high affinity immunoglobulin E receptor and localization at tissue sites adjacent to the microvasculature and at mucosal and epithelial surfaces. This localization of mast cells in both normal and inflamed sites results from their interaction with the laminin component of neural and vascular basement membranes via α6/β1 integrin that serves as a specific laminin receptor.[11]
Volumes of previous literature have evolved pertaining to the role of mast cells in the development of inflammation in the oral mucosa and dental pulp especially in early vasoinductive events and in the transforming stage from acute to chronic inflammation.[5] Mast cells exert their influence locally and systemically by releasing a variety of potent mediators through degranulation. Many of these mediators are stored within cytoplasmic granules (preformed mediators) while others are produced at the time of mast cell stimulation. They have been recognized as a source of a number of cytokines. While the importance and role of mast cells derived cytokines in disease is uncertain, it is conceivable that they may play a significant role in both physiologic and pathologic conditions.[12]
Histopathology of oral reactive lesions usually consists of neovascularization and inflammation depending on the stage of the lesion. Since mast cells contain cytokines that can bring about these actions, their presence in these lesions might help us to have a better understanding of the pathogenesis behind these lesions.[13] The present study includes the comparison of mast cells in oral soft-tissue reactive lesions. According to the results of this study, the mast cell count in all reactive lesions were increased in number compared with the normal oral mucosa and this is in accordance with studies by Günhan et al.[13] and Farahani et al.[14]
In this study, mast cell count was maximum in POF and FH followed by cases of PG and PGCG. The number of mast cells was maximum in POF and FH suggesting that mast cell activation is a characteristic feature of chronic inflammation; a condition that may lead to fibrosis as a result of increased collagen synthesis by fibroblasts. Literature also provides evidence that use of mast cell stabilizer, ketotifen was effective in reducing biomechanical and cellular manifestations of the joint capsule fibrosis in the rabbit model of post-traumatic joint contracture.[15]
One of the main components present inside secretory granules of mast cells is tryptase, an enzyme found exclusively in these cells. Tryptase has been shown to be involved in diverse biological activities such as fibrinogenesis, degradation of vasoactive intestinal peptide and stimulation of proliferation of fibroblast and smooth muscle cells. In addition, tryptase may stimulate the synthesis of collagen and contribute to angiogenesis. The presence of these cells and observation of degranulation activity do not necessarily indicate a destructive role but may suggest their involvement in the repair process.[16]
Many studies have shown the interaction of mast cells with fibroblast and their contribution to collagen synthesis in many diseases and in pathologic conditions such as scleroderma, fibrosis of skin, lung, appendix and kidney and also their association in oral submucous fibrosis, gingival fibromatosis and fibrotic changes in minor salivary gland of patients with Sjogren's syndrome.[17]
Mast cells are an important source of several proangiogenic and angiogenic factors such as histamine, heparin, chymase, basic fibroblast growth factor, vascular endothelial growth factor (VEGF), transforming growth factor-beta and others.[18]
It has been reported that mast cells play a role in normal angiogenesis and in pathological angiogenesis that occurs in inflammatory diseases and tumors. Mast cells have been shown to modulate the function of endothelial cells and to stimulate other cells that facilitate angiogenesis such as fibroblasts, epithelial cells and macrophages secreting angiogenic proteases and cytokines.[19]
Mast cell mediators such as histamine, tryptase, tumor necrosis factor-α (TNF α) and interleukin-4 can increase fibroblast proliferation and also act as chemotactic factor for polymorphonuclear leukocyte. Other factors such as heparin, fibroblast growth factor and VEGF can induce endothelial cell migration and new vessel formation.[13]
In the present study, mast cells were found to increase and degranulate in PG followed by PGCG. The mast cells form the first step in mechanism of neo-vascularization and further inflammatory reactions. Degranulation of mast cells activates endothelium through TNF-dependent mechanism, which may be critical to the elicitation phase of inflammation. Mast cell tryptase has been shown to promote neo-angiogenesis in premalignant and malignant stages of the uterine cervix. Hence, it is more important to know the role of mast cells and their contents in any stage of controlling inflammation and subsequent reactions.[18]
It is pertinent to know the multiple interactions between mast cells, endothelial cells and other immune related system that could provide a basis to further therapies to target mast cell responses.[20]
The findings in this study were in contrast to earlier reports which showed direct relation between the number of mast cells, vascularity and inflammation.[5,20] In cases of moderate/severe vascularity distribution of mast cells were predominantly low as shown in this study. This disparity could be explained as follows. Mast cells have got variable mediators within their granules. Once the mast cells are stimulated they degranulate and bring about required biological action. Therefore, it is only resonate to presume that the predominance of mast cell under degranulation would take place in a stage much before the active stimulation of angiogenesis and subsequent inflammatory reaction. The early stage could be defined as pre-inflammatory stage before the active vascularity and inflammatory cells are increased. Hence, it can be assumed that the pre-inflammatory stage could be the ideal period for intervening with mast cell action during the treatment process.
CONCLUSION
The number of mast cells were more numerous in POF and FH all suggesting that mast cell activation is a characteristic feature of chronic inflammation, a condition that may lead to fibrosis as a result of increased collagen synthesis by fibroblasts.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Effiom OA, Adeyemo WL, Soyele OO. Focal reactive lesions of the gingiva: An analysis of 314 cases at a tertiary health Institution in Nigeria. Niger Med J. 2011;52:35–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Nartey NO, Mosadomr HA, Al-Cailani M, AlMobeerik A. Localised inflammatory hyperplasia of the oral cavity: Clinico-pathological study of 164 cases. Saudi Dent J. 1994;6:145–50. [Google Scholar]
- 3.Zarei MR, Chamani G, Amanpoor S. Reactive hyperplasia of the oral cavity in Kerman province, Iran: A review of 172 cases. Br J Oral Maxillofac Surg. 2007;45:288–92. doi: 10.1016/j.bjoms.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kfir Y, Buchner A, Hansen LS. Reactive lesions of the gingiva. A clinicopathological study of 741 cases. J Periodontol. 1980;51:655–61. doi: 10.1902/jop.1980.51.11.655. [DOI] [PubMed] [Google Scholar]
- 5.Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14:188–98. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 6.Jahanshahi G, Sabaghian M. Comparative immunohistochemical analysis of angiogenesis and mast cell density in oral normal mucosa and squamous cell carcinoma. Dent Res J (Isfahan) 2012;9:8–12. doi: 10.4103/1735-3327.92920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahanshahi G, Ghalayani P, Maleki L. Mast cells distribution and variations in epithelium thickness and basement membrane in oral lichen planus lesion and oral lichenoid reaction. Dent Res J (Isfahan) 2012;9:180–4. doi: 10.4103/1735-3327.95233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell WM, Atterwill CK. Mast cell in neuroimmune function: Neurotoxicological and neuropharmacological perspectives. Neurochemistry. 1995;20:521–32. doi: 10.1007/BF01694534. [DOI] [PubMed] [Google Scholar]
- 9.Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, et al. The human mast cell: Functions in physiology and disease. Front Biosci. 2001;6:D1109–27. doi: 10.2741/krishnas. [DOI] [PubMed] [Google Scholar]
- 10.Kaminer MS, Lavker RM, Walsh LJ, Whitaker D, Zweiman B, Murphy GF. Extracellular localization of human connective tissue mast cell granule contents. J Invest Dermatol. 1991;96:857–63. doi: 10.1111/1523-1747.ep12475169. [DOI] [PubMed] [Google Scholar]
- 11.Walsh LJ, Kaminer MS, Lazarus GS, Lavker RM, Murphy GF. Role of laminin in localization of human dermal mast cells. Lab Invest. 1991;65:433–40. [PubMed] [Google Scholar]
- 12.Sudhakar R, Ramesh V, Balamurali PD, Nirima O, Premalatha B, Karthikshree V. Incidence of mast cells in oral inflammatory lesions: A pilot study. J Oral Maxillofac Pathol. 2005;9:12–5. [Google Scholar]
- 13.Günhan M, Bostanci H, Günhan O. Mast cell counting in fibrous gingival hyperplasias and giant cell granuloma. Ankara Univ Hekim Fak Derg. 1989;16:453–6. [PubMed] [Google Scholar]
- 14.Farahani SS, Navabazam A, Ashkevari FS. Comparison of mast cells count in oral reactive lesions. Pathol Res Pract. 2010;206:151–5. doi: 10.1016/j.prp.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Monument MJ, Hart DA, Befus AD, Salo PT, Zhang M, Hildebrand KA. The mast cell stabilizer ketotifen fumarate lessens contracture severity and myofibroblast hyperplasia: A study of a rabbit model of posttraumatic joint contractures. J Bone Joint Surg Am. 2010;92:1468–77. doi: 10.2106/JBJS.I.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos PP, Nonaka CF, Pinto LP, de Souza LB. Immunohistochemical expression of mast cell tryptase in giant cell fibroma and inflammatory fibrous hyperplasia of the oral mucosa. Arch Oral Biol. 2011;56:231–7. doi: 10.1016/j.archoralbio.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Skopouli FN, Li L, Boumba D, Stefanaki S, Hanel K, Moutsopoulos HM, et al. Association of mast cells with fibrosis and fatty infiltration in the minor salivary glands of patients with Sjögren's syndrome. Clin Exp Rheumatol. 1998;16:63–5. [PubMed] [Google Scholar]
- 18.Michailidou EZ, Markopoulos AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;2:126–32. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulation and inflammation in the oral cavity. J Oral Pathol Med. 1995;24:266–72. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]


