Abstract
Background:
In the recent years, various studies have shown a link between the free radicals, antioxidants and periodontal diseases. The purpose of the present study was to evaluate the oxidative stress and the antioxidant status present in the gingival tissue and plasma of patients with chronic periodontitis and to evaluate the antioxidant property of taurine.
Materials and Methods:
Periodontal status in 10 chronic periodontitis patients was assessed in terms of gingival index, plaque index, probing pocket depth and clinical attachment level prior to and after oral administration of taurine (500 mg O.D.) for 15 days. The oxidative stress present in the gingival tissue and blood (by measuring thiobarbituric acid reactive substance [TBARS]) and the antioxidants namely glutathione peroxidase (GPX) and reduced glutathione (GSH) were estimated before and after administration of taurine. The changes in the clinical parameters were also reassessed following administration of taurine. Statistical comparisons were performed using the Student's t-test. A level of P < 0.05 was considered as statistically significant.
Results:
The levels of TBARS in plasma and gingival tissue showed a significant reduction (P < 0.001) following administration of taurine. The antioxidant enzyme GPX showed a significant reduction following administration of taurine (P < 0.001), whereas GSH increased significantly (P < 0.001) following administration of taurine. The improvement in the periodontal status following administration of taurine was also significant statistically.
Conclusion:
Based on the biochemical and clinical assessments, taurine seems to exert a protective role against the oxidative stress in the management of patients with chronic periodontitis.
Keywords: Antioxidants, chronic periodontitis, glutathione peroxidase, oxidative stress, reduced glutathione, taurine
INTRODUCTION
There is an increasing body of evidence now available to implicate reactive oxygen species (ROS) in the pathogenesis of a variety of diseases including periodontitis. ROS include molecules such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl) and singlet oxygen (O2), which are capable of free radical formation in the extra- and intra-cellular environments.[1]
The most common approach for measuring free radical activity is to measure the end or intermediate products of lipid peroxidation. The most commonly applied test for the measurement of Malondialdehyde (MDA) is the estimation of thiobarbituric acid reactive substances (TBARS).[2]
A number of antioxidant mechanisms exist in the body, whose specific role is to remove or inactivate free radicals/ROS as soon as they form or to repair damage caused by free radicals/ROS.[1] Recently, the antioxidant status of serum, saliva and gingival crevicular fluid (GCF) in periodontitis patients has been widely explored.[3,4,5,6,7,8,9,10,11,12,13]
Taurine (2-amino ethane sulphonic acid), an amino acid has been investigated by several workers for its antioxidant property.[14,15,16,17] Research demonstrates that taurine can protect the heart from neutrophil-induced reperfusion injury and oxidative stress. Because the respiratory burst activity of neutrophils is also significantly reduced in the presence of taurine, perhaps taurine's protective effect is mediated by its antioxidative properties.[18] A few animal studies have demonstrated taurine to be beneficial in tissue repair in periodontal diseases.[19,20]
Recently, topical application of 1% taurine on the two basement membrane proteins (laminin 5 and type IV collagen expressions) of regenerating oral gingival epithelium demonstrated histologic evidence of rapid reepithelization of human gingival wounds.[21] Based on an in-vitro study, taurolidine, a derivative of taurine has been suggested as a potent antimicrobial agent in non-surgical and surgical periodontal treatment.[22]
The aim of this study was to evaluate the efficacy of taurine as an antioxidant in the management of patients with the chronic periodontitis. The objectives were:
To estimate the levels of TBARS and antioxidants glutathione peroxidase (GPX) and reduced glutathione (GSH) in plasma and gingival tissue before and after administration of taurine.
To evaluate the clinical parameters (plaque index [PI], gingival index (GI), probing pocket depth and clinical attachment level) before and after administration of taurine.
MATERIALS AND METHODS
Study population
A total of 10 chronic periodontitis patients who were non-smoker males belonging to the age group of 35-40 years with 5 mm probing depth and attachment loss in at least four sites in each quadrant were selected for the study. They were free from systemic diseases and had not received periodontal treatment or medication during the previous 6 months. All subjects were informed about the study and consent was obtained from them.
Clinical examination
The clinical status of the patients was assessed based on the following: PI (Silness and Loe),[23] GI (Loe and Silness-),[24] probing depth and clinical attachment level (Ramfjord).[25] Radiographic bone loss was also assessed using full mouth intra-oral periapical radiography.
Study design and sample collection
This double-blind study has been approved by the Institutional Human Ethical Committee. Following selection of patients for the study, phase I therapy was completed and the patients were recalled after 6 weeks for review. Patients with unresolved sites (periodontal pockets) were subjected to modified Widman flap surgery in all the four quadrants in four different appointments. The clinical parameters reassessed in the four quadrants on the day of the first sitting of surgery were considered as baseline values.
Blood was collected from the patients prior to surgery by venipuncture in pre-heparinized blood collection tubes and immediately transported to the laboratory for analysis of the antioxidant status. After blood collection, the gingival tissue obtained from the patients by performing modified Widman flap surgery was transported to the laboratory in ice-cold saline for further analysis.
Following surgery, neither non-steroidal anti-inflammatory drugs nor tetracycline was prescribed to patients and were randomly divided into two groups. One group of patients received the drug taurine 500 mg (antioxidant) once daily initially for a period of 15 days followed by the placebo once daily for 15 days. The other group of patients received placebo once daily initially for a period of 15 days followed by taurine 500 mg once daily, for 15 days. The clinical and biochemical assessments were repeated subsequently, in the quadrants subjected to flap surgery following the drug regimen (15 days intake of taurine and placebo).
To maintain full blinding of the results, the drug code (whether taurine or placebo) was held by one of the authors remotely from all assessments and was not broken until all data had been collected and all analysis had been performed. The participants of the study as well as the investigator (who collected the samples and clinical data from patients and provided them with drugs) were unaware about the details of the drugs (whether it was taurine or placebo). The drug code was concealed by using sequentially numbered, identical-appearing containers of taurine or placebo tablets.
Laboratory investigations
Lipid peroxidation was estimated as evidenced by the formation of TBARS. Lipid peroxides in plasma were assayed calorimetrically at 530-535 nm by the method of Yagi.[26] Lipid peroxidation in tissue was estimated by the method of Ohkawa et al.,[27] GSH was measured in plasma and tissue according to the method of Beutler and Kelley.[28] The activity of GPX was estimated by monitoring glutathione utilization by H2O2 according to the method of Rotruck et al.,[29]
Enzyme activity was expressed as μmoles of glutathione utilized/minute/L for plasma and per gram protein in the gingival tissue.
Statistical analysis
After decoding the drugs, the results obtained were subjected to statistical analysis. The values were expressed as mean ± standard deviation (SD) and statistical comparisons were performed using the Student's t-test. A level of P < 0.05 was considered statistically significant.
RESULTS
The oxidative stress and the antioxidant status were investigated in the plasma and gingival tissue sample of 10 chronic periodontitis patients at baseline and following administration of the drug taurine and placebo.
Table 1 shows the mean and SD of the levels of TBARS and the antioxidants glutathione and GPX in plasma at baseline and following administration of taurine and placebo [Figures 1–3]. The difference between the baseline values and following administration of taurine was compared with the difference between baseline values and following administration of placebo using Student's t-test (4 vs. 5). We noticed a statistically significant reduction (P < 0.001) in the levels of TBARS and GPX in plasma following administration of taurine while there was a statistically significant increase in the levels of GSH (P < 0.001).
Table 1.
Levels of TBARS and antioxidants in plasma at baseline and following administration of taurine and placebo

Figure 1.

Levels of plasma thiobarbituric acid reactive substances at baseline and following administration of taurine and placebo. Baseline: 10.71, After taurine: 5.86, After placebo: 8.99
Figure 3.

Levels of plasma reduced glutathione at baseline and following administration of taurine and placebo. Baseline: 31.92, After taurine: 36.76, After placebo: 35.42
Figure 2.

Levels of plasma glutathione peroxidase at baseline and following administration of taurine and placebo. Baseline: 261.27, After taurine: 210.62, After placebo: 237.27
Table 2 shows the mean and SD of the levels of TBARS, GSH and GPX in gingival tissue at baseline and following administration of taurine and placebo [Figures 4–6]. The difference between the baseline values and following administration of taurine was compared with the difference between baseline values and following administration of placebo using Student's t-test (4 vs. 5). We observed a statistically significant reduction (P < 0.001) in the levels of TBARS and GPX in gingival tissue following administration of taurine while there was a statistically significant increase in the levels of GSH (P < 0.001).
Table 2.
Levels of TBARS and antioxidants in gingival tissue at baseline and following administration of taurine and placebo

Figure 4.

Levels of tissue thiobarbituric acid reactive substances at baseline and following administration of taurine and placebo. Baseline: 206.95, After taurine: 164.96, After placebo: 185.5
Figure 6.

Levels of tissue reduced glutathione at baseline and following administration of taurine and placebo. Baseline: 5.87, After taurine: 7.49, After placebo 6.57
Figure 5.
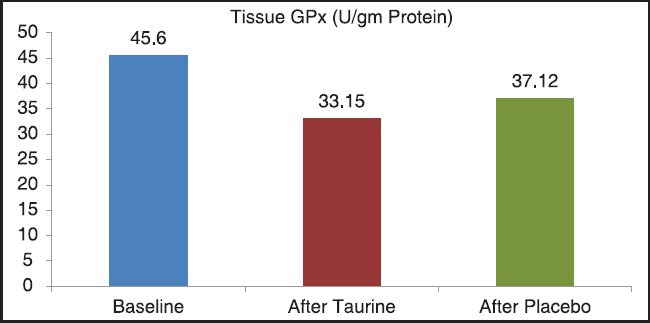
Levels of tissue glutathione peroxidase at baseline and following administration of taurine and placebo, Baseline: 45.6, After taurine 33.15, After placebo: 37.12
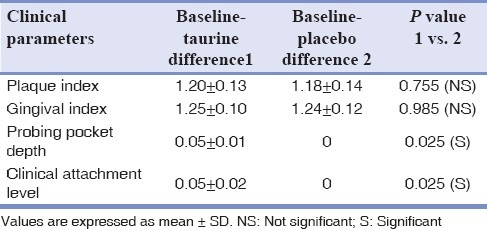
Table 3 shows the mean and SD of the difference between the values of the clinical parameters at baseline and following administration of taurine and placebo. Student's t-test (1 vs. 2) showed that the changes in the clinical parameters namely PI and GI following administration of taurine and placebo were statistically insignificant. However, the changes in the probing pocket depth and clinical attachment level following administration of taurine and placebo were statistically significant (P < 0.025).
Table 3.
Clinical parameters at baseline and following administration of taurine and placebo
DISCUSSION
It is widely agreed that, increased generation of ROS may cause toxic effects by oxidative damage of macromolecules, such as proteins, lipids and deoxyribonucleic acid. Oxidative stress has been implicated as a major contributor in over 100 disorders and more recently periodontitis.[12] Previous studies exploring the levels of antioxidants and the oxidative stress existing in the serum, saliva and GCF of chronic periodontitis patients have reported elevated lipid peroxidation and a disturbed antioxidant status.[3,4,5,6,7,8,9,10,11,12,13] However, limited reports investigating the same in the periodontally diseased gingival tissue are available.[30,31]
Recent medical and dental researches are geared towards the prevention of free radical mediated diseases by using specific nutrient antioxidants.[32] The present study focuses on investigating the efficacy of taurine as an antioxidant in chronic periodontitis patients. Lipid peroxidation and the levels of the antioxidant enzyme GPX and its substrate GSH were estimated before and after administration of taurine.
In various clinical trials conducted in epileptic patients, the dosage of taurine ranged from 375 to 8,000 mg/day. In our study, 500 mg taurine was given once daily for 2 weeks expecting favorable results.
The levels of TBARS in plasma and gingival tissue of chronic periodontitis patients in our study, showed a significant reduction following administration of taurine. This finding is consistent with animal studies conducted by Lim et al.,[14] and Balkan et al.,[15] who also demonstrated a decrease in levels of MDA in hepatic tissue while exploring the effect of taurine in diabetic model mice[14] and in mice with induced liver cirrhosis.[15]
Similarly, Nandhini et al.,[16] also observed that taurine was effective in reducing the peroxidative damage in plasma and liver tissue of rats fed with the high sucrose diet. Hence, the observed reduction in the levels of TBARS in plasma and gingival tissue following administration of taurine in our study can be attributed to the antioxidant potential of taurine.
In the present study there was a reduction in levels of GPX and an increase in levels of GSH in plasma and gingival tissue following administration of taurine. On the contrary, animal studies conducted by Lim et al.,[14] and Balkan et al.,[15] following administration of taurine showed either unaltered or increased levels of GPX in the hepatic tissue of diabetic model mice[14] and mice with induced liver cirrhosis.[15]
It can be hypothesized from the present study that taurine being an antioxidant, has led to a decreased generation of free radicals resulting in decreased levels of H2O2 and lipid peroxides as evident from decreased levels of TBARS in plasma and gingival tissue. Hence, the need for GPX is reduced. The reduction in levels of GPX in plasma and gingival tissue might be attributed to decreased synthesis of GPX or increased degradation of GPX or inhibition of GPX activity and it needs further studies to explore the mechanism of decrease in GPX activity by taurine administration.
Li et al.,[17] who evaluated the protective effect of taurine on HOCl toxicity to rat hepatocyte nuclei, demonstrated that taurine provided more powerful protection against HOCl than GSH.
This finding is in favour of the increase in levels of GSH in plasma and gingival tissue samples following administration of taurine in this study. It can be hypothesized that the administration of taurine would have led to decreased utilization of GSH resulting in the presence of higher levels of GSH in plasma and gingival tissue.
Among the clinical parameters, though PI and GI showed statistically insignificant changes, there was a statistically significant reduction in probing pocket depth and clinical attachment level following administration of taurine.
Short term duration of the study and limited sample size are a few limitations of the study. Along with the evaluation of the antioxidant efficacy of taurine, its impact on the levels of inflammatory mediators like tumor necrosis factor-α, interleukin-1β, etc., can also be investigated. Enrolling a larger number of patients including those with gingivitis, aggressive periodontitis, etc., are the future avenues of research using taurine.
Of course, future trials with topical application of taurine for gingival massage as well as using taurine as a local drug delivery agent in diseased periodontium may throw light on the beneficial effects of taurine in the treatment of periodontal diseases.
CONCLUSION
Based on our data, it can be concluded that taurine seems to improve the antioxidant status of chronic periodontitis patients by influencing the levels of lipid peroxidation products (TBARS) and the antioxidant enzymes GPX and GSH. The apparent improvement observed in the clinical parameters (namely probing pocket depth and clinical attachment level) following administration of taurine also reflects the same.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Tüter G, Kurtiº B, Serdar M. Interleukin-1beta and thiobarbituric acid reactive substance (TBARS) levels after phase I periodontal therapy in patients with chronic periodontitis. J Periodontol. 2001;72:883–8. doi: 10.1902/jop.2001.72.7.883. [DOI] [PubMed] [Google Scholar]
- 3.Battino M, Ferreiro MS, Bompadre S, Leone L, Mosca F, Bullon P. Elevated hydroperoxide levels and antioxidant patterns in Papillon-Lefèvre syndrome. J Periodontol. 2001;72:1760–6. doi: 10.1902/jop.2001.72.12.1760. [DOI] [PubMed] [Google Scholar]
- 4.Chapple IL, Brock G, Eftimiadi C, Matthews JB. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. Mol Pathol. 2002;55:367–73. doi: 10.1136/mp.55.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapple IL, Mason GI, Garner I, Matthews JB, Thorpe GH, Maxwell SR, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem. 1997;34:412–21. doi: 10.1177/000456329703400413. [DOI] [PubMed] [Google Scholar]
- 6.Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994;21:417–25. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 7.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105:167–72. doi: 10.1042/CS20030031. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–84. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurtiº B, Tüter G, Serdar M, Pinar S, Demirel I, Toyman U. Gingival crevicular fluid prostaglandin E(2) and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen. J Periodontol. 2007;78:104–11. doi: 10.1902/jop.2007.060217. [DOI] [PubMed] [Google Scholar]
- 10.Akalin FA, Baltacioğlu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34:558–65. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 11.Khalili J, Biloklytska HF. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008;14:754–60. doi: 10.1111/j.1601-0825.2008.01464.x. [DOI] [PubMed] [Google Scholar]
- 12.Canakci CF, Cicek Y, Yildirim A, Sezer U, Canakci V. Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur J Dent. 2009;3:100–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–8. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim E, Park S, Kim H. Effect of taurine supplementation on the lipid peroxide formation and the activities of glutathione-related enzymes in the liver and islet of type I and II diabetic model mice. Adv Exp Med Biol. 1998;442:99–103. doi: 10.1007/978-1-4899-0117-0_13. [DOI] [PubMed] [Google Scholar]
- 15.Balkan J, Doğru-Abbasoğlu S, Kanbağli O, Cevikbaş U, Aykaç-Toker G, Uysal M. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum Exp Toxicol. 2001;20:251–4. doi: 10.1191/096032701678227758. [DOI] [PubMed] [Google Scholar]
- 16.Nandhini AT, Balakrishnan SD, Anuradha CV. Response of liver antioxidant system to taurine in rats fed high fructose diet. Indian J Exp Biol. 2002;40:1016–9. [PubMed] [Google Scholar]
- 17.Li JX, Pang YZ, Tang CS, Li ZQ. Protective effect of taurine on hypochlorous acid toxicity to nuclear nucleoside triphosphatase in isolated nuclei from rat liver. World J Gastroenterol. 2004;10:694–8. doi: 10.3748/wjg.v10.i5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birdsall TC. Therapeutic applications of taurine. Altern Med Rev. 1998;3:128–36. [PubMed] [Google Scholar]
- 19.Koide M, Okahashi N, Tanaka R, Kazuno K, Shibasaki K, Yamazaki Y, et al. Inhibition of experimental bone resorption and osteoclast formation and survival by 2-aminoethanesulphonic acid. Arch Oral Biol. 1999;44:711–9. doi: 10.1016/s0003-9969(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 20.Ozmeriç N, Ozcan G, Haytaç CM, Alaaddinoğlu EE, Sargon MF, Senel S. Chitosan film enriched with an antioxidant agent, taurine, in fenestration defects. J Biomed Mater Res. 2000;51:500–3. doi: 10.1002/1097-4636(20000905)51:3<500::aid-jbm26>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Gültekin SE, Sengüven B, Sofuoğlu A, Taner L, Koch M. Effect of the topical use of the antioxidant taurine on the two basement membrane proteins of regenerating oral gingival epithelium. J Periodontol. 2012;83:127–34. doi: 10.1902/jop.2011.100568. [DOI] [PubMed] [Google Scholar]
- 22.Eick S, Radakovic S, Pfister W, Nietzsche S, Sculean A. Efficacy of taurolidine against periodontopathic species — An in vitro study. Clin Oral Investig. 2012;16:735–44. doi: 10.1007/s00784-011-0567-2. [DOI] [PubMed] [Google Scholar]
- 23.Silness J, Loe H. Periodontal disease in pregnancy: II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 25.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51. [Google Scholar]
- 26.Yagi K. Lipid peroxides and human diseases. Chem Physiol Lipids. 1978;45:337–51. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E, Kelly BM. The effect of sodium nitrite on red cell GSH. Experientia. 1963;19:96–7. doi: 10.1007/BF02148042. [DOI] [PubMed] [Google Scholar]
- 29.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 30.Panjamurthy K, Manoharan S, Ramachandran CR. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett. 2005;10:255–64. [PubMed] [Google Scholar]
- 31.Borges I, Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm 2007. 2007 doi: 10.1155/2007/45794. 45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–76. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]


