Abstract
Background:
Dental pulp stem cells can be used in regenerative endodontic therapy. The aim of this study was to introduce an efficient method for dental pulp stem cells isolation.
Materials and Methods:
In this in-vitro study, 60 extracted human third molars were split and pulp tissue was extracted. Dental pulp stem cells were isolated by the following three different methods: (1) digestion of pulp by collagenase/dispase enzyme and culture of the released cells; (2) outgrowth of the cells by culture of undigested pulp pieces; (3) digestion of pulp tissue pieces and fixing them. The cells were cultured in minimum essential medium alpha modification (αMEM) medium supplemented with 20% fetal bovine serum(FBS) in humid 37°C incubator with 5% CO 2. The markers of stem cells were studied by reverse transcriptase polymerase chain reaction (PCR). The student t-test was used for comparing the means of independent groups. P <0.05 was considered as significant.
Results:
The results indicated that by the first method a few cell colonies with homogenous morphology were detectable after 4 days, while in the outgrowth method more time was needed (10-12 days) to allow sufficient numbers of heterogeneous phenotype stem cells to migrate out of tissue. Interestingly, with the improved third method, we obtained stem cells successfully with about 60% efficiency after 2 days. The results of RT-PCR suggested the expression of Nanog, Oct-4, and Nucleostemin markers in the isolated cells from dental pulps.
Conclusion:
This study proposes a new method with high efficacy to obtain dental pulp stem cells in a short time.
Keywords: Dental pulp stem cells, isolation, method, molar
INTRODUCTION
Pulp necrosis and interruption of tooth development, which result in incompletely formed roots with wide open apices, reduced root length, and fragile thin dentinal walls, may be the consequences of physical trauma or endodontic infection of a tooth with an immature apex.[1] Apexification is a treatment used to seal an open apex by inducing a calcified barrier at the root. Calcium hydroxide and mineral trioxide aggregate (MTA) are the most widely accepted materials for apexification procedures, but none of them is able to stimulate regeneration of pulp tissue and continued root development, so a root with considerably thin walls at increased risk of fracture remains and may lead eventually to loss of the tooth.[2] Tooth loss has long been associated with accelerated aging and has considerable negative consequences such as vertical and horizontal positional changes.[3] One of the most common traditional treatment modalities include fixed or removable prosthetic therapy, which may lead to many complications such as continuous alveolar bone resorption.[4] The concept of osseointegration was introduced in the 1950s by Per-Ingvar Branemark. Nowadays, endosseous implants are a commonly accepted treatment option. However, osseointegration represents a direct connection between the implant and bone tissue and lacks the periodontium tissue present in natural teeth, which is regarded as an elastic isotropic material and modulate the mechanical stress of mastication.[5] Therefore, the need for alternative tooth replacement therapies is quite evident.
Conventional root canal treatment of a necrotic pulp is not a sufficient management. Some endodontic filling materials and sealers or medicaments have the potential to discolor the tooth crown or weaken the tooth.[6] Furthermore, dental pulp contains proprioceptors and therefore provides important sensory feedback on masticatory forces, a function that may reduce the potential for tooth fracture.[7]
The creation and delivery of new tissues to be a substitute for diseased or traumatized pulp or missing teeth is referred to as regenerative therapies. The success of regenerative endodontic therapy is the ability to constitute a technique that will allow clinicians to create a functional pulp tissue. The source of pulp tissue may come from stem cell therapy, which involves the delivery of autologous stem cells into root canals; dental pulp constructs, which involves the surgical implantation of synthetic pulp tissue grow in the laboratory; or root canal revascularization.[8] A clinical limitation to a blood clot revascularization approach is that concentration and composition of cells trapped in the fibrin clot are unpredictable that may lead to variations in treatment outcome.[9]
Stem cells are considered the most valuable cells for regenerative medicine. Stem cells can be classified as either embryonic or postnatal stem cells. Both can contribute to the development of regenerative medicine. The immunological and ethical concerns due to allogenic embryonic stem cells may be overwhelmed by the use of postnatal stem cells.[10] Furthermore, there have been many advances in postnatal stem cell research. Newer studies suggest that adult stem cells may have much greater plasticity than previously thought. There are various sources of stem cells in adult tissues, such as bone marrow, blood, brain, the eye, skeletal muscle, liver, dental pulp, skin, the lining of the gastrointestinal tract, and pancreas.[11,12,13] Adult stem cells are rare, difficult to identify, purify, and culture. They can be classified as hematopoietic or mesenchymal stem cells. Bone marrow – derived mesenchymal stem cells have been the most studied mesenchymal stem cells.[14] However, it is unclear if these cells have the capacity for dentinogenic differentiation.[15]
Gronthos et al. were the first to isolate dental pulp stem cells (DPSCs) in 2000.[15] Further investigations revealed that these cells have intensive capacity of differentiation into neuron-like cells, adipocytic, osteocytic, and chondrocytic cells.[16,17,18] Moreover, DPSCs demonstrated a higher developmental potential and longer life span than bone marrow stromal stem cells. This has been found that the number of proliferating cells as well as the frequency of colony-forming cells was significantly higher in DPSCs when compared to bone marrow stromal stem cells too.[19] On the other hand, DPSCs can be cryopreserved and used for regenerative purposes later.[20] Based upon these findings, dental pulp stem cells may be considered appropriate cell types for developing tissue engineering strategies and finding ways to culture them outside the body with high efficiency is a great priority of stem cell research.
Unfortunately, the stem cell population in the pulp is very small; approximately 1% of the total cells and the effect of aging reduce the cell pool available.[21] As an important issue concerning the therapeutic use of stem cells is the quantity of cells necessary in order to achieve the best effect, harvesting and expanding the stem cells in culture are indispensable steps for regenerative medicine.[22] Hence, the goal of the present study was to design a modified dental pulp stem cell isolation method with high in vitro expansion potential and optimize the culture conditions for their increased proliferation.
MATERIALS AND METHODS
Sampling
Sixty impacted third molars used in this study were surgically removed from 45 healthy patients (18-30 years of age) by an oral surgeon. Informed consent was obtained from the patients after receiving approval by the Institutional Ethics Committee of Kerman University of Medical Sciences (Code: K/88/220). Before extraction, each subject was screened for systemic diseases by a health history and oral questioning. After a rinse with 0.2% chlorhexidine for 60 s a topical anesthetic gel was applied and teeth were anesthetized using lidocaine 2% with epinephrine 1/80 000 (Daroupakhsh, Tehran, Iran). Teeth that were cut during surgery or presenting a localized infection in the region were excluded from the study.
Isolation and culture of stem cells from dental pulp
The teeth were immersed in sterile phosphate buffer saline (PBS), stored on ice pack and immediately transported to the cell culture lab for sample processing. After cleaning the surface and disinfection with iodine, a horizontal groove was cut along the cementum-enamel junction using diamond fissure bur (DandZ., Wiesbaden, Germany) with high speed handpiece and copious water supply mounted on a high-speed hand piece to split the teeth and obtain the pulp tissue under sterile condition. All pulp tissues were minced into approximately 1.5 × 2 × 1 mm fragments. The teeth were randomly divided into three groups. Out of 60 samples, 20 were included in group I (digestion of pulp pieces by collagenase/dispase enzyme (Roche, Germany) and culture of the released cells following centrifugation); 20 in group II (outgrowth of the cells by culture of undigested pulp pieces) and the remaining 20 samples were in group III (digestion of pulp pieces and fixing them under a cover slip in the medium). In groups I and III, the fragments were digested in a solution of 1 mg/ml collagenase/dispase for 30 min at 37°C and centrifuged at 500 g for 5 min. Cell suspensions were seeded in 60 mm culture dishes containing minimum essential medium alpha modification (α-MEM); with 20% fetal bovine serum (FBS), 100 U/ml penicillin-G, 100 μg/ml streptomycin, and 1 μg/ml amphotrypsin B.[15] Groups II and III received a coverslip to fix the tissue and prevent it from movement in the medium. All specimens were incubated at 37°C and 5% CO2 in the incubator. The medium was changed every 3 days. The cells were passaged 1:5 with 0.25% trypsin/1 mM EDTA every 5 days. The cells were counted and their viability was determined by Ttrypan Blue staining. Cells were cryopreserved in a freezing medium composed of 65% α-MEM medium, 30% FBS, and 5% DMSO and vials were stored in liquid nitrogen tank until used.[15] The student t-test was used for comparing the mean values of three independent groups. P value <0.05 was considered significant.
Mycoplasma detection
The cells were cultured on cover slips, fixed with methanol–acetone and stained with Hoechst 33558 (sigma) as recommended by the company and observed under fluorescent microscope (Axioplan 2, Zeiss) to reveal any contaminant mycoplasma. Images were captured with a digital camera (Powershot A260, Canon).
Extraction of Total RNA and cDNA synthesis
RNA-Easy Kit (Qiagen, Germany), according to manufacturer's protocol was used. RNA measuring 0.5 μg was treated with RNase-free DNase I (Fermentas, Litany) to remove residual contamination with genomic DNA. Total DNA mixed with 0.2 μg of random hexamer and heated at 70°C for 5 min. The mixture was immediately chilled on ice for 5 min followed by the addition of 6.5 μl of a reverse transcription mixture prepared in a total volume of 19 μl containing 4 μl of 5× reaction buffer, 2 μl of 10mM dNTP, 0.5 μl RNase inhibitor. The mixture was incubated at 25°C for 5 min and then 1 μl M-ML V RT was added. The cDNA-synthesis reaction was performed at 42°C for 60 min.
Polymerase chain reaction and electrophoresis
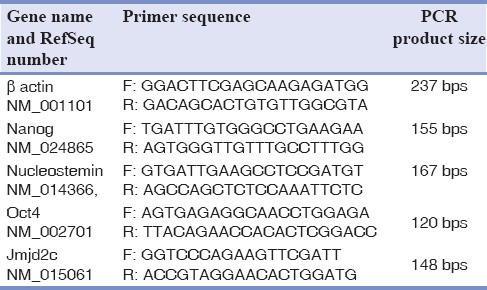
Subsequent polymerase chain reaction (PCR) was as follows: 1 μl CDNA, 1.5 mM MgCl2, 200 μM dNTP, 0.4 μm of each specific primers and 1.25 unit/25 μl reaction Taq DNA polymerase (Cinnagen, Iran). Amplification was undertaken by initial at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s and final extension at 72°C for 5 min. Each experiment was repeated three times. Specific primer pairs [Table 1] were designed by the primer 3 program written by the Whitehead Institute and oligonucleotides were synthesized by Isogen.
Table 1.
The sequences of primers designed and used for regular RT-PCR experiments
PCR products were size-fractionated on a 1.5% agarose gel and visualized by ethidium-bromide straining. The intensities of the PCR products were analyzed by Gene Tools software (Syngene, Cambridge, UK). Each experiment was repeated at least for three samples to obtain reproducible data.
RESULTS
Cell growth and proliferation on culture dishes
The results demonstrated morphological differences in different groups. In group I a few cell colonies were found within 4 days after initial plating. Cells were homogenous fibroblast like in morphology [Figure 1a].
Figure 1.
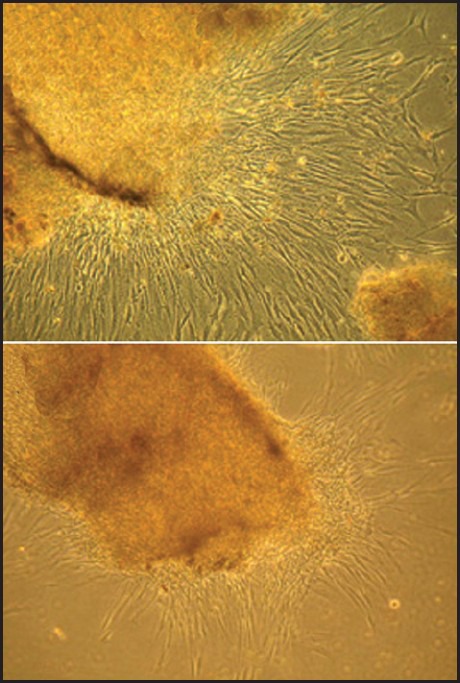
Digestion of pulp segments with collagenase/dispase destroys pulp scaffold, the spindle like cells are observed 2-3 days after primary culture (Figure a, 100X). Undigested pulp segments show an intact edge (Figure b, 100X) and yield cell later than digested tissue
In the outgrowth method 10-12 days were needed to allow sufficient numbers of cells. Cell migration through peripheral was also noticeable in this group [Figure 1b]. Colonies were heterogeneous in appearance with a barrel-shaped spindle and round configuration. Cells isolated by this method had the slowest proliferation rate.
Surprisingly, with the improved third method, only 1 or 2 days after the passage, heterogeneous fast growing populations of stem cells appeared. In this considerably high efficacy group, all the specimens exhibited increased rate of stem cell growth. The result demonstrated that the third method was more efficient (more than 60%) and the second method was less efficient (about 10%) in stem cell isolation and this differences were statistically significant (P < 0.05). To check the cells for mycoplasma contamination we used Hoechst staining solution. Fluorescence microscopy revealed that the nucleus of the stem cells appeared as a blue ellips, which demonstrated that the cells were not contaminated.
Gene expression patterns
We sought to examine the expression of Nucleostemin, Oct-4, jmj2c and Nanog as proliferation and self-renewal regulatory factors for stem cells. Based on the RT-PCR and agarose gel electrophoresis, the cells from all groups expressed all the genes [Figure 2].
Figure 2.
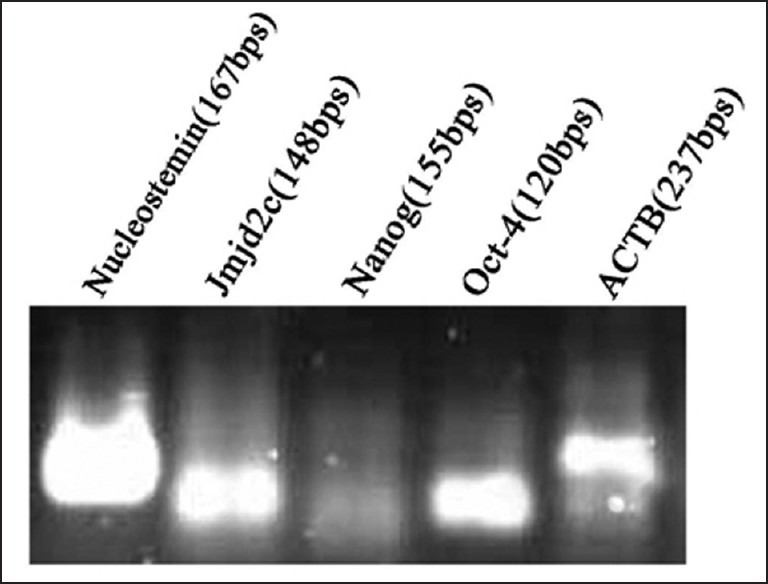
Expression of stem cell markers Nucleostemin, Jmjd2c, Nanog and Oct-4, in the stem cells derived from human adult dental pulp
DISCUSSION
The third molar is the last tooth to develop; it is normally in an early stage of development. Studies have shown that extracted nondecayed impacted third molars are capable of producing an optimum quantity of dental pulp tissue for the isolation of DPSCs.[23,24] Another positive point is that harvesting stem cells from this unavailing tissue after extraction causes no ethical controversy.
It is not possible to identify stem cells definitely in any tissue. Some indirect properties such as surface proteins, rapid cell cycle, clonogenicity, or an undifferentiated state offer significant implications for accuracy of diagnosis.[25] In this study, the expression of some genes including Nucleostemin, Oct-4, and Nanog, which are implicated in the proliferation and self-renewal of stem cells, was studied by RT-PCR assay.
Nucleostemin is a nucleolar protein cloned and described by Tsai and McKay.[26] It is described as a P53 binding protein in the nucleoli of cancer cells, neural and embryogenic stem cells and placenta tissue, but not in terminally differentiated cells. It is thought to be involved in regulation of self-renewal and cell cycle.[26,27] However, an increase of nucleostemin expression by RT-PCR could simply be the result of the proliferation of nucleostemin-positive cells, and not the result of promoting nucleostemin expression within each cell.[28] However, the expression of nucleostemin is reported as strong signals from the nucleoli, which is much more specific compared to p63, and therefore, may be superior as a progenitor marker.[28]
Nanog is a 305 amino acid protein with a conserved homeodomain belonging to the homeobox gene family. It was first described as a key transcription regulator defining human embryonic stem cell identity and self-renewal by both activating repressors of and suppressing activators of differentiation.[29] Down-regulation of Nanog induces differentiation,[30] and over-expression of Nanog induces pluripotency,[31] as shown by the capacity for multi-lineage differentiation and perpetual self-renewal of cells expressing this gene.
Oct-4 is a transcription factor that is coded for by the Pou5f1v gene. The Oct-4 gene influences several genes expressed during early embryonic development, and thus may play a vital role in the processes of development and cell differentiation.[32] Maintaining Oct-4 expression within a certain range is required for stem cell renewal. However, increased Oct-4 expression provokes differentiation to endoderm or mesoderm and decreased Oct-4 expression is related to dedifferentiation to trophectoderm.[33]
As a whole, the expression of these genes in the isolated dental pulp cells indicates a potential role for them in controlling proliferation, survival, and self-renewal. Due to the expression of nucleostemin, Oct-4 and Nanog in the cells from all groups, our results suggest that there exists a population of stem cells in the pulp. This finding is in line with many previous reports, and it implies that the cells isolated from dental pulp are bona fide stem cell.[15,23,24,34]
An important issue relevant to the therapeutic use of stem cells is the quantity of cells essential to attain an optimal effect. Expanding stem cells in culture is a crucial step for regenerative medicine, and a considerable attempt has been made to assess the consequences of the cultivation on stem cell behavior.[22]
Two methods usually have been employed to culture dental pulp stem cells. The first is the enzyme digestion method and the second one is the explant outgrowth method. Due to the decreased cell viability related to the enzyme treatment method, the explant outgrowth one has been suggested as the method of choice.[35] In this experiment, the isolation method had a bright effect on population expansion and our improved third method provided the greatest in vitro expansion than the two mentioned methods and may provide the most cells for biotechnological and biomedical applications. Huang et al. compared the enzyme digestion and outgrowth methods and found that cells isolated by enzyme digestion had a higher proliferation rate than those isolated by the other one.[35] Although our result is similar to Huang et al. study, it seems that the cells isolated by our improved method were less damaged and were therefore healthy enough to propagate longer in vitro than the other methods. Perhaps the other methods damage the cells or alternatively removed critical elements from the endogenous niche.
With our improved method, only 1 to 2 days after the primary culture, heterogeneous fast growing populations of stem cells appeared. There are different types of cells in the pulp. Odontoblasts, whose biological function is dentinogenesis, is part of the outer surface of the dental pulp. Capillaries and small nerve fibers ramify in the subodontoblastic layer. The cell-rich zone contains fibroblasts and undifferentiated cells. Central pulp zone includes the large vessels and nerves. Most of the cells of the pulp are fibroblasts. These cells exhibit wide variation in their degree of differentiation.[36,37] Perhaps multiple stem cells are capable of forming all these cells. We hypothesize that possibly, there exists more than one type of stem cells in the pulp and different methods of isolation could result in different cell types.
CONCLUSION
In conclusion, the results of the current study indicates that adult dental pulp contain stem cells that can be isolated and expanded efficiently. These cells are probably one of the sources of regeneration after pulp injury. They could be valuable in cell based therapeutic approaches too.
We suggest that further work in this area may provide an increased understanding of what is happening to these cells at harvest and over time in culture. This information is important in developing cell isolation procedures that produce the best chance for establishing the DPSC cells in vitro for later use in experiments and for biotechnology or cell therapies.
ACKNOWLEDGMENTS
The authors would especially like to thank Dr. Babak Mohammadi at the Department of Oral and Maxillofacial Surgery for his tireless effort in providing the samples. This research would not have been possible without the financial support of the Research Committee of Kerman University of Medical Sciences.
Footnotes
Source of Support: Research committee of Kerman University of Medical Sciences
Conflict of Interest: The authors deny any potential conflict of interest related to the contents of this paper.
REFERENCES
- 1.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 2.El-Meligy OA, Avery DR. Comparison of apexification with mineral trioxide aggregate and calcium hydroxide. Pediatr Dent. 2006;28:248–53. [PubMed] [Google Scholar]
- 3.Holm-Pedersen P, Schultz-Larsen K, Christiansen N, Avlund K. Tooth loss and subsequent disability and mortality in old age. J Am Geriatr Soc. 2008;56:429–35. doi: 10.1111/j.1532-5415.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 4.Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. J Prosthet Dent. 2003;89:427–35. doi: 10.1016/s0022-3913(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 5.Lin C, Dong QS, Wang L, Zhang JR, Wu LA, Liu BL. Dental implants with the periodontium: A new approach for the restoration of missing teeth. Med Hypotheses. 2009;72:58–61. doi: 10.1016/j.mehy.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein SD, Horowitz AJ, Man M, Wu H, Foran D, Vena DA, et al. Outcomes of endodontic therapy in general practice: A study by the Practitioners Engaged in Applied Research and Learning Network. J Am Dent Assoc. 2012;143:478–87. doi: 10.14219/jada.archive.2012.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randow K, Glantz PO. On cantilever loading of vital and non-vital teeth. An experimental clinical study. Acta Odontol Scand. 1986;44:271–7. doi: 10.3109/00016358609004733. [DOI] [PubMed] [Google Scholar]
- 8.Gotlieb EL, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. An ultrastructural investigation of tissue-engineered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc. 2008;139:457–65. doi: 10.14219/jada.archive.2008.0189. [DOI] [PubMed] [Google Scholar]
- 9.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: A case series. J Endod. 2010;36:536–41. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Henon PR. Human embryonic or adult stem cells: An overview on ethics and perspectives for tissue engineering. Adv Exp Med Biol. 2003;534:27–45. doi: 10.1007/978-1-4615-0063-6_3. [DOI] [PubMed] [Google Scholar]
- 11.Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287:289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 12.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 17.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 18.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–95. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–9. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 20.Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62. doi: 10.1111/j.1365-2591.2009.01622.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith AJ, Patel M, Graham L, Sloan AJ, Cooper PR. Dentine regeneration: The role of stem cells and molecular signalling. Oral Biosci Med. 2005;2:127–32. [Google Scholar]
- 22.Perin EC, Geng YJ, Willerson JT. Adult stem cell therapy in perspective. Circulation. 2003;107:935–8. doi: 10.1161/01.cir.0000057526.10455.bd. [DOI] [PubMed] [Google Scholar]
- 23.Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, et al. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: Implications in neovascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2010;10:105–13. doi: 10.1038/tpj.2009.40. [DOI] [PubMed] [Google Scholar]
- 24.Atari M, Barajas M, Hernández-Alfaro F, Gil C, Fabregat M, Ferrés Padró E, et al. Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol. 2011;26:1057–70. doi: 10.14670/HH-26.1057. [DOI] [PubMed] [Google Scholar]
- 25.Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 26.Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–20. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima M, Kawakita T, Yoshida S, Shimmura S, Tsubota K. Nucleostemin as a possible progenitor marker of corneal epithelialcells. Mol Vis. 2009;15:1162–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Govindasamy V, Abdullah AN, Ronald VS, Musa S, Ab Aziz, Zain RB, et al. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J Endod. 2010;36:1504–15. doi: 10.1016/j.joen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, et al. Pluripotential competence of cells associated with Nanog activity. Mech Dev. 2005;122:67–79. doi: 10.1016/j.mod.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Vodyanik MA, Smuga OK, Antosiewicz BJ, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Wei X, Ling J, Wu L, Xiao Y. Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J Endod. 2011;37:466–72. doi: 10.1016/j.joen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 34.Sloan AJ, Smith AJ. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151–7. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–36. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 36.Avery JK. Structural elements of the young normal human pulp. Oral Surg Oral Med Oral Pathol. 1971;32:113–25. doi: 10.1016/0030-4220(71)90257-x. [DOI] [PubMed] [Google Scholar]
- 37.Luisi SB, Barbachan JJ, Chies JA, Filho MS. Behavior of human dental pulp cells exposed to transforming growth factor-beta1 and acidic fibroblast growth factor in culture. J Endod. 2007;33:833–5. doi: 10.1016/j.joen.2007.04.002. [DOI] [PubMed] [Google Scholar]


