Abstract
A novel approach for biological control of insect pests could be the use of the endophytic entomopathogenic Beauveria bassiana isolate ATP-02. For the utilization of the endophyte as a commercial biocontrol agent, the fungus has to be mass-produced. B. bassiana was raised in shake flask cultures to produce high concentrations of total spores (TS), which include blastospores (BS) and submerged conidiospores (SCS). The highest concentration of 1.33×109 TS/mL and the highest yield of 5.32×1010 TS/g sucrose was obtained in the TKI broth with 5% sugar beet molasses which consists of 50% sucrose as a carbon source. In spite of the lower sugar concentration (2.5%) the amount of TS could be increased up to 11-times in contrast to the cultivation with 5% sucrose. The scale-up to a 2 L stirred tank reactor was carried out at 25°C, 200–600 rpm and 1 vvm at pH 5.5. A TS yield of 5.2×1010 TS/g sucrose corresponding to a SCS yield of 0.2×1010 SCS/g sucrose was obtained after 216 h. With regards to the culture medium the cost of 1012 TS amounts to 0.24 €. Plutella xylostella larvae, which were fed with oilseed rape leaves treated with spores from fermentation resulted in 77 ± 5% mortality. Moreover, spores from submerged cultivation were able to colonize oilseed rape leaves via leaf application. This is the first report of fermentation of an endophytic B. bassiana strain in a low-cost culture medium to very high yields of TS.
Keywords: Submerged culture, Fermentation, Beauveria bassiana, Endophyte, Blastospores, Submerged conidiospores, Biological control
Introduction
In the past decades, many microorganisms have been isolated and investigated for use as a biocontrol agent. Now, many promising strains are available for release into the environment and especially with the renewed interest in biocontrol await further exploitation for large-scale application in agriculture (Glare et al. [2012]). The first step for commercialization of a biocontrol agent like Beauveria bassiana is the mass-production by fermentation (Burges [1998]; Ravensberg [2011]). B. bassiana strains that were applied to the insect and act on the outer surface of the plant show efficacy against a wide range of insect pests and have the potential of becoming a cost-effective biocontrol agent (Khachatourians [1986]). However, the approved products of B. bassiana contain aerial conidia (AC), which are produced by either a solid-state or a diphasic fermentation. These processes are in classical biotechnology considered to be labour-intensive and unsuitable for conventional production of fungal biomass (Feng et al. [1994]; Patel et al. [2011]; Ravensberg [2011]; Rombach et al. [1988]). In contrast to these propagules, blastospores (BS) and submerged conidiospores (SCS) would be produced in submerged cultivations in a shorter time with higher yields and state-of-the-art process control. Furthermore, it was shown that BS and SCS of a B. bassiana isolate are as virulent to grasshoppers as the AC (Hegedus et al. [1992]). Until today, no products with BS or SCS of B. bassiana are available. A few reports on growth requirements and shake flask culture of B. bassiana strains show the best growth and germination in complex media (Bidochka et al. [1987]; Chong-Rodríguez et al. [2011]; Hegedus et al. [1990]; Humphreys et al. [1990]; Pham et al. [2009]; Rombach [1989]; Safavi et al. [2007]; Samsináková [1966]; Thomas et al. [1987]; Vega et al. [2003]). However, only a few publications deal with the production of mycelium (Núñez-Ramírez et al. [2012]) and the production of BS in complex (Humphreys et al. [1989,1990]) and mineral media (Lane et al. [1991]) by submerged fermentation, respectively. Endophytic B. bassiana strains can exist asymptomatically in a variety of plants like banana (Akello et al. [2008]), opium poppies (Quesada-Moraga et al. [2006]), maize (Bing and Lewis [1992]) and sorghum (Tefera and Vidal [2009]). The recently isolated endophytic B. bassiana strain ATP-02 showed great potential for a novel plant control measure in a variety of crops (Tefera and Vidal [2009]). However, it remained unknown if this strain can be mass-produced to high yields and if the spores from a submerged fermentation are able to colonize plants. That is why the objective of the present work was to produce spores of endophytic B. bassiana ATP-02 in a cost-effective culture medium on lab-scale and to scale-up the process to a 2 L stirred-tank reactor. Finally the virulence of the produced spores was checked in a bioassay with Plutella xylostella and their potential to colonize oilseed rape leaves via a leaf application was investigated.
Materials and methods
All materials used were purchased from Merck KGaA (Darmstadt, Germany), Carl Roth GmbH (Karlsruhe, Germany) or AppliChem GmbH (Darmstadt, Germany), if not mentioned otherwise. Sugar beet molasses with a dry matter content of 80% consisting of 50% sucrose was purchased from Suedzucker AG (Mannheim, Germany). All concentrations are given as (w/w).
Strain
B. bassiana isolate ATP-02, DSM 24665, was provided by Prof. Stefan Vidal, Georg-August-University, Department of Crop Sciences/Agricultural Entomology, Goettingen, Germany. The strain was raised at 25°C on SDA agar containing 1% casein peptone, 2% glucose and 1.5% agar-agar at pH 5.5. Temperature optimum was found at 25°C and pH optimum at 5.5 (data not shown).
Cultivation in shake flask culture
Different liquid media were used to cultivate B. bassiana: TKI medium with 5% carbon source (Thomas et al. [1987]), Czapek-Dox medium (Kučera [1971]), YPG medium and PG medium (Bidochka et al. [1987]), Vogel’s medium (Vogel [1956]), SD medium (Odds [1991]), CGM medium containing 1% glucose, 1% corn steep liquor, 0.5% NaCl, 0.1% NaNO3, 1% CaCO3 (Samsináková [1966]), PWG medium containing 1% glucose, 8.75% whey powder, 0.25% peptone (Kassa et al. [2008]), YG medium containing 1% glucose, 1% yeast extract (Leckie et al. [2008]), YSM medium containing 2% sucrose, 0.5% yeast extract, 0.15% KH2PO4, 0.05% MgSO4∙7H2O, 0.001% CaCl2, 0.000003% H3BO3, 0.000004% MnSO4∙4H2O, 0.0000025% Na2MoO4∙2H2O, 0.000008% CuSO4∙5H2O, 0.00004% ZnSO4∙7H2O, 0.00005% FeCl3∙6H2O, 0.00004% CoCl2∙6H2O (Rombach 1988, 1989) and YS medium containing 2.5% sucrose, 2.5% yeast extract (Rombach [1989]). In each case 50 mL medium was placed in 250 mL DURAN® baffled flasks. The pH values of the media were adjusted to 5.5 with 0.5 M NaOH. As a starter inoculum AC from SDA agar (see above) were used. The AC were isolated by flooding the plates with 2 × 5 mL of sterile 0.1% Tween 80 and gently raking the plates with a sterile bristle brush. The shake flask cultures were inoculated with the spore suspension to give an initial spore density of 5.0×104 AC/mL. The flasks were incubated at 25°C on a rotary shaker at a speed of 150 rpm for 8–10 days. Every day, 1 mL samples were taken to check developmental stage and the concentration of the spores with a Thoma counting cell chamber under 400 × magnification (photomicroscope, Carl Zeiss AG, Oberkochen, Germany).
Fermentation
Batch fermentation was carried out in a 2 L BIOSTAT® Bplus stirred tank reactor (Sartorius Stedim System GmbH, Guxhagen, Germany) with a working volume of 1.5 L. The basal salts were dissolved in 1200 mL ddH2O and were autoclaved in the bioreactor. Also, a few drops of the anti-foam agent Pluronic® PE 8100 (BASF SE, Ludwigshafen, Germany) were added before fermentation started. Likewise, 300 mL of a carbon source stock solution (75 g carbon source) were autoclaved separately and were inoculated with 7.5×108 aerial conidia (5.0×104 spores/mL). To start the fermentation, the inoculum suspension was added to the bioreactor. Temperature was maintained at 25°C and fermentation time was between 8–10 days.
Analysis
The metabolic respiratory quotient (RQ) is an on-line parameter for the formation of biomass was calculated from the ratio of the generated carbon dioxide and the consumed oxygen, which were measured with an O2 and CO2 sensor (BlueSens GmbH, Herten, Germany) in the exhaust air. For the determination of fungal dry biomass 15 mL samples were centrifuged for 10 min at 20,000 g, washed two times with ddH2O and centrifuged again. The pellets were suspended in 5–7 mL of ddH2O. The cell suspensions were dried at 115°C to constant weight using a moisture analyzer (Sartorius AG, Goettingen, Germany). Each time, determination of fungal dry biomass was carried out in two replicates.
The colony forming units (CFU) of BS and SCS were determined by spreading 100 μL of diluted samples on SDA plates (Odds [1991]) and incubating at 25°C for 4–6 days. To ensure that a sample will yield CFU in a range between 50 and 150 colonies requires several 10-fold dilutions of the sample with 0.9% NaCl. The CFU were determined on duplicate samples. The CFU/ml was calculated as follows:
| (1) |
Insect virulence assay
Bioassays were conducted with 30 second instar larvae of Plutella xylostella L. (Yponomeutidae: Lepidoptera), which were provided by Prof. Stefan Vidal, Georg-August-University, Department of Crop Sciences/ Agricultural Entomology, Goettingen, Germany. The culture broth of the fermentation was centrifuged for 5 min at 20,000 g, washed two times with ddH2O and centrifuged again. The washed spore mix, consisting of 95% BS and 5% SCS, as well as pure AC from a two-weeks-old SDA culture were suspended in 0.1% Triton-X114 to obtain a final concentration of 106 viable spores/mL. Aliquots of 1 mL of the suspensions were brushed on the adaxial side of secondary oilseed rape leaves with an area of 80 ± 10 cm2. The control leaves were treated with 0.1% Triton-X114 only. High 500 mL beakers were filled with 100 mL water agar (1.0% agar-agar). In each case three stalks of the treated leaves were drilled into the solid water agar. The upper surface of the water agar was covered with sterile filter paper to prevent the larvae from getting stuck. Afterwards, ten larvae were transferred into each of the beakers with and without spores, respectively. The beakers were closed with silk gauze and incubated at room temperature.
After 14 days, the dead larvae were surface sterilized with 70% ethanol for 2 min, 5% sodium hypochlorite for 3 min and 70% ethanol for 2 min, rinsed twice in sterile distilled water, and then placed on sterile tissue paper in a laminar airflow cabinet. The larvae were placed on a modified B. bassiana selective medium, consisting of 1% casein peptone, 4% glucose, 0.1375% Syllit® (Spiess-Urania Chemicals GmbH, Hamburg, Germany), 0.0005% chlortetracycline, 0.0005% crystal violet and 1.5% agar-agar (Chase et al. [1986]; Rangel et al. [2010]), and were incubated at 25°C for 1 week. To evaluate the efficacy of the surface sterilization method the water used to rinse the tissues after surface sterilization was plated on selective medium and was incubated, too.
After re-isolation, the DNA of the mycelium was extracted. In each case 50 mg mycelium were cooled on ice, and 1 mL CTAB buffer (0.02 M Na-EDTA, 126 mM sorbitol, 36.8 mM n-laurylsarcosine, 22 mM CTAB, 90 mM polyvinylpyrrolidone, 10 mM Tris, 0.8 M NaCl at pH 8.0), 2 μL β-mercaptoethanol and 1 μL proteinase K (0.1 g/L) were added. The samples were incubated at 42°C for 10 min, at 65°C for 10 min, and then 800 μL chloroform:isoamylalcohol 24:1 were added, mixed, stored on ice for 10 min and centrifuged at 8,000 rpm for 10 min. The supernatants were carefully taken, mixed with 100 μL 5 M NaCl and 200 μL 30% PEG, stored for 10 min at room temperature and centrifuged at 14,000 rpm for 15 min. The pellets were washed twice with 600 μL 75% ethanol and dried in a thermoblock at 65°C. Afterwards, the DNA pellet was dissolved in 100 μL TE buffer (10 mM Tris at pH 8.0) and stored at −20°C. All samples were assessed by PCR using specific primers namely RD1-F (5′- TGGGTATAGGCCGCAGCAC-3′) and RD1-R (5′- CTCTAAGGGTGACAGGGATAG-3′) which amplify a 208-bp region of the ITS1-5.8S-ITS2 region of B. bassiana. The primer sequences were compared with the NCBI nucleotide database using BLAST and were predicted to be 100% homologous to B. bassiana isolate DAOM210087 as well as ATP-02. All amplifications were performed in a TProfessional (Biometra GmbH, Goettingen, Germany) thermocycler. PCR reactions consisted of a reaction mix (final volume 10 μL) of 1 μl 10 x reaction buffer, 2.5 mM MgCl2, 100 μM dNTPs, 0.3 μM RD1-F primer, 0.3 μM RD1-R primer, 0.3 U Taq polymerase (5 Prime GmbH, Hilden, Germany) and 1 μl template DNA (30 ng). The cycling program included an initial denaturation step of 5 min at 95°C, followed by 35 cycles of 1 min denaturation at 95°C, 1 min annealing at 59°C, and 1 min extension at 72°C. Amplification products were mixed with GelRed™ (Biotium Inc., Hayward, Canada), separated by electrophoresis in 1.5% agarose gels in 1× TBE buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA at pH 8.0) for 60 min at 100 V and visualized under UV radiation.
All tests were run for 14 days and each test was repeated thrice. The mortality data were analyzed statistically using one-way ANOVA test.
Penetration assay
Native oilseed rape (Brassica napus L.) cultivar “PULSAR” was obtained from the Deutsche Saatveredelung AG (Lippstadt, Germany). The seeds were planted singly in pots containing sterile soil/sand substrate (1:3 (v/v)) (Fruhstorfer Erde Type T25, HAWITA Group GmbH, Vechta, Germany) and were cultivated under greenhouse conditions, The plants were maintained at 18-22°C, 40–60% RH for 16 weeks and with a 12-h photoperiod (SON-T Agro 400 W, Philips, Amsterdam, Netherlands).
Formulation components consisting of 0.1% Triton X-114 as a wetter, 0.1% gelatine 280 Bloom (Gelita AG, Goeppingen, Germany) as a humectant, 1% sugar beet molasses as nutrient and 1% titanium dioxide as a UV protection agent were autoclaved for 20 min at 121°C. The spore suspension from a submerged fermentation was centrifuged for 5 min at 20,000 g, washed twice with ddH2O and centrifuged again. Afterwards the spores were suspended in 0.9% NaCl and added to the formulation components up to a final concentration of 106 spores/mL. The control formulation was free of fungal biomass. Then, the formulations were brushed onto an area of approximately 3 cm of the tips of 9th secondary leaves oilseed rape plants. To increase the relative humidity up to 95%, the treated leaves were wrapped with plastic bags for the first 48 h. After 7 days the leaf tips were cut off and the untreated base of the leaves were harvested for the detection of endophytic colonization with B. bassiana by microscopy and PCR.
For microscopy, cross-sections of the leaf mid rip were stained with 0.5% rose bengal dissolved in 5% aqueous ethanol for 15 sec and were washed with ddH2O (Saha et al. [1988]). Growth of B. bassiana in the plant tissue was detected at 200-fold magnification with a light microscope. Afterwards, the leaves were surface sterilized as mentioned above and then placed on sterile tissue paper in a laminar airflow cabinet. In a preliminary test it was shown that this surface sterilization method kills all spores which were applied onto oilseed rape leaves.
For DNA extraction which was described above, approximately 400 mg plant tissue from the surface sterilized leaves and stems was taken. The plant tissue was crushed in a MM400 ball mill using a sterile 5 mm steel ball (Retsch GmbH, Haan, Germany) for 5 min at 30 Hz. To isolate B. bassiana DNA from a SDA culture, 50 mg fungal biomass were directly used for DNA extraction. All tissue samples of both treated and untreated oilseed rape plants were assessed by PCR.
Results
Screening of media in shake flask culture
The entomopathogenic and endophytic fungus B. bassiana isolate ATP-02 was cultivated in shake flasks. The different liquid media described above were used to study the effect of various nutrients, basal salts and other complex components on submerged spore formation. In Figure 1 the concentrations and yields of total spores (TS) with regard to the different culture media are illustrated. The most promising culture medium with regard to a maximum growth and optimum sporulation was the TKI medium with 5% sucrose as a carbon source: After a cultivation time of 168 h B. bassiana produced TS in a concentration of 1.10 ± 0.01×108 TS/mL consisting of 100% BS. In this TKI medium a yield of 2.20 ± 0.02×109 TS/g sucrose was obtained. Due to the lower substrate concentration of 2% sucrose and the also high concentration of 1.09 ± 0.08×108 TS/mL in the YSM medium a yield of 5.43 ± 0.38×109 TS/g sucrose was obtained. However, in contrast to the TKI medium, the YSM medium contains 0.5% yeast extract as a complex component. In all cases, the achieved concentration of SCS was lower than 11% of the TS. The highest, but still low SCS concentration of 0.29 ± 0.04×106 SCS/mL was obtained in the YG medium.
Figure 1.
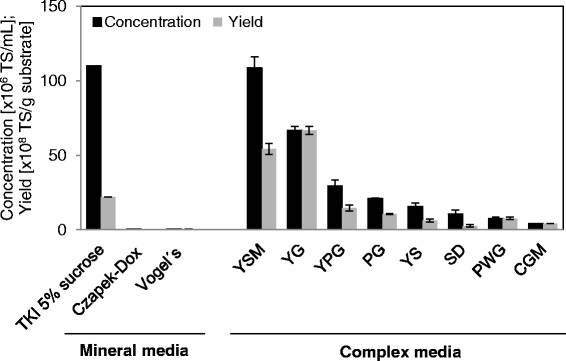
Influence of different culture media on spore formation.B. bassiana was cultivated in 250 mL shake flasks (n = 2). Mean (±SD) concentrations and mean (±SD) yields of TS 168 h after inoculation.
Influence of different carbon sources on spore formation of B. bassiana
B. bassiana ATP-02 was cultured in TKI medium which was supplemented with 5% of different pure carbon sources according to Thomas et al. ([1987]). Furthermore, TKI medium was supplemented with 5% sugar beet molasses as a complex carbon source which consisted of 50% sucrose according to manufacturer specification (Suedzucker AG, Mannheim, Germany). In Figure 2 a and b the concentrations of TS and SCS with regard to the different carbon sources are illustrated. The highest concentration of 1.17 ± 0.05×109 TS/mL corresponding to the highest yield of 4.68 ± 0.20×1010 TS/g sucrose was obtained in the TKI medium with 5% sugar beet molasses. Furthermore, in this medium B. bassiana also produced the highest concentration of 2.00 ± 0.50×107 SCS/mL corresponding to a SCS yield of 8.00 ± 2.00×108 SCS/mL 168 h after inoculation. However, the biomass consisted of more than 98% BS. In spite of the lower sugar concentration of the molasses (2.5%) the concentration of TS could be increased up to 11-times in contrast to the cultivation with 5% sucrose. Due to the lower sugar concentration the yield of TS could be even increased up to 21-times.
Figure 2.
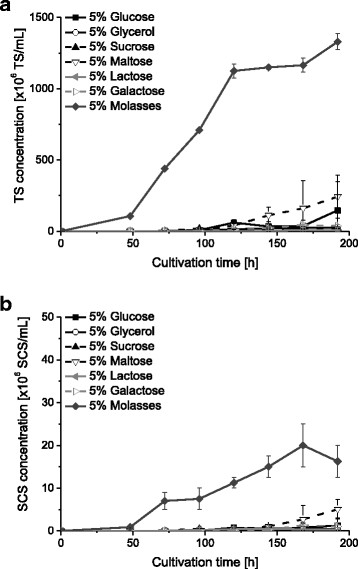
Influence of different carbon sources of the TKI medium on spore formation.B. bassiana was cultivated in 250 mL shake flasks (n = 2). (a) Mean (±SD) concentration of BS. (b) Mean (±SD) concentration of SCS.
Interestingly, when TKI basal salts were omitted from the 5% molasses medium, TS concentration decreased by 96% compared to cultivation in the original TKI medium (data not shown).
Fermentation of B. bassiana ATP-02
B. bassiana ATP-02 was raised in a 2 L stirred tank reactor to produce high concentrations of TS. Based on the cultivations in shake flasks B. bassiana ATP-02 was cultivated in the mineral TKI medium with 5% sugar beet molasses and were inoculated with 5×104 AC/mL. The fermentation conditions and growth parameters are given in Table 1. During 48 h fermentation time, 12.6 g/L dry biomass was produced. Maximal specific growth rate μmax was 0.14 h−1. Average doubling time was 14.7 h. The minimal doubling time of 4.9 h was reached between 48 and 72 h after inoculation. The results of the fermentation could be verified in 7 sequentially performed runs, which are shown in Table 2.
Table 1.
Cultivation of B. bassiana ATP-02 in a 2 L stirred tank reactor: fermentation conditions and growth parameters
| Fermentation conditions | |
|---|---|
| Inoculum [aerial conidia/mL] |
5×104 |
| Start pH [−] |
5.5 |
| Stirrer speed [rpm] |
600 |
| Aeration rate [vvm] |
1.0 |
| Aeration rate [L/min] |
1.5 |
| Temperature [°C] |
25 |
|
Growth parameters |
|
| Biomass produced (after 48 h) [g/L] |
12.6 |
| Max. specific growth rate μmax [h−1] |
0.14 |
| Min. doubling time [h] |
4.9 |
| Mean doubling time [h] | 14.7 |
Table 2.
Cultivation of B. bassiana ATP-02 in 7 sequentially performed fermentation runs
| No. | Total spores [x10 9 TS/mL] | Submerged conidiospores [x10 9 SCS/mL] |
|---|---|---|
| 1 |
1.11 ± 0.00 |
0.04 ± 0.00 |
| 2 |
1.45 ± 0.01 |
0.10 ± 0.01 |
| 3 |
1.12 ± 0.01 |
0.06 ± 0.01 |
| 4 |
2.08 ± 0.02 |
0.12 ± 0.01 |
| 5 |
1.89 ± 0.01 |
0.09 ± 0.02 |
| 6 |
1.39 ± 0.04 |
0.08 ± 0.01 |
| 7 | 1.11 ± 0.09 | 0.04 ± 0.01 |
Achieved mean (±SD) concentrations of TS and SCS 168 h after inoculation. Standard deviations were calculated from two technical replicates.
The Figure 3a and b show the details of the fermentation no. 1. The fermentation process can be subdivided in two phases: a phase of mycelium formation and a following phase of spore formation.
Figure 3.
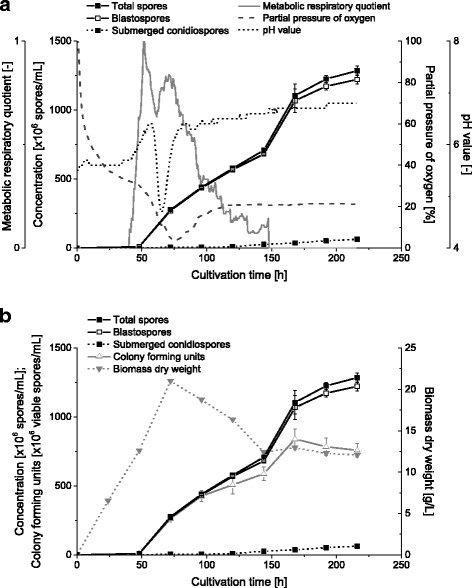
Cultivation ofB. bassianain a 2 L stirred tank reactor. The figure shows the mean (±SD) concentrations of TS, BS and SCS. (a) Process parameters. (b) Correlation of spore counts with biomass and mean (±SD) CFU. In each case, standard deviations resulted from two technical replicates.
At the beginning of the fermentation the amount of dry biomass increased because the fungus produced mycelium. After 62 h a sudden decrease of the RQ to 0.6 followed by a sharp decrease of pH to 4.7, a short recuperation of RQ and a decrease of pO2 to 4% was observed. A sample taken shortly thereafter at 72 h still yielded 21 g biomass/L and the broth was still visibly viscous. Then, biomass dry weight decreased which was accompanied by a visible reduction of mycelium. Preliminary HPLC data indicated a formation of oxalate (data not shown). At this time the concentration of TS started to increase up to 1.29 ± 0.04×109 TS/mL corresponding to a yield of 5.16 ± 0.16×1010 TS/g sucrose at the end of the fermentation. However, the biomass consisted of more than 95% BS. 96 h after inoculation the viability of TS started to decrease. The maximum concentration of 0.84×109 viable spores/mL corresponding to a yield of 3.36×1010 viable spores/g sucrose was obtained 168 h after inoculation. During the further fermentation process the concentration of viable spores decreased to 0.78×109 TS/mL corresponding to a yield of 3.12×1010 TS/g sucrose at the end of the fermentation. Besides, the biomass dry weight decreased during the spore formation phase from 21 g/L to 12 g/L. Furthermore, 80 h after inoculation the RQ decreased continuously and the pO2 reached a non-critical value of 20% and a clogging of the pO2 electrode was noted.
Insect virulence assay
The spore mixture from a submerged fermentation, consisting of 95% BS and 5% SCS as well as pure AC harvested from a petri dish were applied in a virulence test against the diamondback moth, P. xylostella. After 14 days, in the control without fungal spores 93 ± 5% of the larvae developed into viable adult insects. However, 77 ± 5% of larvae fed with spore mix-treated leaves as well as 90 ± 8% of larvae fed with AC-treated leaves died within a week (Figure 4). The dead larvae were surface sterilized, placed on a B. bassiana selective medium and mycelium grew out of all larvae treated with fungal spores and the mycelium was identified as B. bassiana by PCR. The mycelium which grew out of dead larvae not treated with B. bassiana was clearly not B. bassiana, so that these larvae did not die by B. bassiana induced mycosis. It was observed that the spores from submerged fermentation (P < 0.01; F1,4 = 220.5) as well as the pure AC (P < 0.01; F1,4 = 156.3) significantly affected the mortality of larvae. Besides, the number of dead larvae was not significantly affected by the type of spores.
Figure 4.
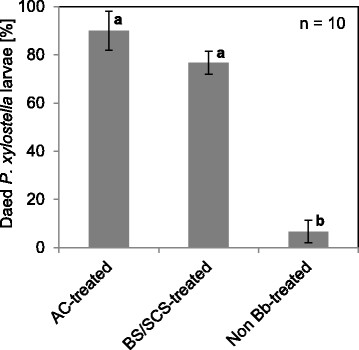
Virulence test withP. xylostellalarvae. The larvae were fed with AC-treated as well as BS/SCS-treated (95% BS and 5% SCS) and non B. bassiana (Bb)-treated oilseed rape leaves. Means (±SD) followed by different letters are significantly different at P < 0.01 using one-way ANOVA test. In each case, standard deviations resulted from three replicates with 10 larvae.
Penetration assay
The influence of a formulation with B. bassiana spores (106 TS/mL) on the penetration of oilseed rape leaves was investigated. The formulation was brushed onto the 9th secondary leaf tips of seven oilseed rape plants and afterwards, endophytic B. bassiana was detected in the tissue of the untreated leaf base by PCR and microscopy. After 7 days, no hyphae growth was observed microscopically in control leaves treated without B. bassiana (n = 2). However, hyphae growth was observed in the mid rip cross-sections of 100% of leaves treated with the formulation. A randomly selected cross-section of these leaf mid rips is illustrated in Figure 5a. To verify that the microscopically detected mycelium was B. bassiana, a PCR was performed. B. bassiana was detected in all untreated areas of the leaves treated with the formulation by PCR and subsequent gel electrophoresis. The positive PCR signals of five randomly selected leaves are shown in Figure 5b and no PCR amplification was observed in control plants.
Figure 5.
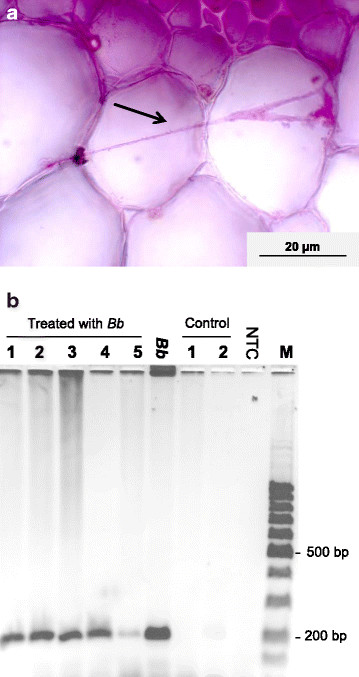
Penetration assay withB. bassianaATP-02. Spray formulations with (n = 7) and without (n = 2) B. bassiana (Bb) were brushed onto the leaf tips. (a) Microscopic detection of Bb in a cross-section of a leaf mid rip from a Bb-treated leaf at 200-fold magnification. The arrow marks the hyphae in the leaf tissue. (b)Bb was detected in the untreated area of the Bb-treated leaves by PCR and subsequent gel electrophoresis. Lane 1–5: DNA from Bb-treated leaves; Lane 6: Bb DNA from pure SDA culture; Lane 7–8: DNA from control-treated leaves; Lane 9: no template control; Lane 10: 100 bp DNA ladder (AppliChem GmbH, Darmstadt, Germany).
Discussion
This study deals with the fermentation aspects of the endophytic B. bassiana ATP-02 which might prepare the way to exploit endophytes as commercial biocontrol agents.
Screening of media in shake flask culture
In the past, mass-production of B. bassiana has focused on AC, but the production through surface cultivation or a two-stage process in which the fungus is allowed to develop under submerged conditions and subsequently transferred to a solid media to sporulate requires long cultivation times, large amounts of space and can be labour-intensive (Hall and Papierok [1982]). The obvious advantages of a submerged cultivation are that the fungus produces spores in a relatively short time with high yields under controlled sterile conditions as well as a simpler scale-up in contrast to solid-state fermentation (Feng et al. [1994]; Hegedus et al. [1992]; Patel et al. [2011]). It was previously known that B. bassiana strains grow in a variety of liquid mineral and complex media, but that the conidiation of the fungi under submerged conditions may be strain-specific (Kassa et al. [2008]) and needs to be investigated for each strain in detail. Furthermore, it was hypothesized that the endophytic B. bassiana strain ATP-02 does not grow in the same way as the established B. bassiana strains that are applied in classic biocontrol on the surface of plants or in the soil.
However, results on cultivation in shake flasks showed that B. bassiana ATP-02 was able to produce BS in the described culture media. Generally, in a submerged cultivation B. bassiana can produce two types of spores, namely BS and SCS. BS are relatively large, thin-walled and single-celled hyphal bodies (Bidochka et al. [1987]). SCS, on the other hand, are small, spherical, more uniform in size and show a higher shelf life than BS (Thomas et al. [1987]; Hegedus et al. [1992]; Holder et al. [2007]). They arise from the fungal mycelia or directly from BS in a process known as microcycle conidiation (Smith et al. [1981]). Thomas et al. ([1987]) describe the direct formation of SCS from BS in the mineral TKI medium with 5% glucose after a cultivation time of 96 h. This phenomenon was not observed in the present work. From the biotechnological point of view the most important properties of the culture medium are high yields of TS and SCS, respectively. Although a higher TS yield was obtained in the YSM medium consisting of basal salts, 2% sucrose and 0.5% yeast extract a 2.5-fold, the mineral TKI medium without expensive complex components was further optimized because of cost-effectiveness.
Influence of different carbon sources on spore formation of B. bassiana
The highest concentrations and yields of TS and SCS were obtained in the TKI medium with 5% sugar beet molasses as a carbon source. It should be pointed out that the concentration of TS could be increased up to 11-times in contrast to the cultivation with 5% sucrose in spite of the lower sucrose concentration of 2.5% in the molasses. The utilized sugar beet molasses consisted of 50% sucrose and only traces of other sugars like glucose, fructose and raffinose as well as different proteins and basal salts according to manufacturer specification (Suedzucker AG, Mannheim, Germany). Furthermore, it could be shown that in addition to the present basal salts of the sugar beet molasses the TKI medium is necessary for optimal growth of B. bassiana. Sugar beet molasses is a residue of the agricultural industry and consequently, it is a low-cost source, which is a big advantage compared to other carbon sources. Therefore, the cost of 1 L TKI basal medium amended with 5% sugar beet molasses amounts to only 0.31 €.
Fermentation of B. bassiana ATP-02
After the optimized cultivation of B. bassiana ATP-02 in shake flasks the process was scaled-up to a 2 L stirred tank reactor. Based on the cultivations in shake flasks B. bassiana ATP-02 was cultivated in the TKI mineral medium with 5% sugar beet molasses. In a preliminary test the fermentation was inoculated with a 5-days old shake flask culture of B. bassiana, which contained only BS. During the fermentation the fungus produced only mycelium (data not shown). Since the objective of cultivation was a high concentration of TS, further fermentations were inoculated with 5.0×104 AC/mL. The achieved concentrations and yields of TS and SCS were comparable with the cultivation of B. bassiana ATP-02 in shake flasks and could be verified in 7 sequentially performed fermentation runs.
An unusual point during all fermentations is the sudden decrease of biomass dry weight in correlation with the low concentration of oxygen in the culture broth 72 h after inoculation. At this point it was observed that the finely dispersed mycelium lysed and the pH and the viscosity of the culture broth decreased. HPLC analysis indicated presence of oxalate. The reason for the rapid pH decrease in our fermentation is not clear, but it can be presumed that intracellular oxalate was suddenly released due to the lysis of mycelium. The following increase of pH suggests that oxalate was then metabolized by the growing spores. These presumptions are supported by other studies which also indicate that B. bassiana strains are able to produce, secrete and metabolize oxalate in vitro (Bidochka and Khachatourians [1993]; Kirkland et al. [2005]).
During the fermentation process the pH value was not regulated due to the fact that fluctuating pH values between 4.0 and 6.5 have no considerable impact on the growth of B. bassiana (Padmavathi et al. [2003]; Thomas et al. [1987]). The typical limitation of oxygen can be prevented by increase of the stirrer speed or agitation rate (Patel et al. [2011]). But the primary objective of this fermentation process was not the production of finely dispersed mycelium but rather the mass-production of sprayable TS without any mycelium.
The recurring decrease of biomass dry weight in the spore formation phase cannot be explained in detail. It may be hypothesized that the mycelium is decreasing but the spore formation does not compensate the weight loss. It can be ruled out that spore biomass was lost during sample preparation as biomass was not filtered but centrifuged.
Furthermore, the achieved TS concentration and yield of the described fermentation process was higher than those obtained by other investigators in studies of liquid shake flask cultivations of epiphytic B. bassiana strains. For example, Thomas et al. ([1987]) reported a maximum concentration of 5.0×108 TS/mL corresponding to a yield of 1.00×1010 TS/g glucose, Rombach ([1989]) described that B. bassiana produced TS in a maximum concentration of 0.17×109 TS/mL corresponding to a yield of 0.85×1010 TS/g sucrose, Vega et al. ([2003]) obtained a maximum concentration of 1.24×109 BS/mL corresponding to a yield of 1.65×1010 BS/g glucose and Pham et al. ([2009]) reported a maximum concentration of 0.85×109 BS/mL. The highest described concentration of BS was reported by Chong-Rodríguez et al. ([2011]), who obtained an inconsistent concentration of 6.38×109 ± 3.63×109 BS/ml in a somewhat costly complex medium, which consisted of 5% sucrose, 2% corn steep liquor and basal salts. In comparison to other published data on cultivation of B. bassiana isolates in a solid-state or submerged cultivation it can be shown that the described fermentation process is very economical with regard to the achieved concentration and yield of TS. Furthermore, an obvious advantage of the fermentation process is that the cost of 1012 TS amounts to only 0.24 € with regard to the utilized culture medium. A further increase of the TS concentration can likely be realized by fed-batch fermentation.
Insect virulence assay
The mortality was 77 ± 5% for P. xylostella larvae, which were fed with oilseed rape leaves treated with BS and SCS, and 90 ± 5% for larvae fed with AC-treated leaves. These larvae mortalities are in accordance with those obtained by other investigators. Godonou et al. ([2009]) reported that AC of B. bassiana caused P. xylostella larvae mortality ranging from 20 to 94%. BS which were sprayed on P. xylostella larvae showed a mortality ranging from 95 to 100% (Fargues et al. [1983]). In addition, Chong-Rodríguez et al. ([2011]) described that BS of B. bassiana maintained for six months at 4°C showed a mortality of more than 80% against third-instar P. xylostella larvae 8 days after application. Furthermore, Ortiz-Urquiza et al. [2010] observed that the composition of the culture medium affected the virulence of AC from B. bassiana because of an increased or decreased secretion of virulent proteins. But the influence of the culture media on the virulence of B. bassiana was not investigated in this work. Since no further mortality tests were conducted with pure BS and pure SCS, it can only be hypothesized that BS must have killed the larvae, because the spore mix consisted of 95% BS which are the preferred propagule of B. bassiana in the haemocoel of infected insects (Jackson et al. [2010]; Shimizu et al. [1993]; Sieglaff et al. [1997]). Furthermore, BS are highly infective against a number of insect pests and have a lower LD50 when compared to AC or SCS (Hegedus et al. [1992]). Finally, it was shown here that spores from a submerged cultivation are as virulent to P. xylostella larvae as AC.
Penetration assay
The simple penetration assay indicated that the spores from submerged fermentation show endophytic properties. This is in line with studies on AC that were applied to leaves and could to some extent colonize plants (Bing and Lewis [1991,1992]; Gurulingappa et al. [2010]; Landa et al. [2013]; Posada et al. [2007]; Quesada-Moraga et al. [2006]; Quesada-Moraga et al. [2009]; Tefera and Vidal [2009]; Wagner and Lewis [2000]). Many questions about endophytism remain that are not within the scope of this fermentation study.
To the best of our knowledge, this is the first report of fermentation of an endophytic B. bassiana strain in a low-cost culture medium to very high yields of TS, which are able to penetrate oilseed rape leaves via a leaf application. This should further encourage the recent activities to exploit the biocontrol potential of endophytic entomopathogenic fungi. Besides, the evidence that the endophytic strain grows in simple cultivation conditions much like the classic biocontrol strains is further proof that in nature some microorganisms are facultative endophytes, which can optionally live inside plants and other habitats (Hardoim et al. [2008]). The results also clearly suggest to further explore submerged cultivation for entomopathogenic fungi in general. Further studies are required to produce higher amounts of SCS of B. bassiana, which show a higher shelf life than thin-walled BS and may also persist longer when sprayed onto plant leaves.
Abbreviations
AC: Aerial conidia:
BS: Blastospores:
SCS: Submerged conidiospores:
TS: Total spores:
RQ: Respiratory quotient:
CFU: Colony forming units:
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
RL designed and carried out the cultivations, fermentations and mortality tests, analyzed the data and wrote the manuscript. DJS carried out the molecular biology studies. AVP conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rieke Lohse, Email: rieke.lohse@fh-bielefeld.de.
Desiree Jakobs-Schönwandt, Email: desiree.jakobs@fh-bielefeld.de.
Anant V Patel, Email: anant.patel@fh-bielefeld.de.
Acknowledgements
We would like to thank Prof. S. Vidal (Georg-August-University of Goettingen, Department of Crop Sciences/Agricultural Entomology) for providing the endophytic B. bassiana strain ATP-02 and the P. xylostella larvae. The research performed in this study was funded by the German Federal Ministry of Education and Research (FKZ 17 N1510).
References
- Akello J, Dubois T, Coyne D, Kyamanywa S. Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot. 2008;4:1437–1441. doi: 10.1016/j.cropro.2008.07.003. [DOI] [Google Scholar]
- Bidochka MJ, Khachatourians GG. Oxalic acid hyperproduction in Beauveria bassiana mutants is related to a utilizable carbon source but not to virulence. J Invertebr Pathol. 1993;4:53–57. doi: 10.1006/jipa.1993.1073. [DOI] [Google Scholar]
- Bidochka MJ, Pfeifer TA, Khachatourians GG. Development of the entomopathogenic fungus Beauveria bassiana in liquid cultures. Mycopathologia. 1987;4:77–83. doi: 10.1007/BF00436909. [DOI] [Google Scholar]
- Bing LA, Lewis LC. Suppression of Ostrinia nubilalis (Hubner)(Lepidoptera: pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol. 1991;4:1207–1211. [Google Scholar]
- Bing LA, Lewis LC. Endophytic Beauveria bassiana (Balsamo) Vuillemin in corn: the influence of the plant growth stage and Ostrinia nubilalis (Hübner) Biocontrol Sci Techn. 1992;4:29–47. [Google Scholar]
- Burges HD. Formulation of microbial pesticides. Kluwer Academic Publishers, Dordrecht, The Netherlands; 1998. [Google Scholar]
- Chase AR, Osborne LS, Ferguson VM. Selective isolation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae from an artificial potting medium. Fla Entomol. 1986;4:285–292. doi: 10.2307/3494930. [DOI] [Google Scholar]
- Chong-Rodríguez MJ, Maldonado-Blanco MG, Hernández-Escareño JJ, Galán-Wong LJ, Sandoval-Coronado CF. Study of Beauveria bassiana growth, blastospore yield, desiccation-tolerance, viability and toxic activity using different liquid media. Afr J Biotechnol. 2011;4:5736–5742. [Google Scholar]
- Fargues J, Reisinger O, Robert PH, Aubart C. Biodegradation of entomopathogenic hyphomycetes: influence of clay coating on Beauveria bassiana blastospore survival in soil. J Invertebr Pathol. 1983;4:131–142. doi: 10.1016/0022-2011(83)90212-4. [DOI] [Google Scholar]
- Feng MG, Poprawski TJ, Khachatourians GG. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Techn. 1994;4:3–34. doi: 10.1080/09583159409355309. [DOI] [Google Scholar]
- Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J, Marrone P, Morin L, Stewart A. Have biopesticides come of age? Trends Biotechnol. 2012;4:250–258. doi: 10.1016/j.tibtech.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Godonou I, James B, Atcha-Ahowé C, Vodouhè S, Kooyman C, Ahanchédé A, Korie S. Potential of Beauveria bassiana and Metarhizium anisopliae isolates from Benin to control Plutella xylostella L. (Lepidoptera: plutellidae) Crop Prot. 2009;4:220–224. doi: 10.1016/j.cropro.2008.10.009. [DOI] [Google Scholar]
- Gurulingappa P, Sword GA, Murdoch G, McGee PA. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control. 2010;4:34–41. doi: 10.1016/j.biocontrol.2010.06.011. [DOI] [Google Scholar]
- Hall RA, Papierok B. Fungi as biological control agents of arthropods of agricultural and medical importance. Parasitology. 1982;4:205–240. doi: 10.1017/S0031182000053658. [DOI] [Google Scholar]
- Hardoim PR, van Overbeek LS, van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;4:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Hegedus DD, Bidochka MJ, Khachatourians GG. Beauveria bassiana submerged conidia production in a defined medium containing chitin, two hexosamines or glucose. Appl Microbiol Biotechnol. 1990;4:641–647. doi: 10.1007/BF00604930. [DOI] [Google Scholar]
- Hegedus DD, Bidochka MJ, Miranpuri GS, Khachatourians GG. A comparison of the virulence, stability and cell-wall-surface characteristics of three spore types produced by the entomopathogenic fungus Beauveria bassiana. Appl Microbiol Biotechnol. 1992;4:785–789. doi: 10.1007/BF00172195. [DOI] [Google Scholar]
- Holder DJ, Kirkland BH, Lewis MW, Keyhani NO. Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology. 2007;4:3448–3457. doi: 10.1099/mic.0.2007/008524-0. [DOI] [PubMed] [Google Scholar]
- Humphreys AM, Matewele P, Trinci APJ. Effects of water activity on morphology, growth and blastospore production of Metarhizium anisopliae, Beauveria bassiana and Paecilomyces farinosus in batch and fed-batch culture. Mycol Res. 1989;4:257–264. doi: 10.1016/S0953-7562(89)80063-2. [DOI] [Google Scholar]
- Humphreys AM, Matewele P, Cunliffe B, Trinci APJ. Comparison of sporulation of Paecilomyces farinosus and Beauveria bassiana in batch and fed-batch culture. Mycol Res. 1990;4:1046–1050. doi: 10.1016/S0953-7562(09)81331-2. [DOI] [Google Scholar]
- Jackson MA, Dunlap CA, Jaronski ST. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. Biocontrol. 2010;4:129–145. doi: 10.1007/s10526-009-9240-y. [DOI] [Google Scholar]
- Kassa A, Brownbridge M, Parker BL, Skinner M, Gouli S, Guo M, Lee F, Hata T. Whey for mass production of Beauveria bassiana and Metarhizium anisopliae. Mycol Res. 2008;4:583–591. doi: 10.1016/j.mycres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Khachatourians GG. Production and use of biological pest control agents. Trends Biotechnol. 1986;4:120–124. doi: 10.1016/0167-7799(86)90144-7. [DOI] [Google Scholar]
- Kirkland BH, Eisa A, Keyhani NO. Oxalic acid as a fungal acaracidal virulence factor. J Med Entomol. 2005;4:346–351. doi: 10.1603/0022-2585(2005)042[0346:OAAAFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kučera M. Toxins of the entomophagous fungus Beauveria bassiana: II effect of nitrogen sources on formation of the toxic protease in submerged culture. J Invertebr Pathol. 1971;4:211–215. doi: 10.1016/0022-2011(71)90093-0. [DOI] [PubMed] [Google Scholar]
- Landa BB, Lopez-Diaz C, Jimenez-Fernandez D, Montes-Borrego M, Munoz-Ledesma FJ, Ortiz-Urquiza A, Quesada-Moraga E. In-planta detection and monitorization of endophytic colonization by a Beauveria bassiana strain using a new-developed nested and quantitative PCR-based assay and confocal laser scanning microscopy. J Invertebr Pathol. 2013;4:128–138. doi: 10.1016/j.jip.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Lane BS, Trinci APJ, Gillespie AT. Endogenous reserves and survival of blastospores of Beauveria bassiana harvested from carbon- and nitrogen-limited batch cultures. Mycol Res. 1991;4:821–828. doi: 10.1016/S0953-7562(09)80045-2. [DOI] [Google Scholar]
- Leckie BM, Ownley BH, Pereira RM, Klingeman WE, Jones CJ, Gwinn KD. Mycelia and spent fermentation broth of Beauveria bassiana incorporated into synthetic diets affect mortality, growth and development of larval Helicoverpa zea (Lepidoptera: noctuidae) Biocontrol Sci Techn. 2008;4:697–710. doi: 10.1080/09583150802262906. [DOI] [Google Scholar]
- Núñez-Ramírez DM, Valencia-López JJ, Calderas F, Solís-Soto A, López-Miranda J, Medrano-Roldán H, Medina-Torres L. Mixing analysis for a fermentation broth of the fungus Beauveria bassiana under different hydrodynamic conditions in a bioreactor. Chem Eng Technol. 2012;4:1954–1961. doi: 10.1002/ceat.201200130. [DOI] [PubMed] [Google Scholar]
- Odds FC. Sabouraud(’s) agar. J Med Vet Mycol. 1991;4:355–359. doi: 10.1080/02681219180000581. [DOI] [PubMed] [Google Scholar]
- Ortiz-Urquiza A, Riveiro-Miranda L, Santiago-Álvarez C, Quesada-Moraga E. Insect-toxic secreted proteins and virulence of the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. 2010;4:270–278. doi: 10.1016/j.jip.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Padmavathi J, Uma Devi K, Uma Maheswara Rao C. The optimum and tolerance pH range is correlated to colonial morphology in isolates of the entomopathogenic fungus Beauveria bassiana – a potential biopesticide. World J Microb Biot. 2003;4:469–477. doi: 10.1023/A:1025151000398. [DOI] [Google Scholar]
- Patel AV, Jakobs-Schönwandt D, Rose T, Vorlop KD. Fermentation and microencapsulation of the nematophagous fungus Hirsutella rhossiliensis in a novel type of hollow beads. Appl Microbiol Biotechnol. 2011;4:1751–1760. doi: 10.1007/s00253-010-3046-9. [DOI] [PubMed] [Google Scholar]
- Pham TA, Kim JJ, Kim SG, Kim K. Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology. 2009;4:218–224. doi: 10.4489/MYCO.2009.37.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada F, Aime MC, Peterson SW, Rehner SA, Vega FE. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: hypocreales) Mycol Res. 2007;4:748–757. doi: 10.1016/j.mycres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Quesada-Moraga E, Landa BB, Muñoz-Ledesma J, Jiménez-Diáz RM, Santiago-Álvarez C. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia. 2006;4:323–329. doi: 10.1007/s11046-006-0014-0. [DOI] [PubMed] [Google Scholar]
- Quesada-Moraga E, Muñoz-Ledesma FJ, Santiago-Alvarez C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: hypocreales) Environ Entomol. 2009;4:723–730. doi: 10.1603/022.038.0324. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Dettenmaier SJ, Fernandes EKK, Roberts DW. Susceptibility of Metarhizium spp. and other entomopathogenic fungi to dodine-based selective media. Biocontrol Sci Techn. 2010;4:375–389. doi: 10.1080/09583150903518370. [DOI] [Google Scholar]
- Ravensberg WJ. A roadmap to the successful development and commercialization of microbial pest control products for control of arthropods. Springer, Dordrecht, The Netherlands; 2011. [Google Scholar]
- Rombach MC. Production of Beauveria bassiana [Deuteromycotina: hyphomycetes] sympoduloconidia in submerged culture. Entomophaga. 1989;4:45–52. doi: 10.1007/BF02372586. [DOI] [Google Scholar]
- Rombach MC, Aguda RM, Roberts DW. Production of Beauveria bassiana [Deuteromycotina: hyphomycetes] in different liquid media and subsequent conidiation of dry mycelium. Entomophaga. 1988;4:315–324. doi: 10.1007/BF02372621. [DOI] [Google Scholar]
- Safavi SA, Shah FA, Pakdel AK, Rasoulian GR, Bandani AR, Butt TM. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol Lett. 2007;4:116–123. doi: 10.1111/j.1574-6968.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- Saha DC, Jackson MA, Johnsoncicalese JM. A rapid staining method for detection of endophytic fungi in turf and forage grasses. Phytopathology. 1988;4:237–239. doi: 10.1094/Phyto-78-237. [DOI] [Google Scholar]
- Samsináková A. Growth and sporulation of submersed cultures of the fungus Beauveria bassiana in various media. J Invertebr Pathol. 1966;4:395–400. doi: 10.1016/0022-2011(66)90056-5. [DOI] [Google Scholar]
- Shimizu S, Tsuchitani Y, Matsumoto T. Production of an extracellular protease by Beauveria bassiana in the haemolymph of the silkworm, Bombyx mori. Lett Appl Microbiol. 1993;4:291–294. doi: 10.1111/j.1472-765X.1993.tb00360.x. [DOI] [Google Scholar]
- Sieglaff DH, Pereira RM, Capinera JL. Pathogenicity of Beauveria bassiana and Metarhizium flavoviride (Deuteromycotina) to Schistocerca americana (Orthoptera : acrididae) J Econ Entomol. 1997;4:1539–1545. [Google Scholar]
- Smith JE, Anderson JG, Deans SG, Berry DR. In: Biology of conidial fungi. Cole GT, Kendrick B, editor. Academic Press, New York, NY; 1981. Biochemistry of microcylce conidiation; pp. 329–353. [DOI] [Google Scholar]
- Tefera T, Vidal S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. Biocontrol. 2009;4:663–669. doi: 10.1007/s10526-009-9216-y. [DOI] [Google Scholar]
- Thomas KC, Khachatourians GG, Ingledew WM. Production and properties of Beauveria bassiana conidia cultivated in submerged culture. Can J Microbiol. 1987;4:12–20. doi: 10.1139/m87-003. [DOI] [Google Scholar]
- Vega FE, Jackson MA, Mercadier G, Poprawski TJ. The impact of nutrition on spore yields for various fungal entomopathogens in liquid culture. World J Microb Biot. 2003;4:363–368. doi: 10.1023/A:1023924304456. [DOI] [Google Scholar]
- Vogel HJ. A conventional growth medium for Neurospora (Medium N) Microb Genet Bul. 1956;4:42–44. [Google Scholar]
- Wagner BL, Lewis LC. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl Environ Microbiol. 2000;4:3468–3473. doi: 10.1128/AEM.66.8.3468-3473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


