Abstract
Background
Heat-tolerant rice cultivars have been developed as a countermeasure to poor grain appearance quality under high temperatures. Recent studies showed that elevated CO2 concentrations (E-[CO2]) also reduce grain quality. To determine whether heat-tolerant cultivars also tolerate E-[CO2], we conducted a free-air CO2 enrichment (FACE) experiment with 12 rice cultivars differing in heat tolerance.
Results
The percentage of undamaged grains of five standard cultivars (Akitakomachi, Kinuhikari, Koshihikari, Matsuribare, Nipponbare) averaged 61.7% in the ambient [CO2] (AMB) plot and 51.7% in the FACE plot, whereas that of heat-tolerant cultivars (Eminokizuna, Wa2398, Kanto 257, Toyama 80, Mineharuka, Kanto 259, Saikai 290) averaged 73.5% in AMB and 71.3% in FACE. This resulted in a significant [CO2] by cultivar interaction. The percentage of white-base or white-back grains increased from 8.4% in AMB to 17.1% in FACE in the sensitive cultivars, but from only 2.1% in AMB to only 4.4% in FACE in the heat-tolerant cultivars.
Conclusion
Heat-tolerant cultivars retained their grain appearance quality at E-[CO2] under present air temperatures. Further improvements in appearance quality under present conditions will be needed to achieve improvements under E-[CO2], because E-[CO2] will likely lower the threshold temperature for heat stress.
Keywords: Chalky grains, Climate change, FACE (free-air CO2 enrichment), Heat-tolerant cultivars, Oryza sativa
Background
High temperatures during grain filling often cause serious damage to the grain quality of rice (Oryza sativa L.) (Terashima et al. [2001]; Lanning et al. [2011]; Kondo et al. [2012]), reducing the proportion of first-grade rice (Hasegawa et al. [2009]) or milling quality (Lyman et al. [2013]). One of the main reasons for the reduction is the decreased percentage of undamaged grains, which is due to increased proportions of chalky and unfilled grains. The occurrence of chalky grains under high temperatures is attributable mainly to the inhibition of starch accumulation (Morita [2008]; Morita and Nakano [2011]).
Daily mean temperatures >26°C during the grain-filling period cause chalkiness in the grains of japonica cultivars (Morita [2008]). The cumulative temperature above 26°C within 15 days after heading can be used as an index of the extent of chalky grains (Lur et al. [2009]). Daily mean air temperatures of >26°C during grain filling are becoming more frequent in Japan. Ishigooka et al. ([2011]) analyzed temperature records during the 20 days after heading in the Kanto, Hokuriku, Tokai, and Kinki regions from 1978 to 2010. Large areas in Tokai and Kinki experienced an increasing frequency of high temperatures, with low interannual variability. Kanto and Hokuriku experienced high interannual variability, with ≥80% of paddy fields under heat stress (1994, 2007, 2010 in Kanto; 1985, 1994, 2010 in Hokuriku).
Because grain quality under heat stress varies substantially among cultivars (Nishimura et al. [2000]; Wakamatsu et al. [2007]), improvement of heat tolerance is one of the most effective countermeasures (Morita [2008]). In fact, breeders have already developed some heat-tolerant cultivars in Japan, including ‘Nikomaru’ (Sakai et al. [2007]; [2010]), ‘Kumasannochikara’ (Fujii et al. [2009]), ‘Genkitsukushi’ (Wada et al. [2010]), and ‘Akisakari’ (Tanoi et al. [2010]), and there are a number of ongoing efforts to develop cultivars with improved heat tolerance.
The atmospheric CO2 concentration ([CO2]) is increasing. [CO2] has increased from 280 μmol mol−1 in 1800 to 396 μmol mol−1 in March 2013 (NOAA [2013]). Its continued increase is expected to have a big influence on crop production. Elevated [CO2] (E-[CO2]) is expected to increase the grain yield of rice (Kobayashi et al. [2006]; Hasegawa et al. [2007], Ainsworth [2008]), but it decreases the protein content (Lieffering et al. [2004]; Terao et al. [2005]; Yang et al. [2007]; Taub et al. [2008]; Seneweera [2011]) and the proportion of undamaged grains (Yang et al. [2007]). These studies suggest that production of high-quality rice grains will become even more difficult under the expected higher air temperatures and [CO2], but attempts to cope with both factors have yet to begin.
The mechanism of the reduction in undamaged grains under E-[CO2] is not understood, but a possible mechanism is a higher canopy temperature in E-[CO2] than in ambient [CO2] (AMB) conditions, which was observed in a free-air CO2 enrichment (FACE) study (Yoshimoto et al. [2005]). If higher canopy temperature due to E-[CO2] is the main reason for the reduced grain appearance quality, heat-tolerant cultivars might also be resistant to the occurrence of chalky grains under E-[CO2], but this hypothesis has never been tested. Therefore, we conducted a FACE experiment using several rice cultivars to determine whether heat-tolerant cultivars could maintain high grain quality under E-[CO2].
Results
Temperatures conditions and heading dates
The daily mean air temperature throughout the growing season in 2012 was 23.7°C, slightly higher than the 30-year average of 23.2°C obtained at the nearest weather station (AMeDAS at Tsukuba; 36°3.4′N, 140°7.5′E, 25 m above sea level). Heading date ranged from 24 July to 7 August, depending on cultivar (Table 1), but the daily mean air temperature averaged over the 20 days after heading (T20DAH), an important determinant of grain quality, varied only in a limited range from 26.5 to 26.9°C, which exceeded the threshold for the occurrence of chalky grains (Morita [2008]). Heading date was significantly earlier in the FACE plot than in the AMB plot (P < 0.05), but by only 1 day, and did not affect T20DAH (Table 1).
Table 1.
Maturation, heading date, average temperature of T 20DAH , tolerance rank, and notes for rice cultivars under test
|
Cultivargroup
A
|
Cultivar(CV) |
Heading date |
T
20DAH
B
°C |
Tolerancerank
C
|
Year ofrelease |
Note |
||
|---|---|---|---|---|---|---|---|---|
| FACE | AMB | FACE | AMB | |||||
| ST |
Akitakomachi |
7/24 |
7/26 |
26.8 ± 0.3 |
26.9 ± 0.3 |
S |
1982 |
Standard cultivar in this study |
| |
Kinuhikari |
7/30 |
7/30 |
26.7 ± 0.3 |
26.7 ± 0.3 |
S |
1983 |
Big grains, Standard cultivar in this study |
| |
Koshihikari |
7/31 |
8/1 |
26.6 ± 0.3 |
26.5 ± 0.3 |
MT |
1953 |
Standard cultivar in this study |
| |
Nipponbare |
8/5 |
8/6 |
26.6 ± 0.3 |
26.6 ± 0.3 |
MT |
1961 |
Standard cultivar in this study |
| |
Matsuribare |
8/6 |
8/7 |
26.6 ± 0.3 |
26.6 ± 0.3 |
S |
1990 |
Standard cultivar in this study |
| HT |
Mineharuka |
7/29 |
7/30 |
26.8 ± 0.3 |
26.7 ± 0.3 |
VT |
2002 |
Long & thin grains (Saka et al., [2007]) |
| |
Toyama 80 |
7/30 |
7/31 |
26.7 ± 0.3 |
26.6 ± 0.3 |
VT |
2012 |
Apq1 on chromosome 7 (Ebitani et al., [2013]) |
| |
Eminokizuna |
7/31 |
7/31 |
26.6 ± 0.3 |
26.6 ± 0.3 |
VT |
2008 |
Small grains, useful for sushi |
| |
Wa2398 |
7/31 |
7/31 |
26.6 ± 0.3 |
26.6 ± 0.3 |
VT |
not released |
QTL on chromosome 2. Derived from ‘Ma Li Xian’ |
| |
Kanto 257 |
8/1 |
8/1 |
26.5 ± 0.3 |
26.5 ± 0.3 |
T |
2011 |
Derived from Mineharuka × Ikuhikari |
| |
Kanto 259 |
8/5 |
8/5 |
26.6 ± 0.3 |
26.6 ± 0.3 |
MT– T |
2012 |
High-yielding cultivar |
| |
Saikai 290 |
8/6 |
8/6 |
26.6 ± 0.3 |
26.6 ± 0.3 |
VT |
2012 |
High-yielding cultivar; good quality, good eating quality |
| Source of variation |
df |
Statistical significance |
||||||
| Block |
3 |
|
|
|
|
|||
| CO2 |
1 |
* |
|
|
|
|||
| Main plot error |
3 |
|
|
|
|
|
|
|
| CV |
11 |
*** |
|
|
|
|||
| GroupD |
1 |
P = 0.055 |
|
|
|
|||
| STD |
4 |
*** |
|
|
|
|||
| HTD |
6 |
*** |
|
|
|
|||
| CO2 × CV |
11 |
* |
|
|
|
|||
| CO2 × GroupE |
1 |
ns |
|
|
|
|||
| CO2 × STE |
4 |
** |
|
|
|
|||
| CO2 × HTE |
6 |
ns |
|
|
|
|||
| Split plot error | 66 | |||||||
AST, standard cultivars; HT, heat-tolerant cultivars.
BT20DAH, air temperatures averaged for 20 days after heading ± standard error of mean.
CVT, very tolerant; T, tolerant; MT, moderately tolerant; S, susceptible.
DSub-division of variation among the cultivars.
ESub-division of variation among the CO2 × CV interaction.
***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant.
Grain appearance quality
The percentage of undamaged grains (UDG) was lower in FACE than in AMB by 5.4 percentage points (Table 2, P = 0.083), averaged over all cultivars, but with highly significant cultivar differences (P < 0.001). The reduction in UDG by E-[CO2] also differed significantly among cultivars, as evidenced by a highly significant [CO2] × cultivar interaction (P < 0.001; Table 2). This interaction was largely associated with the interaction with cultivar group (P < 0.001), whereas [CO2] × HT cultivars was not significant. Namely, the five standard (ST) cultivars (Akitakomachi, Koshihikari, Kinuhikari, Matsuribare, and Nipponbare) showed a large reduction of 10.0 percentage points (from 61.7% to 51.7%), whereas the heat-tolerant (HT) cultivars (Eminokizuna, Wa2398, Kanto 257, Toyama 80, Mineharuka, Kanto 259, Saikai 290) showed a reduction of only 2.2% (from 73.5% to 71.3%). The reduction in UDG was also different among the ST cultivars, as indicated by a significant [CO2] × ST cultivars interaction (P < 0.01, Table 2).
Table 2.
The effect of E-[CO 2 ] on the percentage of grains categorized by appearance
| |
|
Grain appearance category
B
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cultivar group
A
|
Cultivar (CV) |
Undamaged |
Cracked |
Milky-white |
Basal-white & White-Back |
White-belly |
|||||
| |
|
(%) |
(%) |
(%) |
(%) |
(%) |
|||||
| FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | ||
| ST |
Akitakomachi |
40.2 |
58.0 |
1.6 |
1.8 |
5.7 |
4.8 |
28.9 |
10.8 |
4.3 |
2.3 |
| |
Kinuhikari |
68.7 |
72.5 |
0.5 |
0.9 |
3.3 |
2.9 |
8.0 |
4.1 |
1.5 |
2.0 |
| |
Koshihikari |
68.4 |
74.7 |
1.2 |
1.4 |
3.0 |
2.0 |
9.8 |
5.1 |
1.9 |
1.5 |
| |
Nipponbare |
52.9 |
60.8 |
0.4 |
0.2 |
10.4 |
6.6 |
12.8 |
6.9 |
5.3 |
2.4 |
| |
Matsuribare |
28.2 |
42.4 |
0.1 |
0.0 |
15.4 |
8.8 |
26.2 |
15.2 |
7.6 |
4.4 |
| HT |
Mineharuka |
68.2 |
71.3 |
0.1 |
0.2 |
3.4 |
2.1 |
5.6 |
2.6 |
1.1 |
0.9 |
| |
Toyama 80 |
71.9 |
75.6 |
4.1 |
4.1 |
2.8 |
2.1 |
3.9 |
1.3 |
1.4 |
1.8 |
| |
Eminokizuna |
85.0 |
85.7 |
0.1 |
0.0 |
1.6 |
0.7 |
1.1 |
0.7 |
0.8 |
0.5 |
| |
Wa2398 |
80.4 |
82.7 |
0.3 |
0.2 |
1.7 |
1.2 |
3.2 |
1.3 |
1.6 |
1.2 |
| |
Kanto 257 |
74.2 |
76.1 |
0.2 |
0.1 |
2.8 |
2.0 |
3.6 |
1.7 |
2.0 |
1.8 |
| |
Kanto 259 |
55.7 |
57.6 |
0.2 |
0.1 |
8.0 |
5.8 |
8.3 |
4.5 |
4.3 |
2.4 |
| |
Saikai 290 |
63.8 |
65.3 |
0.7 |
0.6 |
5.4 |
4.1 |
5.2 |
2.6 |
6.2 |
4.8 |
| |
ST mean |
51.7 |
61.7 |
0.8 |
0.9 |
7.6 |
5.0 |
17.1 |
8.4 |
4.1 |
2.5 |
| |
HT mean |
71.3 |
73.5 |
0.8 |
0.8 |
3.6 |
2.6 |
4.4 |
2.1 |
2.5 |
1.9 |
| 12 cultivars mean |
63.1 |
68.5 |
0.8 |
0.8 |
5.3 |
3.6 |
9.7 |
4.7 |
3.2 |
2.2 |
|
| Source of variation |
df |
Statistical significance |
|||||||||
| Block |
3 |
|
|
|
|
|
|||||
| CO2 |
1 |
P = 0.083 |
ns |
* |
* |
* |
|||||
| Main plot error |
3 |
|
|
|
|
|
|
|
|
|
|
| CVC |
11 |
*** |
*** |
*** |
*** |
*** |
|||||
| Group |
1 |
*** |
* |
*** |
*** |
*** |
|||||
| STC |
4 |
*** |
*** |
*** |
*** |
*** |
|||||
| HTC |
6 |
*** |
*** |
*** |
*** |
*** |
|||||
| CO2 × CV |
11 |
*** |
ns |
P = 0.068 |
*** |
*** |
|||||
| CO2 × GroupD |
1 |
*** |
ns |
ns |
*** |
* |
|||||
| CO2 × STD |
4 |
** |
ns |
** |
*** |
*** |
|||||
| CO2 × HTD |
6 |
ns |
ns |
ns |
ns |
ns |
|||||
| Split plot error | 66 | ||||||||||
AST, standard cultivars; HT, heat-tolerant cultivars.
BPercentage of grain number by appearance categorized by grain quality inspector.
CSub-division of variation among the cultivars.
DSub-division of variation among the CO2 × CV interaction.
***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant; values indicate 0.05 < P < 0.1.
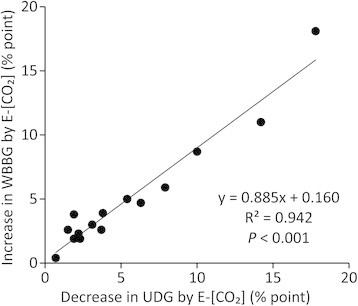
The reduction in UDG was due mainly to an increase in the percentage of chalky grains (Figure 1), in particular, of white-back and white-base grains (WBBG: with a chalky area in the back of the grain or close to the basal part). WBBG increased from 8.4% in AMB to 17.1% in FACE in the ST group, but from only 2.1% to only 4.4% in the HT group (Table 2). This resulted in a highly significant [CO2] × cultivar group interaction (P < 0.001) and a non-significant [CO2] × HT cultivars interaction (Table 2).
Figure 1.
Relationship between decrease in percentage undamaged grain (UDG) and increase in white-base and white-back grains (WBBG) by E-[CO 2 ] among the 12 rice cultivars.
Other types of chalky grains were also increased by E-[CO2]: the percentage of milky-white grains was 3.6% in AMB and 5.3% in FACE (P < 0.05, Table 2), and that of white-belly grains was 2.2% in AMB and 3.2% in FACE (P < 0.05), with highly significant cultivar differences (P < 0.001). The [CO2] × cultivar group interaction was significant for white-belly grains (P < 0.05), whereas the CO2 × HT cultivar interaction was not significant, but the magnitudes of the E-[CO2] effect and the interaction with cultivars were much smaller than in WBBG.
Grain shape and weight
Averaged over all cultivars, E-[CO2] increased grain volume by 0.2 mm3 or 1.4% (P < 0.05, Table 3). Among the three components of grain volume, the effect of E-[CO2] was significant only for thickness (P < 0.001, Table 3), which accounted for most of the increase in volume due to E-[CO2]. The main effect of E-[CO2] on grain length was not significant, but there was a significant [CO2] × cultivar interaction (P < 0.01). There were also significant [CO2] × cultivar interactions for volume (P < 0.01) and thickness (P < 0.001), indicating that E-[CO2] had different effects on these traits, depending on cultivar. However, these traits are similar between the ST and HT groups among and across the CO2 treatments as evidenced by non-significant [CO2] × cultivar group interaction (Table 3) for any of the grain shape components.
Table 3.
The effect of E-[CO 2 ] on shape, protein content, 1000-grain weight and brown rice yield
| |
|
Grain volume(mm
3
) |
Grain shape |
1000-grain weight
B
(g) |
Gain protein content
B
(mg g
−1
) |
Brown rice yield
B
(g m
−2
) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cultivar Group
A
|
Cultivar (CV) |
Length(mm) |
Width(mm) |
Thickness(mm) |
|||||||||||
| FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | FACE | AMB | ||
| ST |
Akitakomachi |
14.3 |
14.2 |
5.08 |
5.08 |
2.71 |
2.71 |
1.99 |
1.98 |
22.0 |
22.2 |
63.5 |
63.9 |
581 |
567 |
| |
Kinuhikari |
14.5 |
14.4 |
4.95 |
4.97 |
2.80 |
2.80 |
2.00 |
1.98 |
22.6 |
22.2 |
64.3 |
66.0 |
577 |
517 |
| |
Koshihikari |
14.1 |
14.3 |
5.01 |
5.05 |
2.77 |
2.79 |
1.94 |
1.95 |
21.6 |
21.8 |
57.4 |
63.0 |
679 |
604 |
| |
Nipponbare |
14.6 |
13.9 |
5.04 |
4.96 |
2.76 |
2.74 |
2.01 |
1.96 |
22.1 |
21.4 |
58.0 |
61.3 |
415 |
374 |
| |
Matsuribare |
13.4 |
13.0 |
4.96 |
4.92 |
2.67 |
2.68 |
1.94 |
1.89 |
20.7 |
19.4 |
60.1 |
59.9 |
580 |
419 |
| HT |
Mineharuka |
15.0 |
14.8 |
5.39 |
5.38 |
2.62 |
2.62 |
2.03 |
2.01 |
22.5 |
22.2 |
56.4 |
63.1 |
535 |
518 |
| |
Toyama 80 |
14.1 |
14.1 |
4.96 |
4.99 |
2.77 |
2.76 |
1.96 |
1.95 |
21.7 |
21.6 |
53.3 |
56.6 |
578 |
557 |
| |
Eminokizuna |
14.0 |
13.9 |
5.01 |
5.02 |
2.64 |
2.65 |
2.02 |
2.00 |
21.4 |
21.8 |
74.9 |
72.5 |
535 |
496 |
| |
Wa2398 |
14.2 |
14.1 |
5.00 |
5.02 |
2.76 |
2.77 |
1.96 |
1.94 |
21.3 |
21.6 |
65.1 |
67.4 |
540 |
457 |
| |
Kanto 257 |
14.8 |
14.8 |
5.34 |
5.33 |
2.69 |
2.70 |
1.97 |
1.97 |
22.7 |
23.3 |
55.0 |
59.8 |
564 |
538 |
| |
Kanto 259 |
14.7 |
13.9 |
5.21 |
5.12 |
2.72 |
2.72 |
1.98 |
1.91 |
22.6 |
21.3 |
54.6 |
57.8 |
506 |
410 |
| |
Saikai 290 |
14.8 |
14.5 |
4.96 |
4.93 |
2.87 |
2.86 |
1.99 |
1.96 |
22.5 |
21.8 |
59.5 |
58.8 |
583 |
475 |
| |
ST mean |
14.2 |
14.0 |
5.01 |
5.00 |
2.74 |
2.74 |
1.98 |
1.95 |
21.8 |
21.4 |
60.7 |
62.8 |
566 |
496 |
| |
HT mean |
14.5 |
14.3 |
5.12 |
5.11 |
2.72 |
2.72 |
1.99 |
1.96 |
22.1 |
21.9 |
59.8 |
62.3 |
549 |
493 |
| 12 cultivars mean |
|
14.4 |
14.2 |
5.08 |
5.06 |
2.73 |
2.73 |
1.98 |
1.96 |
22.0 |
21.7 |
60.2 |
62.5 |
556 |
494 |
| Source of variation |
df |
Statistical significance |
|||||||||||||
| Block |
3 |
|
|
|
|
|
|
|
|||||||
| CO2 |
1 |
* |
ns |
ns |
*** |
P = 0.060 |
P = 0.083 |
P = 0.066 |
|||||||
| Main plot error |
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CV |
11 |
*** |
*** |
*** |
*** |
*** |
*** |
*** |
|||||||
| GroupC |
1 |
*** |
*** |
*** |
** |
*** |
ns |
ns |
|||||||
| STC |
4 |
*** |
*** |
*** |
*** |
*** |
*** |
*** |
|||||||
| HTC |
6 |
*** |
*** |
*** |
*** |
*** |
*** |
P = 0.053 |
|||||||
| CO2 × CV |
11 |
** |
** |
ns |
*** |
*** |
** |
ns |
|||||||
| CO2 × GroupD |
1 |
ns |
ns |
ns |
ns |
ns |
ns |
ns |
|||||||
| CO2 × STD |
4 |
** |
** |
ns |
** |
** |
P = 0.095 |
ns |
|||||||
| CO2 × HTD |
6 |
* |
* |
ns |
** |
*** |
** |
ns |
|||||||
| Split plot error | 66 | ||||||||||||||
AST, standard cultivars. HT, heat-tolerant cultivars.
BExpressed on a 15% moisture content basis.
CSub-division of variation among the cultivars.
DSub-division of variation among the CO2 × CV interaction.
***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant. Values indicate 0.05 < P < 0.1.
Grain weight was also increased by E-[CO2] (P = 0.060, Table 3), with highly significant cultivar differences (P < 0.001) and a highly significant [CO2] × cultivar interaction (P < 0.001). In each cultivar group, there was also considerable variation in the response of grain weight to the CO2 treatment (P < 0.01 for the [CO2] × ST interaction and P < 0.001 for the [CO2] × HT interaction, Table 3), but the response was similar across cultivar groups.
Grain protein content
The grain protein content (expressed on a 15% moisture content basis) averaged over the 12 cultivars dropped, from 62.5 mg g−1 in AMB to 60.2 mg g−1 in FACE (P = 0.083, Table 3). The effect of E-[CO2] differed significantly among cultivars, as evidenced by a [CO2] × cultivar interaction (P < 0.01), but the reduction in protein content by E-[CO2] was similar between ST and HT cultivars: from 62.8 mg g−1 in AMB to 60.7 mg g−1 in FACE in the ST cultivars, and from 62.3 mg g−1 in AMB to 59.8 mg g−1 in FACE in the HT cultivars (ns for the [CO2] × cultivar group interaction, Table 2).
Yield
The average brown rice yield of all 12 cultivars increased from 494 g m−2 in AMB to 556 g m−2 in FACE (12.5%, P = 0.066). The yield enhancement in the two cultivar groups was similar—14.2% in ST and 11.3% in HT—and there was no [CO2] × cultivar interaction.
Relationships among chalky grain percentage, protein content, and grain shape
Multiple-regression analysis showed that grain shape (length, width, and thickness), weight, and protein content were not associated with cultivar differences in the UDG response to E-[CO2] (data not shown).
Discussion
E-[CO2] significantly reduced grain appearance quality by increasing the percentage of chalky grains, as was reported in a previous FACE experiment in China (Yang et al. [2007]). Here, we showed that the effect of E-[CO2] on grain appearance differed widely among cultivars, and that newly developed heat-tolerant cultivars retained high quality under E-[CO2]. These results suggest that the current efforts in breeding for heat tolerance will also be effective under projected climate change.
The mechanism by which the grain appearance quality is reduced by E-[CO2] is not yet understood. E-[CO2] might be expected to improve appearance quality because of higher rates of photosynthesis and of assimilate supply to the grains. In fact, a growth chamber study revealed a modest but positive effect of E-[CO2] (Ward [2007]). However, this advantage did not appear under the FACE conditions, likely because of various indirect effects of E-[CO2] on the physiology of the crop grown in open field conditions.
Among various types of chalky grains that degrade grain appearance quality, we observed significant increases in the percentage of WBBG at E-[CO2] (Table 2), which is known to increase under high temperatures and limited N (Kondo et al. [2006]; Wakamatsu et al. [2007]). We applied the same amount of N fertilizers to all cultivars under both [CO2] conditions, but E-[CO2] decreased grain protein content (Table 3), as observed in many other studies (Lieffering et al. [2004]; Terao et al. [2005]; Yang et al. [2007]; Taub et al. [2008]; Seneweera [2011]; Zhang et al. [2013]). The reason for the different responses in the occurrences of chalky grain to E-[CO2] among cultivars is not clear. If N-related processes are involved in the occurrence of E-[CO2] induced chalkiness, one can hypothesize that cultivars with a smaller loss in grain protein content under E-[CO2] may show a smaller reduction in grain quality. However, we did not observe noticeable differences in the grain protein response to E-[CO2] between ST and HT groups. Some HT cultivars with a small reduction of grain quality also showed a slightly larger reduction of protein content than ST cultivars. This suggests that the quantity of protein does not have direct relevance to the response of appearance quality to E-[CO2]. While we need further studies to determine whether the quantity or activity of specific proteins or enzymes plays a role in the response, the rates of chalkiness could be reduced even with a lower protein content, which is highly important for eating quality.
E-[CO2] reduces leaf stomatal conductance, which is commonly observed across many plant species (reviewed by Ainsworth and Rogers [2007]). This could result in a higher canopy temperature under E-[CO2] than under ambient [CO2] because of reduced transpirational cooling. We did not measure canopy or panicle temperatures in this study, but a previous rice FACE study reported a 0.2 to 1°C increase in canopy temperature by E-[CO2] (Yoshimoto et al. [2005]), which likely occurred in our FACE experiment. Some recent studies highlighted the importance of canopy temperature rather than air temperature in heat stress (Matsui et al. [2007]; Julia and Dingkuhn [2013]; Zhao and Fitzgerald [2013]). These results suggest that E-[CO2] induces chalky grains through increased canopy or panicle temperature. Evidence also exists that canopy temperatures vary significantly among genotypes (Takai et al. [2010]), so the difference in foliage temperatures may partially account for the cultivar differences in the response to E-[CO2]. The canopy microclimate of different cultivars could also be an important subject for future study.
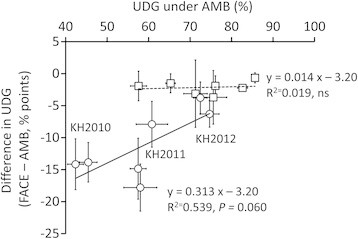
Grain appearance quality often varies from year to year with seasonal and weather conditions. The loss of grain quality due to hot summers has been reported recently both in Japan and elsewhere (e.g., Kondo et al. [2012]; Lyman et al. [2013]). At the Tsukuba FACE site, we tested Koshihikari in 2010 and 2011 also, when the temperatures during the grain-filling period were higher than in 2012. As expected, UDG in AMB in these two years was smaller than in 2012. When we combined these two additional data points with those of the ST group in the 2012 season, the difference in UDG between FACE and AMB became larger as UDG in AMB decreased (Figure 2). This suggests that the negative effect of E-[CO2] on UDG can be exacerbated in hotter summers. In the HT group, the same relationship was not significant, suggesting that these cultivars are likely to show a smaller loss of grain quality under E-[CO2], but this needs to be tested under harsher environments.
Figure 2.
Relationship between the percentage of undamaged grains (UDG) in AMB and difference in UDG between FACE and AMB. KH = Koshihikari (tested in 2010–2012). ○, Standard cultivar; □, Heat-tolerant cultivar. Error bars represent standard error of the mean (n = 4).
E-[CO2] induced WBBG-type chalkiness, which has long been known as a result of high sensitivity to heat (Nagato and Ebata [1965]). All of the HT cultivars showed good appearance quality under both [CO2] conditions, indicating that selection for heat tolerance can be effective for improving grain appearance quality under E-[CO2]. The recent identification of a number of QTLs for heat-induced increases in white-back or white-base chalkiness (e.g., Kobayashi et al. [2007]; [2013]; Ebitani et al. [2008]) will support marker-assisted selection for high appearance quality under high temperatures and possibly under E-[CO2]. However, some of these QTLs may not be effective above a certain threshold; for instance, a T20DAH of 27°C (Kobayashi et al. [2007]). Our results suggest that projected rises in [CO2] will lower the threshold for heat-induced chalkiness, so continued efforts will be needed to increase the level of heat tolerance. In the meantime, we need to improve our mechanistic understanding of how heat-tolerant cultivars could be buffered against loss of grain quality under elevated [CO2] in order to identify promising traits for future breeding programs.
Under the projected future climate change, both high productivity and high quality will be needed. In our previous study, we suggested the potential for improving productivity under E-[CO2] by showing cultivar differences of 3% to 36% in grain yield responses to E-[CO2] (Hasegawa et al. [2013]). Here, we did not observe a significant interaction in grain yield between [CO2] and cultivars, but neither did we find a negative relationship between quality and grain yield (r2 = 0.0281, ns). This suggests that both productivity and quality traits can be improved separately or simultaneously, but continued efforts are needed.
Conclusions
These results indicate that heat-tolerant cultivars retain their grain quality under E-[CO2] better than standard cultivars. Thus, an improvement in grain quality under present conditions will achieve an improvement under E-[CO2]. However, continued efforts will be needed to improve heat tolerance, because E-[CO2] will likely decrease the threshold temperature for heat stress.
Methods
Study site
We conducted the FACE experiment at the Tsukuba FACE site, which was established in farmers’ fields in Tsukubamirai City, Ibaraki Prefecture, Japan (35°58′N, 139°60′E, 10 m above sea level). The climate is humid subtropical with an average annual temperature of 13.8°C and annual precipitation of 1280 mm. The soil is a Fluvisol, which is typical of alluvial areas. The soil properties at the site are described by Hasegawa et al. ([2013]); in brief, the soil contains 21.4 mg g−1 total C and 1.97 mg g−1 total N, has a bulk density of 0.87 Mg m−3, and has a composition of 36% sand, 40% silt, and 23% clay.
CO2 treatment
The method for controlling [CO2] in an open field is described by Nakamura et al. ([2012]). In brief, four blocks (replicates) were established in paddy fields, each consisting of two octagonal plots (240 m2, 17 m across): an ambient [CO2] (AMB) plot and an E-[CO2] (FACE) treatment plot. The FACE plots were equipped with emission tubes around the perimeter, which released CO2 from the windward side to keep the [CO2] measured at the central point at ca. 200 μmol mol−1 above the AMB level. The season-long daytime average [CO2] in 2012 was 392 μmol mol−1 in the AMB plots and 577 μmol mol−1 in the FACE plots.
Cultural practices and growth conditions
We applied compound fertilizer to supply 4.36 g m−2 of phosphorus (P) and 8.30 g m−2 of potassium (K) on 9 April 2012, before plowing. We applied a total of 8 g m−2 of nitrogen (N) fertilizer: 2 g m−2 as urea, 4 g m−2 as one controlled-release fertilizer (type LP100), and 2 g m−2 as another controlled-release urea (type LP140, JCAM Agri. Co., Tokyo, Japan). Right after N application, we puddled (tilled) the field for uniformity on 17 May in 2012.
In each plot, we planted 7 heat-tolerant cultivars and 5 standard cultivars (Table 1). Seedlings were transplanted by hand on 23–25 May 2012 at a spacing of 30 cm × 15 cm (22.2 hills m−2). Seedlings of Akitakomachi and Koshihikari, which we sampled during the growing season for other studies, were planted in areas of 2.7 m × 1.95 m (Akitakomachi) and 3 m × 5.4 m (Koshihikari) at three seedlings per hill. The others were planted in areas of 1.2 m × 0.45 m (12 hills) per cultivar in each plot; they were randomly allocated within the plot.
Measurement
We sampled 21 hills of Akitakomachi and Koshihikari and 12 hills of the others (11 of Wa2398 in one FACE plot) in each of the four replicates for grain yield components and quality measurement. After the materials were dried under a rain shelter, we measured the total aboveground plant weight and panicle number. After threshing, we measured the total weight of the spikelets. Each spikelet sample was then split into three subsamples. One subsample was dehulled to determine the brown rice weight; this sample was then used for grain quality measurement in this study. The other 2 subsamples were used in another study. We measured the moisture content of the grains with a grain moisture tester (Riceter f, Kett Electric Laboratory, Tokyo, Japan). The brown rice yield and 1000-grain weight were expressed on a 15% moisture content basis.
We measured grain appearance quality (different types of chalky grains) and grain shape (width, length, thickness) with a grain quality inspector (RGQI20A; Satake Corp., Hiroshima, Japan) equipped with image analysis software. White-back and white-base grains were counted together, and are reported as WBBG. The grain volume was calculated as for an ellipsoidal body: 4/3 × π × length/2 × width/2 × thickness/2. N was measured with an NC analyzer (Sumigraph NC-22 F; Sumica Chemical Analysis Service, Tokyo, Japan). The protein content (%) was calculated as N (%) × 5.95 (MEXT [2005]) and corrected to 15% moisture content.
Statistics
We conducted an analysis of variance for the 12 cultivars, using a split-plot design, where [CO2] was treated as the main factor and cultivar as the split factor, with four replications, in the SAS software, using the GLM procedure (SAS Institute Inc., Cary, NC, USA). To test differences between cultivar groups, we separated the sums of squares for the split-plot components into the following sources of variation; between-cultivar groups, within HT and ST groups and their interactions with [CO2] (Tables 1, 2, 3). To examine whether cultivar differences in the UDG response to E-[CO2] were associated with grain shapes (length, width, thickness), grain weight and grain protein content, we conducted multiple regression of the increase in UDG by elevated [CO2] on the log-transformed response ratio (FACE/ambient) of grain shape parameters. Statistical significance is indicated for P < 0.001, 0.01, or 0.05, and actual P values are presented where 0.05 < P < 0.1.
Abbreviations
AMB: Ambient:
FACE: Free-air CO2 enrichment:
HT: Heat-tolerant cultivars:
ST: Standard cultivars:
T20DAH: Air temperatures averaged for 20 days after heading:
UDG: Percentage of non-damaged grains:
WBBG: White-based and white-back grains:
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TH planned and supervised the project. HS and TT designed the experimental plot. YU, HS, TT, HN, and TH conducted the FACE experiment. HN managed the [CO2] treatments and monitored the environmental variables. YU collected and analyzed the data. HN advised on grain quality measurements and interpretation of the results. YU and TH wrote the paper. All authors read and approved the final manuscript.
Contributor Information
Yasuhiro Usui, Email: yasuusui@affrc.go.jp.
Hidemitsu Sakai, Email: hsakai@niaes.affrc.go.jp.
Takeshi Tokida, Email: tokida@affrc.go.jp.
Hirofumi Nakamura, Email: 1623nakamura@gmail.com.
Hiroshi Nakagawa, Email: nakagawa16@affrc.go.jp.
Toshihiro Hasegawa, Email: thase@affrc.go.jp.
Acknowledgements
We thank Dr. Hiroshi Nemoto, Mr. Yoshihiro Sunohara, and Dr. Yoshinobu Takeuchi of the National Agricultural Research Organization, and Dr. Takeshi Ebitani of the Toyama Prefectural Agricultural, Forestry and Fisheries Research Center for advice on the selection of cultivars and for providing seeds. We also thank Dr. Tetsuhisa Miwa of the National Institute for Agro-Environmental Sciences for his advice on statistical methods and team member of Tsukuba FACE for their help in the field experiments. This work was supported in part by the Ministry of Agriculture, Forestry and Fisheries, Japan, through a research project entitled ‘Development of technologies for mitigation and adaptation to climate change in agriculture, forestry and fisheries’, and in part by a Grant-in-Aid for Scientific Research on Innovative Areas (no. 24114711) by the Japan Society for the Promotion of Science, as part of the project entitled “Comprehensive studies of plant responses to high CO2 world by an innovative consortium of ecologists and molecular biologists.”
References
- Ainsworth EA. Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biol. 2008;7:1642–1650. doi: 10.1111/j.1365-2486.2008.01594.x. [DOI] [Google Scholar]
- Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanism and environmental interaction. Plant Cell Environ. 2007;7:258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Ebitani T, Yamamoto Y, Yano M, Funane M. Identification of quantitative trait loci for grain appearance using chromosome segment substitution line in rice. Breeding Res. 2008;7:91–99. doi: 10.1270/jsbbr.10.91. doi.org/10.1270/jsbbr.10.91. [DOI] [Google Scholar]
- Ebitani T, Murata Y, Iyama Y, Yamaguchi T, Fujita T. Development of the novel line, Toyama 80, with Koshihikari genetic background can produce superior grains for appearance quality under high temperature stress. Breeding Res. 2013;7:233. [Google Scholar]
- Fujii Y, Mitsukawa M, Sakanashi J, Ueno I, Izumi K, Hatakeyama S, Araki S, Kurata K, Tanaka M. Breeding and characters of a new cultivar ‘Kumasannochikara’ with resistance to high-temperature during ripening period. Res Bull Kumamoto Pref Agric Res Cent. 2009;7:1–10. [Google Scholar]
- Hasegawa T, Kuwagata T, Nishimori M, Ishigooka Y, Murakami M, Yoshimoto M, Kondo M, Ishimaru T, Sawano S, Masaki Y, Matsuzaki H. In: Proceeding of the MARCO Symposium. Hasegawa T, Sakai H, editor. National Institute for Agro-Environmental Sciences, Tsukuba, Japan; 2009. Recent warming trends and rice growth and yield in Japan; pp. 44–51. 5–9 October 2009. [Google Scholar]
- Hasegawa T, Shimono H, Yang LX, Kim HY, Kobayashi T, Sakai H, Yoshimoto M, Lieffering M, Ishiguro K, Wang YL, Zhu JG, Kobayashi K, Okada M. In: Proceedings of the 26th International Rice Conference. Aggarwal P, Ladha J, Singh R, Devakumar C, Hardy B, editor. Los Baños (Philippines) and New Delhi (India): International Rice Research Institute, Indian Council of Agricultural Research, and National Academy of Agricultural Sciences. Macmillan India Ltd, New Delhi, India; 2007. Response of rice to increasing CO2 and temperature: Recent findings from large-scale free-air CO2 enrichment (FACE) experiments; pp. 439–447. 9–12 October 2006. [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu C, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N, Matsunami T, Kaneta Y, Sato T, Takakai F, Sameshima R, Okada M, Mae T, Makino A. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol. 2013;7:148–159. doi: 10.1071/FP12357. doi.org/10.1071/FP12357. [DOI] [PubMed] [Google Scholar]
- Ishigooka Y, Kuwagawa T, Nishimori M, Hasegawa T, Ohno H. Spatial characterization of recent hot summers in Japan with agro-climatic indices related to rice production. J Agric Meteorol. 2011;7:209–224. doi: 10.2480/agrmet.67.4.5. doi.org/10.2480/agrmet.67.4.5. [DOI] [Google Scholar]
- Julia C, Dingkuhn M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Europ J Agronomy. 2013;7:50–60. doi: 10.1016/j.eja.2013.03.006. doi.org/10.1016/j.eja.2013.03.006. [DOI] [Google Scholar]
- Kobayashi A, Bao G, Ye S, Tomita K. Detection of Quantitative Trait Loci for white-back and basal-white kernels under high temperature stress in japonica rice varieties. Breed Sci. 2007;7:107–116. doi: 10.1270/jsbbs.57.107. [DOI] [Google Scholar]
- Kobayashi A, Sonoda J, Sugimoto K, Kondo M, Iwasawa N, Hayashi T, Tomita K, Yano M, Shimizu T. Detection and verification of QTLs associated with heat-induced quality decline of rice (Oryza sativa L.) using recombinant inbred lines and near-isogenic lines. Breed Sci. 2013;7:339–346. doi: 10.1270/jsbbs.63.339. doi.org/10.1270/jsbbs.63.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Okada M, Kim HY, Lieffering M, Miura S, Hasegawa T. Paddy rice responses to free-air [CO2] enrichment. In: Nösberger J, Long SP, Norby RJ, Stitt M, Hendrey GR, Blum H. Managed ecosystems and CO2: Case studies, processes, and perspectives. Springer-Verlag, Berlin; 2006. [Google Scholar]
- Kondo M, Iwasawa N, Yoshida H, Nakagawa H, Ohno H, Nakazono K, Usui Y, Tokida T, Hasegawa T, Kuwagata T, Morita S, Nagta K. Factors influencing the appearance quality in rice under high temperature in 2010. Jpn J Crop Sci. 2012;7(Extra issue 1):120–121. [Google Scholar]
- Kondo M, Morita S, Nagata K, Koyama Y, Ueno N, Hosoi J, Ishida Y, Yamakawa T, Nakayama Y, Yoshida Y, Ohashi Y, Iwai M, Odaira Y, Nakatsu S, Katsuba Z, Hajima M, Mori Y, Kimura H, Sakata M. Effects of Air temperature during ripening and grain protein contents on grain chalkiness in rice. Jpn J Crop Sci. 2006;7(extra issue 2):14–15. [Google Scholar]
- Lanning SB, Siebenmorgen TJ, Counce PA, Ambardekar AA, Mauromoustakos. Extreme nighttime air temperatures 2010 impact rice chalkiness and milling. Field Crops Res. 2011;7:132–136. doi: 10.1016/j.fcr.2011.06.012. doi.org/10.1016/j.fcr.2011.06.012. [DOI] [Google Scholar]
- Lieffering M, Kim HY, Kobayashi K, Okada M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Res. 2004;7:279–286. doi: 10.1016/j.fcr.2004.01.004. doi.org/10.1016/j.fcr.2004.01.004. [DOI] [Google Scholar]
- Lur HS, Wu YC, Chang SJ, Lao CL, Hsu CL, Kondo M. In: Proceeding of the MARCO Symposium. Hasegawa T, Sakai H, editor. National Institute for Agro-Environmental Sciences, Tsukuba, Japan; 2009. Effects of high temperature on yield and grain quality of rice in Taiwan; pp. 38–43. 5–9 October 2009. [Google Scholar]
- Lyman NB, Jagadish KSV, Nalley LL, Dixon BL, Siebenmorgen T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS One. 2013;7:e72157. doi: 10.1371/journal.pone.0072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kobayasi K, Yoshimoto M, Hasegawa T. Stability of rice pollination in the field under hot and dry conditions in the Riverina region of New South Wales, Australia. Plant Prod Sci. 2007;7:57–63. doi: 10.1626/pps.10.57. doi.org/10.1626/pps.10.57. [DOI] [Google Scholar]
- Standard tables of food composition in Japan. ᅟ. 2005;7:ᅟ. http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/toushin/05031802.htm (Fifth revised and enlarged edition- 2005 -, http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/toushin/05031802.htm. Accessed 27 Jan 2014. [Google Scholar]
- Morita S. Prospect for developing measures to prevent high-temperature damage to rice grain ripening. Jpn J Crop Sci. 2008;7:1–12. doi: 10.1626/jcs.77.1. [DOI] [Google Scholar]
- Morita S, Nakano H. Nonstructural carbohydrate content in the stem at full heading contributes to high performance of ripening in heat-tolerant rice cultivar Nikomaru. Crop Sci. 2011;7:818–828. doi: 10.2135/cropsci2010.06.0373. [DOI] [Google Scholar]
- Nagato K, Ebata M. Effects of high temperature during ripening period on the development and the quality of rice kernels. Jpn J Crop Sci. 1965;7:59–66. doi: 10.1626/jcs.34.59. [DOI] [Google Scholar]
- Nakamura H, Tokida T, Yoshimoto M, Sakai H, Fukuoka M, Hasegawa T. Performance of the enlarged rice-FACE system using pure CO2 installed in Tsukuba, Japan. J Agric Meteorol. 2012;7:15–23. doi: 10.2480/agrmet.68.1.2. doi.org/10.2480/agrmet.68.1.2. [DOI] [Google Scholar]
- Nishimura M, Kaji R, Ogawa T. Varietal difference in the occurrence of coarse grain due to the high temperature stress given during the ripening period of rice plant. Breeding Res. 2000;7:17–22. doi: 10.1270/jsbbr.2.17. [DOI] [Google Scholar]
- ᅟ. ᅟ, ᅟ; 2013. [Google Scholar]
- Saka N, Terashima T, Kudo S, Kato T, Sugiura K, Endou I, Shirota M, Inoue M, Otake T. A new high field resistant variety “Mineharuka” for rice blast. Res Bull Aichi Agric Res Ctr. 2007;7:95–109. [Google Scholar]
- Sakai M, Okamoto M, Tamura K, Kaji R, Mizobuchi R, Hirabayashi H, Fukaura S, Nishimura M, Yagi T. ‘Nikomaru’, a new rice variety with excellent palatability and grain appearance developed for warm region of Japan. Breeding Res. 2007;7:67–73. [Google Scholar]
- Sakai M, Okamoto M, Tamura K, Kaji R, Mizobuchi R, Hirabayashi H, Yagi T, Nishimura M, Fukaura S. ‘Nikomaru’, A high-yielding rice variety with superior eating quality and grain appearance under high temperature during ripening. Bull NARO Kyushu Okinawa Agric Res Cent. 2010;7:43–61. [Google Scholar]
- Seneweera S. Effects of elevated CO2 on plant growth and nutrient partitioning of rice (Oryza sativa L.) at rapid tillering and physiological maturity. J Plant Interact. 2011;7:35–42. doi: 10.1080/17429145.2010.513483. [DOI] [Google Scholar]
- Takai T, Yano M, Yamamoto T. Canopy temperature on clear and cloudy days can be used to estimate varietal differences in stomatal conductance in rice. Field Crops Res. 2010;7:165–170. doi: 10.1016/j.fcr.2009.10.019. [DOI] [Google Scholar]
- Tanoi M, Tomita K, Kobayashi A, Hayashi T. Heat tolerance and grain quality of a new rice cultivar ‘Akisakari’. Hokuriku Crop Sci. 2010;7:3–6. [Google Scholar]
- Taub DR, Miller B, Allen H. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol. 2008;7:565–575. doi: 10.1111/j.1365-2486.2007.01511.x. [DOI] [Google Scholar]
- Terao T, Miura S, Yanagihara T, Hirose T, Nagata K, Tabuchi H, Kim H-Y, Lieffering M, Okada M, Kobayashi K. Influence of free-air CO2 enrichment (FACE) on the eating quality of rice. J Sci Food Agric. 2005;7:1861–1868. doi: 10.1002/jsfa.2165. [DOI] [Google Scholar]
- Terashima K, Saito Y, Sakai N, Watanabe T, Ogata T, Akita S. Effects of high air temperature in summer of 1999 on ripening and grain quality of rice. Jpn J Crop Sci. 2001;7:449–458. doi: 10.1626/jcs.70.449. [DOI] [Google Scholar]
- Wada T, Tsubone M, Inoue T, Ogata T, Hamachi Y, Matsue Y, Oosato FK, Yasunaga T, Kawamura Y, Ishizuka A. ‘Genkitsukushi’, a new cultivar with tolerance to high temperature during maturing period and high eating quality. Bull Fukuoka Agric Res Centr. 2010;7:1–9. [Google Scholar]
- Wakamatsu K, Sasaki O, Uezono I, Tanaka A. Effects of high air temperature during the ripening period on the grain quality of rice in warm region of Japan. Jpn J Crop Sci. 2007;7:71–78. doi: 10.1626/jcs.76.71. [DOI] [Google Scholar]
- Ward RM. Potential impact of temperature and carbon dioxide levels on rice quality. The University of Sydney, Dissertation; 2007. [Google Scholar]
- Yang LX, Wang YL, Dong GC, Gu H, Huang JY, Zhu JG, Yang HJ, Liu G, Han Y. The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crops Res. 2007;7:128–140. doi: 10.1016/j.fcr.2007.03.006. doi.org/10.1016/j.fcr.2007.03.006. [DOI] [Google Scholar]
- Yoshimoto M, Oue H, Takahashi N, Kobayashi K. The effects of FACE (free-air CO2 enrichment) on temperatures and transpiration of rice panicles at flowering stage. J Agric Meteorol. 2005;7:597–600. [Google Scholar]
- Zhang G, Sakai H, Tokida T, Usui Y, Zhu C, Nakamura H, Yoshimoto M, Fukuoka M, Kobayashi K, Hasegawa T. The effects of free-air CO2 enrichment (FACE) on carbon and nitrogen accumulation in grains of rice (Oryza sativa L.) J Exp Bot. 2013;7:3179–3188. doi: 10.1093/jxb/ert154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Fitzgerald M. Climate change: implication for the yield of edible rice. PLoS One. 2013;7:e66218. doi: 10.1371/journal.pone.0066218. [DOI] [PMC free article] [PubMed] [Google Scholar]


