Abstract
Cardiorespiratory coupling is an encompassing term describing more than the well-recognized influences of respiration on heart rate and blood pressure. Our data indicate that cardiorespiratory coupling reflects a reciprocal interaction between autonomic and respiratory control systems, and the cardiovascular system modulates the ventilatory pattern as well. For example, cardioventilatory coupling refers to the influence of heart beats and arterial pulse pressure on respiration and is the tendency for the next inspiration to start at a preferred latency after the last heart beat in expiration. Multiple complementary, well-described mechanisms mediate respiration’s influence on cardiovascular function, whereas mechanisms mediating the cardiovascular system’s influence on respiration may only be through the baroreceptors but are just being identified. Our review will describe a differential effect of conditioning rats with either chronic intermittent or sustained hypoxia on sympathetic nerve activity but also on ventilatory pattern variability. Both intermittent and sustained hypoxia increase sympathetic nerve activity after 2 weeks but affect sympatho-respiratory coupling differentially. Intermittent hypoxia enhances sympatho-respiratory coupling, which is associated with low variability in the ventilatory pattern. In contrast, after constant hypobaric hypoxia, 1-to-1 coupling between bursts of sympathetic and phrenic nerve activity is replaced by 2-to-3 coupling. This change in coupling pattern is associated with increased variability of the ventilatory pattern. After baro-denervating hypobaric hypoxic-conditioned rats, splanchnic sympathetic nerve activity becomes tonic (distinct bursts are absent) with decreases during phrenic nerve bursts and ventilatory pattern becomes regular. Thus, conditioning rats to either intermittent or sustained hypoxia accentuates the reciprocal nature of cardiorespiratory coupling. Finally, identifying a compelling physiologic purpose for cardiorespiratory coupling is the biggest barrier for recognizing its significance. Cardiorespiratory coupling has only a small effect on the efficiency of gas exchange; rather, we propose that cardiorespiratory control system may act as weakly coupled oscillator to maintain rhythms within a bounded variability.
Keywords: neural control of heart rate, neural control of sympathetic nerve activity, neural control of respiration, weakly coupled oscillators
1 INTRODUCTION
1.1 History and Definition of Types of “Cardiorespiratory Coupling”
Cardiorespiratory coupling (CRC) encompasses various phenomena which result from shared inputs, common rhythms, and complementary functions. In particular, autonomic and respiratory rhythms are expressed in the other’s neural activity, including both pattern generators and motor activity (Dick et al., 2005). This has led us to conceive of reciprocal interaction between the respiratory and autonomic control systems in the function of gas exchange (Fig. 1). In other words, in addition to the well-recognized respiratory influence on autonomic activity, the autonomic system has an influence on respiratory pattern formation. The respiratory influence on autonomic activity is breath to breath, whereas the autonomic influence on respiration can be considered beat to beat (Larsen et al., 2010; Zhu et al., 2013). The effect of a slower rhythm superimposed on a faster one is recognized easily; no doubt this contributed to the early recognition and general acceptance of the influence of respiration on heart rate (HR) and blood pressure (BP). In contrast, the effect of blood pressure on respiration, referred to as cardioventilatory coupling (CVC), is just being recognized. A fundamental deterrent to accepting CRC as being physiologically significant is that it appears to have only a weak role in determining the efficiency of gas exchange. In light of this, we theorize the reciprocal interaction of CRC relates to its physiologic function in pattern formation determining variability of cycles within limits as described for weakly coupled oscillators (Ermentrout and Ko, 2009; Ermentrout and Saunders, 2006; Ermentrout et al., 2008; Galan et al., 2005, 2010).
FIGURE 1.

Schematic of cardiorespiratory coupling. The bidirectional arrows between each limit cycle (blue (left), respiratory and red (right), cardiac) represent the reciprocal coupling scheme between respiration and autonomic, cardiac rhythms, which are depicted as harmonics (here the cardiac: respiratory rhythm is 4:1 entrainment). Multiple mechanisms mediate the respiratory influence on the cardiac cycle, whereas a single mechanism mediates the influence of the cardiac/sympathetic activity onthe respiratory cycle. The proposed mechanism is through baror eceptors and their beat-to-beat increase in activity.
Heartbeat, blood pressure, and ventilation share common frequencies. Billman (2011) wrote a concise history on the observation and quantification of HR and BP and the influence of respiration on these two variables (also see Larsen et al., 2010). Briefly, in 1733 Rev. Stephen Hales reported that respiration modulates HR and BP. This observation was confirmed by Carl Ludwig (1847) who measured the increases in HR and BP during inspiration. The increase in HR during inspiration is referred to as respiratory sinus arrhythmia (RSA) and the increase in BP as Traube–Hering waves. Heart rate and BP are modulated neurally, and both parasympathetic and sympathetic nerves have respiratory-modulated activity patterns. Multiple factors, including mechanical coupling, underlie the increases in HR and BP. Mechanical coupling in the cardiorespiratory system is due to the location of the lungs and heart in the thoracic cavity. Inspiration relies on a decrease in thoracic pleural pressure which draws blood to the heart and increases venous return, which increases HR and cardiac output. However, HR and BP can increase during inspiration in a perfused in situ preparation in which the thorax is wide open and the lungs are removed (Baekey et al., 2008, 2010; Dick et al., 2009; Julien et al., 2009). Consequently, this review focuses on the neural mechanism (Fig.1).
CVC, a distinct property of CRC was described in the twentieth century by Walter Coleman. He observed animals at the Zoological Gardens in Regent’s Park and reported that the ratio of heart beats to breaths was a whole number (4 and 5:1) in numerous species (Coleman, 1920). More convincingly, a statistical evaluation of the distribution of the time intervals between respiratory phase transitions and the previous or next heart beat identified that the onset of inspiration occurs at a preferred latency after the previous peak in systolic BP (Friedman et al., 2012; Galletly and Larsen, 1999b; Larsen and Galletly, 1999). This interval has the strongest statistical “coupling” compared to the interval between I-onset and the next heart beat and the intervals associated with the inspiratory-to-expiratory phase transition (Friedman et al., 2012). However, CVC is weak and becomes apparent during quiet sleep and anesthesia (Galletly and Larsen, 1997a,b; Larsen and Galletly, 1999). The proposed mechanism for CVC is that systolic peak BP occurring late in expiration initiates inspiration at a preferred latency and is referred to as the baroreceptor-trigger hypothesis (Galletly and Larsen, 1999a). However, our data indicate that baroreceptor input activates expiratory neurons (Dick and Morris, 2004; Dick et al., 2005), including postinspiratory neurons(Baekey et al., 2010). This would act to delay rather than trigger inspiration. Accordingly, the onset of inspiration would occur at a preferred latency because the magnitude of the delay of inspiratory onset depends on the magnitude and timing of arterial pressure pulse, assuming that equivalent arterial pulse pressures occur at two slightly different latencies in late expiration. The later beat would activate less postinspiratory activity and delay the onset of inspiration less than the earlier one, which could recruit more postinspiratory activity because the neurons producing this activity are less hyperpolarized. Thus, the interval between the systolic arterial pressure peak and the inspiratory onset would be more similar. Either way (triggering or delaying), CVC depends on baroreceptors and carotid sinus sensory activity rather than an interaction between brainstem neural networks that generate cardiac, blood pressure, and respiratory patterns.
The reciprocal nature of autonomic and respiratory rhythms reflects a middle ground in the dichotomy regarding the neural control of homeostasis (Feldman and Ellenberger, 1988). At one end of the dichotomy is the opinion that the cardiovascular and respiratory control systems are two separate but parallel entities with distinct effectors, that is, cardiac and smooth muscles under parasympathetic and sympathetic control and striated musculature under respiratory, somatic motor control. At the other end, the cardiovascular and respiratory systems are controlled by a single neural system controlling gas exchange. While reciprocal nature of the coexpression of arterial pulse and respiratory rhythms indicates, at least, a middle ground of coupled control, the magnitude of coupling depends on various factors, which will be explored in this review.
1.2 Heart Rate Variability
Heart rate variability (HRV) can be assessed by many tools that examine distributions in the temporal and frequency domains. A common tool to assess HRV is the power spectral density of a continuous data stream of heart beats. Due to the RSA, the power spectral density has a peak at the respiratory frequency, which is referred to as the “high-frequency component of the power spectral density.” The other components are described in detail in the 1996 white paper published by the joint international committee on HRV (1996). One consensus is that autonomic tone, the balance of sympathetic and parasympathetic activities, can be characterized by an analysis of the HRV (Vinik, 2012; Vinik et al., 2011).
While it is beyond the scope of this review to discuss the applicability of the HRV as a biomarker, the high-frequency component of the HRV power spectral density depends on RSA. Even though decreases in HRV have been recognized as a forecasting pathogenesis and morbidity for 50 years (Hon and Lee, 1963a,b), for example, in predicting a subsequent myocardial infarctions (Buccelletti et al., 2009), we are just beginning to understand the relationship between cardiorespiratory dynamics and disease states that effect brainstem neural networks and control. In this regard, we propose the reciprocal component of cardiorespiratory coupling, CVC, as a bio-marker that complements RSA.
1.3 Physiologic Relevance of Cardiorespiratory Coupling
The physiologic purpose of cardiorespiratory coupling remains obscure. Teleological reasoning leads to the hypothesis that CRC increases the efficiency of gas exchange by matching pulmonary perfusion to ventilation during inspiration (Hayano et al., 1996). Significant increases in the efficiency of gas exchange were found in a creative experiment in which canine HR was controlled by peripheral vagal nerve stimulation (Hayano et al., 1996). The efficiency of gas exchange was measured during three conditions: (1) replicating RSA, vagal stimulation during expiration, thus causing a relative increase in HR during inspiration; (2) reversing RSA, HR increasing during expiration; or (3) no RSA, HR distributed equally throughout the respiratory cycle. Number of heart beats per respiratory cycle was the same in each condition and the blood gases were maintained. Compared to distributing HR evenly, replicating RSA decreased physiological dead space by 10% and the fraction of intrapulmonary shunt by 51%, whereas reversing RSA increased dead space by 14% and intrapulmonary shunt by 64% (Hayano et al., 1996). However, the physiologic effectiveness appears to be weak (Ben-Tal et al., 2012). Recent optimization modeling studies reported that these effects amounted to just a 3% improvement in gas exchange efficiency (Ben-Tal et al., 2012). Is this 3% improvement relevant; after all, cardiorespiratory uncoupling is not one of the four causes of hypoxemia? But it is well known that mammalian species are intrinsically energy efficient and normally work of breathing is highly efficient for many body habitus reasons (Goldman and Mead, 1973; Goldman et al., 1978, 1976), consequently to measurean effect of patterning maybe highly significant. A 3% energy conservation from breath to breath may impact highly trained athletes whose CRC is enhanced and severely ill individuals who lose CRC and die of respiratory failure.
Ben-Tal et al. (2012) proposed that RSA acts to minimize cardiac rather than respiratory work. Even though this theory shifts the focus from the work of breathing to that of beating, it still supports the general concept that RSA acts to make gas exchange efficient. However, even in this broader context, Larsen and coworkers have performed series of studies in humans and have not found support for either RSA or CVC enhancing the efficiency of a gas exchange. For example, in one study (Sin et al., 2010), the efficiency of gas exchange was compared between one group of humans with pacemakers which had a stable HR that was independent of the breathing pattern and another group of normal humans. Values of oxygen consumption and carbon dioxide production were obtained at two respiratory frequencies, normal respiratory rate (15 brths/min) and slow, deep breathing (6 brths/min) to accentuate the RSA. In the normal subjects, HR varied by 10% within the respiratory cycle during slow breathing. Even with this magnitude of RSA, gas exchange efficiency was similar in both groups. While the authors concluded that RSA had no effect, the magnitude of the Traube–Hering waves was similar in both groups; whether this was able to compensate for the absence of RSA and optimize the efficiency of gas exchange in the paced group is unknown.
In summary, while it is unlikely that the CRC is a driving force determining the efficiency of gas exchange, it may be one of the few malleable forces. Further, CRC is reduced in illness increasing the work of breathing.
We propose that a purpose of reciprocally coupled interactions between respiration and cardiovascular systems is identified by the theory of coupled oscillators. Mutual coupling increases the variability of the oscillators’ frequencies allowing them to respond and adapt to external perturbations as well as to develop complex patterns of activity (Kuramoto, 1984; Winfree, 2001). These features are essential for the cardiorespiratory system and are generally properties of neural networks (Hoppensteadt and Izhikevich, 1997). For instance, the theory of coupled oscillators predicts that weakly coupled neurons phase-lock their activity in response to sensory stimuli in a stimulus-dependent manner (Galán, 2009 and subject of the next section). Further, in this context and focusing on CRC as a neurally controlled physiologic property, even though the cardiac influence on respiration is weak, it may provide a source for ventilatory pattern variability. Thus, uncoupling will affect the variability of the ventilatory pattern.
2 HYPOXIC CONDITIONING, ENHANCING, AND DIMINISHING CRC
While simultaneously recording sympathetic and respiratory motor activities from adult male Sprague Dawley rats, we noted that respiratory modulation of splanchnic sympathetic nerve activity (sSNA) increases during and after brief (45 s) exposures to hypoxia (8% O2 in the inhaled gas) (Dick et al., 2004). The persistence of enhanced CRC after the stimulus was our first indication of plasticity; in this case, a form of activity dependent, short-term plasticity existed in the sympathetic control system. Our second indication, a form of long-term plasticity, was evoked by acute intermittent hypoxia which was repetitive (n = 10) hypoxic exposures (45 s of 8% O2 separated by 5 min of 100% O2) (Dick et al., 2007). Acute intermittent hypoxia evoked a progressive increase in sSNA (and phrenic nerve activity (PNA)) that persisted for at least 60 min following the last hypoxic exposure. The recruitment of activity after acute intermittent hypoxia and in the presence of maintained normoxic and eucapnic blood gas levels is referred to as long-term facilitation. Methysergide, a serotonergic (5HT2) receptor antagonist, blocked the development of sSNA (and PNA) long-term facilitation (Dick et al., 2007). Surprisingly, long-term facilitation of sSNA response was more robust and consistent than that of PNA, in particular it was apparent in recordings of sSNA when it was not evident in PNA (Dick et al., 2007; Xing and Pilowsky, 2010; Xing et al., 2013). The evoked sSNA was modulated by the respiratory cycle with the greatest increase in SNA occurred in the postinspiratory phase. In summary, long-term facilitation is present in sSNA, shares properties similar to those of PNA, and results predominantly in recruitment of SNA in post-inspiration (Dick et al., 2007).
We theorized that CRC could provide the neural substrate for the enhanced SNA associated with chronic intermittent hypoxia based on the enhanced sympatho-respiratory coupling that was associated with acute intermittent hypoxia. Conditioning rats with chronic intermittent hypoxia is a model developed by Fletcher (2001) to determine if repetitive hypoxia could evoke hypertension, a common and severe comorbidity associated with sleep apnea. During the conditioning process, rats were housed in their home cages which were placed in a chamber for 8 h each day for 10 days (the other 16 h, the rats were returned to the animal facility). The chamber was flushed for 45 s with 100% N2 lowering the ambient O2 to 5% for 5 s and then flushed with room air for 5 min. An identical neighboring chamber would house control rats and the chamber was flushed with room air rather than nitrogen.
We also conditioned rats to chronic-sustained hypoxia in a hypobaric chamber. The chamber was held a 0.5 atm. for 14 days, which lowered the partial pressure of inspired O2 to values comparable to that of 8% O2 at 1.0 atm. The rationale is that sustained hypoxia unlike intermittent hypoxia does evoke SNA but not hypertension. Thus, we hypothesized that different CRC patterns would emerge after conditioning in chronic intermittent or sustained hypoxia.
We recorded BP, sSNA, and PNA in vagotomized, anesthetized adult, male rats. The conditioning and the experimental protocols from which we obtained the presented data were approved by Institutional Animal Care and Use committee at Case Western Reserve University.
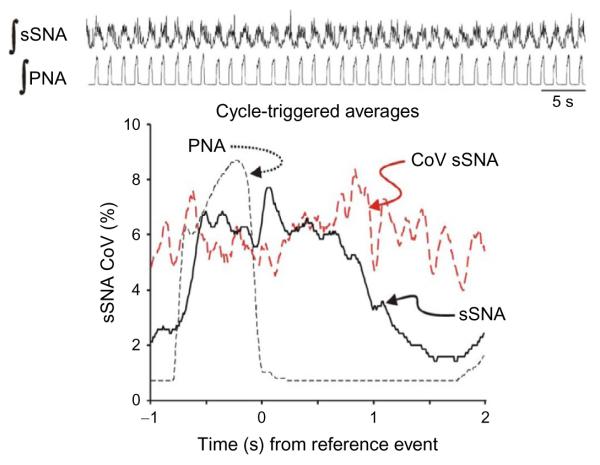
After chronic intermittent hypoxia conditioning, sSNA had well-defined bursts of activity entrained with PNA bursts (Fig. 2). The low coefficient of variation in the cycle-triggered average of sSNA indicated that sSNA had consistent respiratory modulation with every inspiratory burst of PNA. The cycle-triggered average also showed that the peak of sSNA occurred with cessation of PNA in the postinspiratory period. While the postinspiratory peak is consistent with the entrainment pattern of naïve Sprague Dawley rats, the consistency of sSNA from breath to breath and the robust recruitment of sSNA during inspiration were distinct attributes of chronic intermittent hypoxic-conditioned rats (Dick et al., 2004). In contrast to Fig. 2, sSNA was recruited in late expiration after chronic intermittent hypoxic conditioning in Wister rats (Abdala et al., 2009; Zoccal et al., 2008, 2009a,b). The late-E burst of sSNA was coincident with recruited abdominal motor activity. These coincident changes in both the cardiovascular and respiratory motor patterns lead to the development of an appealing model, describing a possible pathophysiologic process of neurogenic hypertension. In this model, chronic intermittent hypoxia sensitizes chemo-sensitivity of the carotid body (Peng et al., 2010, 2003, 2004; Prabhakar and Kumar, 2010; Prabhakar et al., 2007, 2010, 2005). Second-order neurons receive carotid body afferent input project to and excite CO2-chemosensitive neurons in the parafacial neurons in the ventral brainstem (Fortuna et al., 2008; Guyenet and Mulkey, 2010; Guyenet et al., 2009, 2005). The parafacial neurons upregulate expiratory activity in the respiratory central pattern generator which excites barosensitive bulbospinal presympathetic neurons in the rostral ventral lateral medulla (Molkov et al., 2010, 2011; Moraes, 2012). Our data are consistent with this model in the sense that the excitatory drive to sympathetic activity is modulated by the respiratory pattern generator. Further, both data sets emphasize that postinspiratory activity is not upregulated, which is significant because postinspiratory activity is the prominent sympathy–respiratory coupling pattern of naïve, unconditioned rats and is the phase in which sSNA is primarily recruited after acute intermittent hypoxia.
FIGURE 2.
Sympatho-respiratory coupling is high following chronic intermittent hypoxia. The bursts of integrated splanchnic sympathetic nerve activity (∫ sSNA) and integrated phrenic nerve activity (∫ PNA) are highly correlated; this is reflected in the cycle-triggered average of ∫ sSNA in which the nadir is close to 0 and the coefficient of variation (CoV) is low across the respiratory cycle. Traces for this figure and Fig. 3: top, ∫ sSNA and ∫ PNA. Graph: Cycle-triggered averages of ∫ sSNA (black continuous line), ∫ PNA (black dashed line), CoV of ∫ sSNA (red dashed line). Note: The y-axis is scaled for the CoV of ∫ sSNA. Even though the scale of the averages of ∫ sSNA and ∫ PNA is arbitrary because it depends on the amplification of each nerve recording, it does range from zero to a normalized maximum, so the signal-to-ratio is depicted by the averages for that component of the signal that is correlated temporally to the reference event, the offset of inspiration.
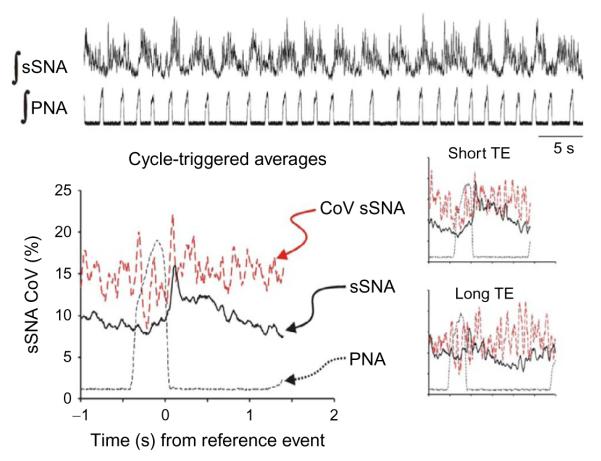
Even though chronic-sustained hypoxia conditioning also upregulated sSNA activity, the bursts of sSNA were not entrained 1:1 with bursts of PNA (Fig. 3). In contrast to chronic intermittent hypoxia, the entrainment pattern of sSNA to PNA was 2:3, although this was not consistent. However, the cycle-triggered average still displayed a peak of sSNA in the postinspiratory phase. But the cycle-triggered average which is used to potentiate the signal-to-noise ratio shows an increase in activity that is not time-locked to inspiratory-to-expiratory phase transition. This is not “tonic” activity as the recording has a clearly defined nadir after the sSNA burst.
FIGURE 3.
Sympatho-respiratory coupling is low following chronic-sustained hypoxia. The bursts of ∫ sSNA do not appear time-locked to ∫ PNA. Instead of a 1:1 coupling pattern, 2 bursts of ∫ sSNA are coupled to three bursts of ∫ PNA. Nevertheless, the cycle-triggered average of ∫ sSNA has a “postinspiratory” burst of activity or a peak of activity associated with the inspiratory–expiratory phase transition; the nadir of ∫ sSNA is not close to 0 with the lowest values of ∫ sSNA occurring after the start of inspiration; and the coefficient of variation (CoV) is two-to-three times that of the chronic intermittent hypoxic-conditioned rat across the respiratory cycle. Insets: Cycle-triggered averages; even selecting the short and long respiratory cycles (duration of expiration, TE) does not improve the signal-to-noise ratio of the ∫ sSNA. Traces as in Fig. 2.
The insets in Fig. 3 are cycle-triggered averages grouping the shortest (top) and longest (bottom) respiratory cycles to determine if differences in the CRCs were apparent. However, in both short and long respiratory cycles, the coupling pattern appears the same with the possible exception that the decrease in sSNA associated with early inspiration appears more prominent with a distinctly lower coefficient of variation in the shortest breaths. But the major finding is the change in coupling pattern between chronic intermittent and sustained hypoxia with the pattern-associated slower bursting pattern of chronic-sustained hypoxia not associated with an increase in arterial BP.
While sSNA differs in CRC patterns after chronic intermittent versus sustained hypoxia conditioning, two caveats should be considered. First, chronic intermittent hypoxia elicits changes through sensory, control, and effector components of the BP system. Multiple factors in the cardiorespiratory system contribute to cardiovascular morbidity, including (1) upregulated carotid body and downregulated baroreceptor sensitivity, (2) altered cytochemistry of brainstem nuclei involved in cardiorespiratory control, (3) increased vascular reactivity and resistance. Although all these factors have been identified, we have focused on just one, patterning of sympathetic activity. Second, although direct translation of these findings to control blood pressure in humans may not be readily possible, the upregulation of chemo- and down-regulation of baroreceptors have been identified already. Indeed, the issue here may be specific to patterning. Slow, deep breathing attempts to utilize changes in respiratory patterning to affect a decrease in sympathetic activity in hypertensive patients. However, recently (Limberg et al., 2013), muscle SNA (mSNA) was recorded and comparison during slow (7 brths/min), normal (14 brths/min), and fast (21 brths/min) rates to determine whether paced breathing had beneficial effects of reducing sympathetic tone. Even though paced breathing was effective at redistributing the pattern of bursts of mSNA with more bursts occurring during inspiration in slow breathing, variables with vascular resistance and flow did not change. Thus, alterations in breathing frequency and tidal volume did not affect blood pressure in these volunteers, which lead to the conclusion that the mean level of sympathetic activity rather than the patterning was critical for hypertension. While this is only a single study in which only a portion (40%) had increased systolic pressure, it draws attention to a major deficit in our knowledge that is essential for understanding the role of patterning of sSNA in neurogenic hypertension. Specifically, the relationship among (1) the patterning of SNA, (2) evoked neurotransmitter release, and (3) smooth muscle reactivity in vessels may be acting differently in normo- and hypertensive subjects.
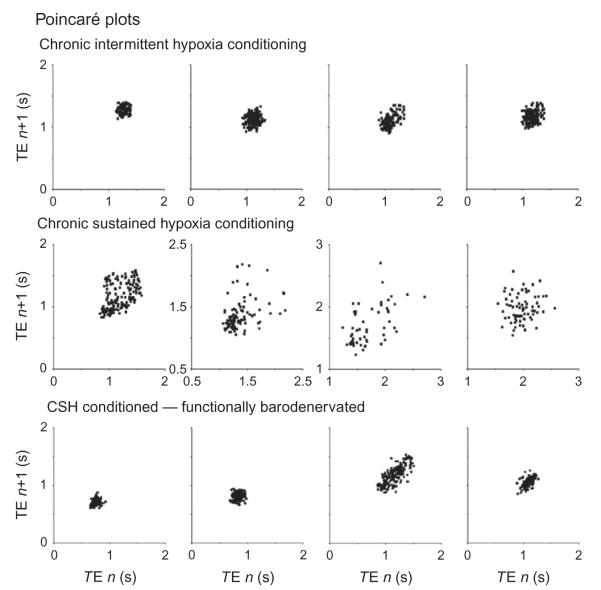
The unexpected finding was the impact that chronic intermittent and sustained hypoxic conditioning had on the breathing pattern (Fig. 4). After chronic-sustained hypoxia, ventilatory pattern variability was greater than that after chronic intermittent hypoxia. This difference in patterning depended on the presence of baro- and chemo-sensory input. Ventilatory pattern variability was reduced after transection of the carotid sinus nerve (the rats were vagotomized). The effectiveness of the barodenervation was tested by administration of phenylephrine which increased blood pressure but did not affect SNA nor elicit an increase in TE (not shown). In this regard, the aortic depressor nerve remained intact, but direct electrical stimulation of this nerve in various pattern had a minor effect on ventilatory pattern variability (McMullan et al., 2009). Similarly, chemoreceptor drive was minimized by maintaining blood gases eucapnic and hyperoxic.
FIGURE 4.
Poincaré plots. The duration of expiration (TE) of the next cycle is plotted against the TE of the current cycle to display cycle-to-cycle variability. In four chronic intermittent hypoxic-conditioned rats, the variability of TE is small and forms a tight cluster points. Compare this to four chronic-sustained hypoxic-conditioned rats in which the points are distributed widely. However, the distribution forms a tight cluster after both the aortic depressor and carotid sinus nerve are transected.
We interpret these data to indicate that the influence of arterial BP on respiration is modifiable. In particular, enhanced sympatho-respiratory coupling in chronic intermittent hypoxia reflexes reduction of stochastic variability in ventilation, whereas the slow rhythm of SNA in chronic-sustained hypoxia may act as a source of variability.
3 CONCLUSION
Cardiorespiratory coupling is reciprocal. Both effects, that of respiration on the cardiovascular activity as well as that of the arterial pressure pulse on respiratory activity, can be identified and quantified. However, the role of cardiorespiratory coupling in homeostasis and pathophysiology remains obscure. The plasticity of cardiorespiratory coupling evoked by hypoxia is an intriguing aspect of the cardiorespiratory control system; it draws our attention to limited knowledge about translating increased sympathetic nerve activity into vascular dynamics and hypertension. Further, the influence of hypoxic conditioning on breath-to-breath variability not only supports the reciprocal nature of cardiorespiratory coupling but also draws attention to the concept that variability in these patterns of activity may subserve more than gas exchange.
Acknowledgments
We gratefully acknowledge that this work was supported by NIH HL-080318, NS-069220, and HL-007913 (R. R. D.).
Abbreviations
- BP
blood pressure, specifically, arterial blood pressure in this review
- CRC
cardiorespiratory coupling
- CVC
cardioventilatory coupling, a property of cardiorespiratory coupling referring to the influence of arterial blood pressure pulse on respiration
- HR
heart rate HRV heart rate variability
- PNA
phrenic nerve activity
- RSA
respiratory sinus arrhythmia, a property of cardiorespiratory coupling referring to the influence of respiration on heart rate
- sSNA
splanchnic sympathetic nerve activity
References
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart. J. 1996;17:354–381. [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J. Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp. Physiol. 2008;93:803–816. doi: 10.1113/expphysiol.2007.041400. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir. Physiol. Neurobiol. 2010;174:135–145. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tal A, Shamailov SS, Paton JF. Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency. J. Physiol. 2012;590:1989–2008. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE. Heart rate variability—a historical perspective. Front. Physiol. 2011;2:86. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, Silveri NG. Heart rate variability and myocardial infarction: systematic literature review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2009;13:299–307. [PubMed] [Google Scholar]
- Coleman WM. On the correlation of the rate of heart beat, breathing, bodily movement and sensory stimuli. J. Physiol. 1920;54:213–217. doi: 10.1113/jphysiol.1920.sp001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J. Physiol. 2004;556:959–970. doi: 10.1113/jphysiol.2003.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh Y-H, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R1121–R1128. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J. Appl. Physiol. 2005;99:691–698. doi: 10.1152/japplphysiol.01124.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh Y-H, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp. Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir. Physiol. Neurobiol. 2009;168:76–85. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Ermentrout B, Saunders D. Phase resetting and coupling of noisy neural oscillators. J. Comput. Neurosci. 2006;20:179–190. doi: 10.1007/s10827-005-5427-0. [DOI] [PubMed] [Google Scholar]
- Ermentrout B, KO TW. Delays and weakly coupled neuronal oscillators. Philos. Transact. A Math. Phys. Eng. Sci. 2009;367:1097–1115. doi: 10.1098/rsta.2008.0259. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Galan RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci. 2008;31:428–434. doi: 10.1016/j.tins.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Ellenberger HH. Central coordination of respiratory and cardiovascular control in mammals. Annu. Rev. Physiol. 1988;50:593–606. doi: 10.1146/annurev.ph.50.030188.003113. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J. Appl. Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J. Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Dick TE, Jacono FJ, Loparo KA, Yeganeh A, Fishman M, Wilson CG, Strohl KP. Cardio-ventilatory coupling in young healthy resting subjects. J. Appl. Physiol. 2012;112:1248–1257. doi: 10.1152/japplphysiol.01424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán RF. The phase oscillator approximation in neuroscience: an analytical framework to study coherent activity in neural networks. In: Perez Velazquez JL, Wennberg R, editors. Coordinated Activity in the Brain: Measurements and Relevance to Brain Function and Behavior. Springer; NY: 2009. pp. 65–90. [Google Scholar]
- Galan RF, Ermentrout GB, Urban NN. Efficient estimation of phase-resetting curves in real neurons and its significance for neural-network modeling. Phys. Rev. Lett. 2005;94:158101. doi: 10.1103/PhysRevLett.94.158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan RF, Dick TE, Baekey DM. Analysis and modeling of ensemble recordings from respiratory pre-motor neurons indicate changes in functional network architecture after acute hypoxia. Front. Comput. Neurosci. 2010;4 doi: 10.3389/fncom.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly DC, Larsen PD. Cardioventilatory coupling during anaesthesia. Br. J. Anaesth. 1997a;79:35–40. doi: 10.1093/bja/79.1.35. [DOI] [PubMed] [Google Scholar]
- Galletly DC, Larsen PD. Coupling of spontaneous ventilation to heart beat during benzodiazepine sedation. Br. J. Anaesth. 1997b;78:100–101. doi: 10.1093/bja/78.1.100. [DOI] [PubMed] [Google Scholar]
- Galletly D, Larsen P. Ventilatory frequency variability in spontaneously breathing anaesthetized subjects. Br. J. Anaesth. 1999a;83:552–563. doi: 10.1093/bja/83.4.552. [DOI] [PubMed] [Google Scholar]
- Galletly DC, Larsen PD. The determination of cardioventilatory coupling from heart rate and ventilatory time series. Res. Exp. Med. (Berl.) 1999b;199:95–99. doi: 10.1007/s004330050136. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Mead J. Mechanical interaction between the diaphragm and rib cage. J. Appl. Physiol. 1973;35:197–204. doi: 10.1152/jappl.1973.35.2.197. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Grimby G, Mead J. Mechanical work of breathing derived from rib cage and abdominal V-P partitioning. J. Appl. Physiol. 1976;41:752–763. doi: 10.1152/jappl.1976.41.5.752. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Grassino A, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: dynamics. J. Appl. Physiol. 1978;44:840–848. doi: 10.1152/jappl.1978.44.6.840. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir. Physiol. Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, Depuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir. Physiol. Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842–847. doi: 10.1161/01.cir.94.4.842. [DOI] [PubMed] [Google Scholar]
- Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. Viii. Patterns preceding fetal death, further observations. Am. J. Obstet. Gynecol. 1963a;87:814–826. [PubMed] [Google Scholar]
- Hon EH, Lee ST. The fetal electrocardiogram. I. The electrocardiogram of the dying fetus. Am. J. Obstet. Gynecol. 1963b;87:804–813. [PubMed] [Google Scholar]
- Hoppensteadt FC, Izhikevich EM. Applied Mathematical Sciences. Springer-Verlag; New York: 1997. Weakly Connected Neural Networks. [Google Scholar]
- Julien C, Parkes MJ, Tzeng SY, Sin PY, Ainslie PN, Van de Borne P, Fortrat JO, Custaud MA, Gharib C, Porta A, Vallais F, Baselli G, Pagani M, Lucini D, Hughson RL, Taylor JA, Tan CO, Baekey DM, Dick TE, Paton JF, Taha B. Comments on point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J. Appl. Physiol. 2009;106:1745–1749. doi: 10.1152/japplphysiol.00196.2009. [DOI] [PubMed] [Google Scholar]
- Kuramoto Y. Chemical Oscillations, Waves, and Turbulence. Springer Verlag; New York: 1984. [Google Scholar]
- Larsen PD, Galletly DC. Cardioventilatory coupling in the anaesthetised rabbit, rat and guinea-pig. Pflugers Arch. 1999;437:910–916. doi: 10.1007/s004240050862. [DOI] [PubMed] [Google Scholar]
- Larsen PD, Tzeng YC, Sin PY, Galletly DC. Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir. Physiol. Neurobiol. 2010;174:111–118. doi: 10.1016/j.resp.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Morgan BJ, Schrage WG, Dempsey JA. Respiratory influences on muscle sympathetic nerve activity and vascular conductance in the steady state. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1615–H1623. doi: 10.1152/ajpheart.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan S, Dick TE, Farnham MM, Pilowsky PM. Effects of baroreceptor activation on respiratory variability in rat. Respir. Physiol. Neurobiol. 2009;166:80–86. doi: 10.1016/j.resp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J. Neurophysiol. 2010;104:2713–2729. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J. Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J. Neurophysiol. 2012;108:882–890. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J. Appl. Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010;174:156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin. Exp. Pharmacol. Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp. Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir. Physiol. Neurobiol. 2010;174:230–234. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin PY, Webber MR, Galletly DC, Ainslie PN, Brown SJ, Willie CK, Sasse A, Larsen PD, Tzeng YC. Interactions between heart rate variability and pulmonary gas exchange efficiency in humans. Exp. Physiol. 2010;95:788–797. doi: 10.1113/expphysiol.2010.052910. [DOI] [PubMed] [Google Scholar]
- Vinik AI. The conductor of the autonomic orchestra. Front. Endocrinol. 2012;3:71. doi: 10.3389/fendo.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet. Med. 2011;28:643–651. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree AT. Interdisciplinary Applied Mathematics. Springer; New York: 2001. The Geometry of Biological Time. [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J. Physiol. 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin. Exp. Pharmacol. Physiol. 2013;40:602–609. doi: 10.1111/1440-1681.12129. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hsieh YH, Dhingra RR, Dick TE, Jacono FJ, Galan RF. Quantifying interactions between real oscillators with information theory and phase models: application to cardiorespiratory coupling. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013;87:022709. doi: 10.1103/PhysRevE.87.022709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp. Physiol. 2009a;94:972–983. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin. Exp. Pharmacol. Physiol. 2009b;36:1188–1196. doi: 10.1111/j.1440-1681.2009.05202.x. [DOI] [PubMed] [Google Scholar]