Abstract
Background
Chronic kidney disease (CKD) and obesity are important public health concerns. We examined the association between anthropomorphic measures and incident CKD and mortality.
Design
Cohort
Setting and Participants
Individual patient data pooled from the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study
Exposures
Waist to hip ratio (WHR), body mass index (BMI)
Outcomes
Incident CKD defined as serum creatinine rise of >0.4 mg/dL with baseline creatinine ≤1.4 mg/dL in men and 1.2 mg/dL in women and final creatinine above these levels, and, in separate analyses, as estimated glomerular filtration rate (eGFR) decline ≥15 mL/min/1.73m2 with baseline eGFR ≥60 and final eGFR <60 mL/min/1.73m2.
Analysis
Multivariable logistic regression to determine the association between waist to hip ratio (WHR), body mass index (BMI) and outcomes. Cox models to evaluate a secondary composite outcome of all-cause mortality and incident CKD.
Results
Among 13,324 individuals, mean WHR was 0.96 in men and 0.89 in women and mean BMI was 27.2 kg/m2 in both men and women. Over 9.3 years, 300 (2.3%) in creatinine-based models and 710 (5.5%) in eGFR-based models developed CKD. In creatinine-based models, each standard deviation increase in WHR was associated with an increased risk of incident CKD [Odds ratio=1.22 (1.05, 1.43)] and the composite outcome [Hazard ratio=1.12 (1.06, 1.18)], while each standard deviation increase in BMI was not associated with CKD [Odds ratio=1.05 (0.93, 1.20)] and appeared protective for the composite outcome [Hazard ratio=0.94 (0.90, 0.99)]. Results of eGFR-based models were similar.
Limitations
Single measures of creatinine, no albuminuria data.
Conclusions
WHR but not BMI is associated with incident CKD and mortality. Assessment of CKD risk should utilize WHR rather than BMI as an anthropomorphic measure of obesity.
Introduction
Chronic kidney disease (CKD) is increasing in incidence and prevalence in the US with approximately 13% of adults in the US affected (1). With the increasing incidence of hypertension and diabetes and the aging of the US population, the number of individuals with CKD will likely continue to rise (1). CKD is now recognized as an independent risk state for myocardial infarction and cardiovascular mortality, and, in its end stage, is responsible for tremendous morbidity, mortality and costs (2).
Obesity is also increasing in the United States and is associated with the comorbid conditions that cause CKD, including hypertension, diabetes and cardiovascular disease (3). Most previous studies evaluating obesity as a risk factor for CKD use body mass index (BMI) to define obesity and have shown discrepant results. For example, Hsu et al demonstrated that higher BMI was a risk factor for kidney failure in an insured US population while Iseki et al demonstrated that higher BMI was an independent risk factor for kidney failure in Japanese men but not Japanese women dwelling in Okinawa (4, 5). BMI is affected by muscle mass, fat mass and bone, and deviations from expected levels of muscle mass in particular can obscure the relationship between BMI and outcomes (6). Waist to hip ratio (WHR), a measure of central obesity and visceral fat, may be a better measure for obesity than other anthropometric measures, particularly in individuals with atypical body habitus (7). Therefore we examined both WHR and BMI as risk factors for development of kidney disease and mortality.
Methods
Study Population
Individual patient data were pooled from 2 limited-access, community-based, longitudinal studies: the Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS). ARIC recruited 15,792 subjects, between the age of 45 to 64 years, from four geographically diverse communities between 1987 and 1989 (8). CHS included 5,201 subjects, 65 years and older, randomly selected from Medicare eligibility files during 1989 and 1990 (9). In both studies, follow-up occurred at 3-4 year intervals. An additional 687 African American participants were recruited in CHS from 1992-1993 (year 5); they were not included here due to limited follow-up. Final serum creatinine measurement occurred at visit 4 in ARIC (1996-1998) and year 9 in CHS (1996-1997). Details of recruitment and follow-up for the studies are described elsewhere (8, 9).
Creatinine Measurement and Calibration
In ARIC, baseline serum creatinine was assessed in 15,582 (99%) subjects, while in CHS it was assessed in 5,716 (97%) subjects. Because serum creatinine assays vary across laboratories, we indirectly calibrated mean individual study creatinine values from ARIC and CHS to mean National Health and Nutrition Examination Survey (NHANES) III values for a given age, race and sex, resulting in adjustments of −0.24 mg/dL (21 μmol/L) in ARIC and −0.11 mg/dL (10 μmol/L) in CHS values (10). Baseline serum creatinine values for the pooled cohort were determined by subtracting these adjustments from measured serum creatinine values. We also adjusted for changes in laboratory measurements over time using previously published calibration factors: in ARIC, 0.18 mg/dL (16 μmol/L) was added to visit 4 measurements, while, in CHS, 0.11 mg/dL (10 μmol/L) was subtracted from visit 3 measurements (11). Estimated GFR was calculated with the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation (10).
Waist to Hip Ratio and Body Mass Index
In both ARIC and CHS, waist circumference was measured in centimeters (cm) by trained personnel using the smallest circumference between the lower ribs and iliac crests. Hip circumference was measured in cm using the greatest circumference between iliac crest and thighs (measured at the level of maximal protrusion of the gluteal muscles). WHR is calculated by dividing waist circumference by hip circumference. BMI was calculated by dividing weight (kg) by height2 (m2). The quality control scheme for anthropometry involved equipment calibration and monitoring, as well as between-technician and within-technician assessments of reliability.
Baseline Covariates
Other baseline variables included demographics (age, sex, race, education status), lifestyle characteristics (smoking, alcohol intake), medication use, past medical history (diabetes, hypertension and cardiovascular disease), examination findings (systolic and diastolic blood pressure, electrocardiogram results); and laboratory variables (total cholesterol, high density lipoprotein (HDL) cholesterol, albumin, glucose, hematocrit). Age in CHS was provided in 2-year groups. We assigned the mean age for that group to create a continuous variable comparable to ARIC. Race was defined as white or African American. Education level was dichotomized according to high school graduation status. Cigarette smoking and alcohol use were dichotomized as current users and non-users. Diabetes was defined as having a self reported history of diagnosis, use of oral hypoglycemic or insulin, or a fasting glucose ≥126 mg/dL (7 mmol/L). Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic ≥ 90 mm Hg or use of antihypertensive medications. Left ventricular hypertrophy (LVH) was defined by electrocardiographic criteria. Baseline cardiovascular disease was defined by history of recognized or silent myocardial infarction, angina based on the Rose questionnaire, stroke, transient ischemic attack, intermittent claudication, and prior coronary angioplasty or bypass procedures.
Study outcomes
The primary study outcome was incident CKD. This was defined as: 1) serum creatinine rise of >0.4 mg/dL (35 μmol/L) with baseline creatinine ≤1.4 mg/dL (124 μmol/L) in men and 1.2 mg/dL (106 μmol/L) in women and final creatinine above these levels, and, in separate analyses, as 2) eGFR decline ≥15 mL/min/1.73m2 (0.25 mL/sec/1.73m2) with baseline eGFR ≥60 (1 mL/sec/1.73m2) and final eGFR <60 mL/min/1.73m2 (11). To account for the semi-competing outcomes of kidney function decline and all-cause mortality, the secondary outcome was a composite of development of kidney disease and time to death. In these models only, time to kidney disease was defined by duration between the initial and final creatinine measurements.
Study Sample
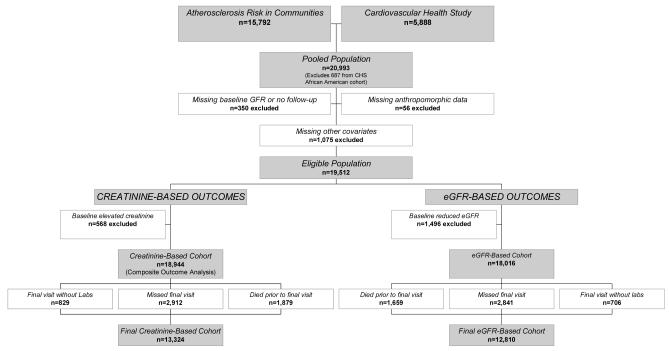
From a pooled sample of 21,680 individuals, we excluded the African American cohort from CHS enrolled at the time of the 2nd visit (n=687). Other exclusions were 340 individuals with missing baseline creatinine, age, sex or race data, 10 individuals with no follow-up data, 56 individuals missing either baseline BMI or WHR (8 without BMI only, 29 without WHR only and 19 without both), and 1,075 individuals missing other baseline covariates. Of these 1,075 individuals, 208 were missing baseline electrocardiograms to define LVH and 551 individuals were missing data on baseline cardiovascular disease; these 551 individuals were from ARIC and similar at baseline to individuals without cardiovascular disease (data not shown). Because creatinine-based and eGFR-based analyses differed in their definitions of baseline kidney disease, final numbers differ by analysis, with 568 excluded for baseline reduced kidney function in the creatinine-based cohort and 1,496 in the eGFR-based cohort. There were 5,620 and 5,206 individuals with missing creatinine levels at the final visit in the creatinine-based and eGFR-based cohorts, respectively. These individuals were not analyzed in the primary analyses but were evaluated in secondary analyses that also assess all-cause mortality (Figure 1). Baseline characteristics in the manuscript are presented for the creatinine-based cohort.
Figure 1.
Derivation of the study cohorts.
Statistical Analysis
Baseline characteristics were compared with analysis of variance for continuous variables and chi-square tests for categorical variables; Pearson correlations among WHR, waist circumference and BMI were assessed. Restricted cubic splines were used to evaluate for non-linear relationships between anthropomorphic measures and outcomes. Multivariable logistic regression models were used to assess risk of incident CKD associated with baseline WHR or BMI adjusting for baseline covariates and study of origin. All models in both WHR and BMI analyses a priori adjusted for age, sex, African American race, high school graduation, prior cardiovascular disease, diabetes, hypertension, current smoking and alcohol consumption, systolic blood pressure, HDL and total cholesterol, albumin, hematocrit, baseline kidney function, and study of origin. To best compare the effects of WHR and BMI, risks are presented both per unit and per standard deviation increase in the independent variable. Because baseline anthropomorphic measurements may differ by sex, interaction terms between sex and both WHR and BMI were tested.
Sensitivity Analyses
As diabetes, hypertension and cholesterol may mediate the relationship between obesity and development of kidney disease, we evaluated parsimonious multivariable models that did not adjust for history of diabetes and hypertension, HDL and total cholesterol, and systolic blood pressure. We also performed subgroup analyses of ARIC and CHS data. Finally, we examined the association of waist circumference (without normalization for hip circumference) and development of CKD in univariate and multivariable models.
Analyses were performed with SAS version 9.1. The Institutional Review Board at Tufts-New England Medical Center approved this research.
Results
Baseline characteristics
Among 13,324 individuals, mean age was 57.4 years. Mean WHR was 0.96 ± 0.05 (median: 0.96) in men and 0.89 ± 0.08 (median: 0.89) in women; mean BMI was 27.2 ± 3.8 (median: 26.8) kg/m2 in men and 27.2 ± 5.6 (median: 26.2) kg/m2 in women. Mean baseline serum creatinine was 0.9 mg/dL (80 μmol/L) and mean GFR 89.8 mL/min/1.73m2 (1.50 mL/sec/1.73m2) (Table 1).
Table 1.
Baseline demographic and clinical data and sex-specific Pearson correlations between waist to hip ratio or body mass index and baseline data.
| Total |
Pearson Correlation
Waist to Hip Ratio |
Pearson Correlation
Body Mass Index |
|||
|---|---|---|---|---|---|
| Demographics | Men | Women | Men | Women | |
| Age | 57.4 ± 9.0 | 0.05 | 0.11 | −0.11 | −0.07 |
| Female | 56.6 | - | - | - | - |
| African American | 18.0 | −0.18 | 0.06 | 0.05 | 0.28 |
| High School Graduate | 80.5 | −0.07 | −0.16 | −0.02† | −0.19 |
| ARIC | 80.4 | 0.01* | 0.01* | 0.10 | 0.07 |
| Medical History | |||||
| Diabetes | 6.5 | 0.12 | 0.20 | 0.16 | 0.20 |
| Hypertension | 39.4 | 0.13 | 0.20 | 0.16 | 0.25 |
| Cardiovascular Disease | 12.7 | 0.06 | 0.06 | 0.02€ | 0.04 |
| Current Smoker | 19.4 | −0.02† | <0.01* | −0.11 | −0.11 |
| Current Alcohol Use | 58.2 | 0.03£ | −0.11 | −0.02€ | −0.20 |
| ACE Inhibitor Use | 4.9 | 0.06† | 0.01* | 0.07£ | 0.08‡ |
| Physical Findings | |||||
| Systolic Blood Pressure | 122.2 ± 18.7 | 0.11 | 0.21 | 0.13 | 0.23 |
| Diastolic Blood Pressure | 72.5 ± 10.7 | 0.06 | 0.13 | 0.19 | 0.26 |
| LVH | 1.6 | <0.01* | 0.04 | 0.02€ | 0.02£ |
| Body Mass Index | 27.2 ± 4.9 | 0.55 | 0.44 | 1.00 | 1.00 |
| Waist to Hip Ratio | 0.92 ± 0.08 | 1.00 | 1.00 | 0.55 | 0.44 |
| Laboratory Results | |||||
| Serum Creatinine | 0.9 ± 0.2 | −0.02† | <0.01* | 0.05 | 0.05 |
| Estimated GFR | 90.4 ± 19.4 | −0.05 | 0.02€ | −0.02* | 0.07 |
| Hematocrit | 41.7 ± 3.8 | 0.16 | 0.11 | 0.10 | 0.05 |
| Total Cholesterol | 213.9 ± 40.4 | 0.06 | 0.15 | 0.06 | 0.03£ |
| HDL Cholesterol | 52.7 ± 16.8 | −0.26 | −0.29 | −0.26 | −0.30 |
| Albumin | 4.1 ± 0.3 | −0.03£ | −0.05 | −0.01* | −0.20 |
ARIC, Atherosclerosis Risk in Communities; ACE, angiotensin converting enzyme; LVH, left ventricular hypertrophy; GFR, glomerular filtration rate; HDL, high-density lipoprotein
Percent using ACE inhibitors is based only on Cardiovascular Health Study (CHS) participants only.
All variables are % or mean ± standard deviation. Age is in years, blood pressure in mm Hg, body mass index in kg/m2, serum creatinine, total and HDL cholesterol in mg/dL, estimated GFR in mL/min/1.73 m2, hematocrit in %, and albumin in g/dL.
To convert creatinine to μmol/L, multiply by 88.4; to convert eGFR to mL/sec/1.73m2, multiply by 0.01667, to convert total and HDL cholesterol to mmol/L, multiply by 0.02586; and to convert albumin to g/L, multiply by 10.
P-value for Pearson correlations between clinical variables and WHR or BMI for all comparisons <0.001 except
>0.2;
<0.2;
<0.1;
<0.05; and
<0.01.
Correlation among WHR, BMI and Waist Circumference
Among men, 51.6% of individuals in the upper quartile of WHR were in the upper quartile of BMI, while, among women, 46.7% of individuals in the upper quartile of WHR were in the upper quartile of BMI (data not shown); Pearson correlation was 0.55 in men and 0.44 in women for these two measures (p<0.0001 for both). However, BMI was highly correlated with waist circumference alone (0.89 in men and 0.87 in women, p<0.0001).
Univariate Analyses
Over 9.3 years, 300 (2.3%) and 710 (5.5%) developed CKD, using creatinine and eGFR-based definitions, respectively. Individuals who developed CKD had significantly worse baseline kidney function and were more likely to be older, diabetic and hypertensive (Table 2).
Table 2.
The distribution of risk factors, stratified by development of kidney disease.
| Creatinine Model | Estimated GFR Model | |||
|---|---|---|---|---|
| No Development N =13,024 (97.7%) |
Development N =300 (2.3%) |
No Development N =12,100 (94.5%) |
Development N =710 (5.5%) |
|
| Demographics | ||||
| Age | 57.3± 9.0 | 61.6 ± 9.6 | 56.8 ± 8.7 | 60.5 ± 8.4 |
| Female | 56.9 | 46.0 | 56.0 | 59.2† |
| Black | 17.7 | 28.7 | 18.9 | 17.2* |
| High School Graduate | 80.8 | 68.0 | 81.0 | 74.5 |
| ARIC | 80.6 | 72.0 | 82.6 | 79.3‡ |
| Medical History | ||||
| Diabetes | 6.1 | 23.7 | 6.0 | 14.2 |
| Hypertension | 38.7 | 68.3 | 37.3 | 60.4 |
| Cardiovascular Disease | 12.4 | 26.7 | 11.8 | 21.3 |
| Current Smoker | 19.4 | 20.0* | 19.9 | 18.5* |
| Current Alcohol Use | 58.5 | 45.0 | 58.8 | 49.6 |
| ACE Inhibitor Use | 5.0 | 3.6* | 4.5 | 2.7* |
| Physical Findings | ||||
| Systolic Blood Pressure | 121.9 ± 18.5 | 134.6 ± 21.0 | 121.3 ± 18.3 | 130.7 ± 21.3 |
| Diastolic Blood Pressure | 72.5 ± 10.7 | 74.8 ± 12.4 | 72.6 ± 10.7 | 73.4 ± 11.9‡ |
| LVH | 1.4 | 6.3 | 1.4 | 3.8 |
| Body Mass Index | 27.2 ± 4.9 | 28.6 ± 5.1 | 27.2 ± 4.9 | 27.7 ± 4.9‡ |
| Waist to Hip Ratio | 0.92 ± 0.08 | 0.95 ± 0.07 | 0.92 ± 0.08 | 0.94 ± 0.07 |
| Baseline Laboratory Results | ||||
| Serum Creatinine | 0.8 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| Estimated GFR | 90.6± 19.4 | 80.8 ± 16.9 | 92.4 ± 18.5 | 82.1 ± 13.4 |
| Hematocrit | 41.7 ± 3.8 | 41.9 ± 4.4* | 41.7± 3.8 | 41.6 ± 3.9* |
| Total Cholesterol | 213.9 ± 40.3 | 215.9 ± 44.0* | 213.4 ± 40.1 | 220.0 ± 44.8 |
| HDL Cholesterol | 52.8 ± 16.8 | 47.8± 15.1 | 52.8 ± 16.8 | 51.2 ± 16.7‡ |
| Albumin | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3‡ |
ARIC, Atherosclerosis Risk in Communities; ACE, angiotensin converting enzyme; LVH, left ventricular hypertrophy; GFR, glomerular filtration rate; HDL, high-density lipoprotein
Percent using ACE inhibitors is based only on Cardiovascular Health Study (CHS) participants only.
All variables are % or mean ± standard deviation. Age is in years, blood pressure in mm Hg, body mass index in kg/m2, serum creatinine, total and HDL cholesterol in mg/dL, estimated GFR in mL/min/1.73 m2, hematocrit in %, and albumin in g/dL.
To convert creatinine to μmol/L, multiply by 88.4; to convert eGFR to mL/sec/1.73m2, multiply by 0.01667, to convert total and HDL cholesterol to mmol/L, multiply by 0.02586; and to convert albumin to g/L, multiply by 10.
P <0.001 for all comparisons within outcomes except
>0.2;
<0.1; and
<0.05.
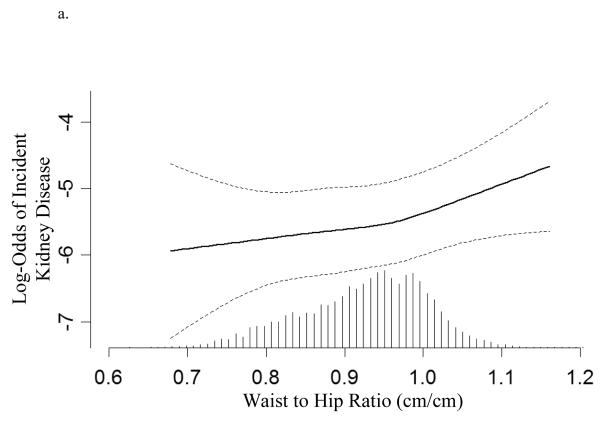
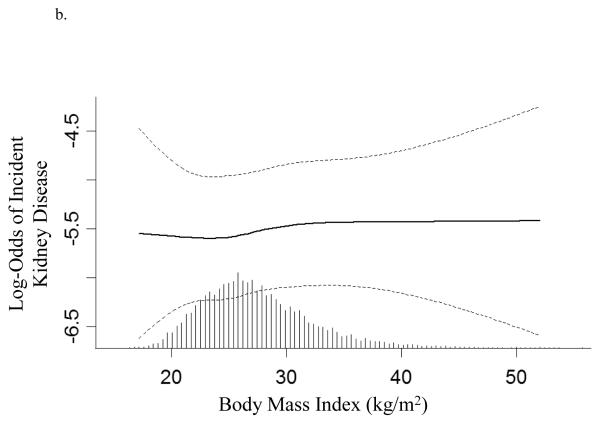
In univariate analysis of creatinine-based outcomes, each 0.1 increase in WHR was associated with an 81% increase in risk of developing CKD [Odds Ratio (OR)=1.81 (1.54, 2.12)] while each two kg/m2 increase in BMI was associated with an 11% increase in the risk of developing incident CKD [OR=1.11 (95% CI: 1.06, 1.15)] (Table 3). Graphical representation was not consistent with a non-linear relationship (not shown).
Table 3.
Unadjusted and adjusted associations between WHR, BMI and incident kidney disease.
| Predictor | Method | Model | Per unit increase | Per SD increase | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| WHR | Creatinine | Unadjusted | 1.81 | 1.54-2.12 | 1.60 | 1.41-1.82 |
|
| ||||||
| Adjusted | 1.29 | 1.07-1.56 | 1.22 | 1.05-1.43 | ||
|
| ||||||
| eGFR | Unadjusted | 1.35 | 1.22-1.49 | 1.27 | 1.17-1.37 | |
|
| ||||||
| Adjusted | 1.17 | 0.99-1.34 | 1.09 | 0.99-1.21 | ||
|
| ||||||
| BMI | Creatinine | Unadjusted | 1.11 | 1.06-1.15 | 1.28 | 1.15-1.41 |
|
| ||||||
| Adjusted | 1.03 | 0.97-1.08 | 1.05 | 0.93-1.20 | ||
|
| ||||||
| eGFR | Unadjusted | 1.04 | 1.01-1.07 | 1.11 | 1.03-1.19 | |
|
| ||||||
| Adjusted | 0.99 | 0.96-1.03 | 0.99 | 0.90-1.08 | ||
WHR, waist to hip ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; OR, odds ratio; SD, standard deviation
One unit increase is 0.1 unit increase in WHR or 1 kg/m2 increase in BMI.
Models adjusted for age, sex, African American race, high school graduation, prior cardiovascular disease, diabetes, hypertension, current smoking and alcohol consumption, systolic blood pressure, HDL and total cholesterol, albumin, hematocrit, baseline kidney function, and study of origin
Multivariable Analyses
In creatinine-based models, each unit increase in WHR but not BMI was associated with 28% increase in the risk of developing kidney disease. In eGFR-based models, WHR trended to significance [OR=1.12 (0.99, 1.27)] while there was no relationship between increasing BMI and incident kidney disease (Table 3, Figures 2a and 2b). To compare the effects of WHR and BMI, the association of each standard deviation increase in WHR and BMI and incident kidney disease are presented in Table 3. Interaction terms between WHR and sex as well as BMI and sex were non-significant in all analyses.
Figure 2.
Log-transformed adjusted odds and 95% confidence interval (dashed line) for developing kidney disease associated with (a) waist to hip ratio and (b) body mass index (based on serum creatinine models). Hatch marks represent the relative proportion of individuals at any given waist to hip ratio or body mass index. Models are plotted as restricted cubic splines with 4 knots and are adjusted for age, sex, race, education, cardiovascular disease, diabetes, hypertension, smoking, alcohol, systolic blood pressure, HDL and total cholesterol, albumin, hematocrit, baseline kidney function, and study of origin. P-values for linearity are 0.6 and 0.9 respectively.
Mortality Analyses
In creatinine-based analyses, there were 1,879 deaths prior to the final visit. Each standard deviation increase in WHR was associated with a 41% increase in the composite outcome of incident CKD and mortality in univariate analysis and a 12% increase in multivariable analysis while each standard deviation increase in BMI was non-significant in univariate analysis and was associated with a 6% decreased risk of the composite outcome in multivariable models (Table 4). Models using eGFR were similar.
Table 4.
Unadjusted and adjusted associations between WHR, BMI and the composite outcome of incident kidney disease and mortality.
| Predictor | Method | Model | Per unit increase | Per SD increase | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| WHR | Creatinine | Unadjusted | 1.54 | 1.45- 1.63 | 1.41 | 1.35- 1.48 |
|
| ||||||
| Adjusted | 1.15 | 1.08- 1.23 | 1.12 | 1.06- 1.18 | ||
|
| ||||||
| eGFR | Unadjusted | 1.45 | 1.37- 1.53 | 1.35 | 1.29- 1.41 | |
|
| ||||||
| Adjusted | 1.11 | 1.04- 1.18 | 1.09 | 1.03- 1.14 | ||
|
| ||||||
| BMI | Creatinine | Unadjusted | 0.99 | 0.97- 1.01 | 0.98 | 0.94- 1.02 |
|
| ||||||
| Adjusted | 0.98 | 0.96- 1.00 | 0.94 | 0.90- 0.99 | ||
|
| ||||||
| eGFR | Unadjusted | 0.99 | 0.97- 1.01 | 0.98 | 0.94- 1.02 | |
|
| ||||||
| Adjusted | 0.97 | 0.95- 0.99 | 0.93 | 0.88- 0.97 | ||
WHR, waist to hip ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval; SD, standard deviation
One unit increase is 0.1 unit increase in WHR or 1 kg/m2 increase in BMI.
Models adjusted for age, sex, African American race, high school graduation, prior cardiovascular disease, diabetes, hypertension, current smoking and alcohol consumption, systolic blood pressure, HDL and total cholesterol, albumin, hematocrit, baseline kidney function, and study of origin
Sensitivity Analyses
Analyses that excluded potential mediators of obesity on kidney disease revealed a statistically significant risk of outcomes using creatinine-based models with both WHR and BMI [OR=1.48 (1.28, 1.70) and OR=1.26 (1.12, 1.41) per standard deviation rise, respectively], although the association with WHR was more marked. WHR [Hazard Ratio (HR)=1.22 (1.16, 1.29)] but not BMI [HR=1.03 (0.99, 1.08)] was independently associated with the composite outcome [HR=1.22 (1.16, 1.29) and HR=1.03 (0.99, 1.08) per standard deviation rise, respectively]. Subgroup analyses using ARIC and CHS data had similar results, and, although the term for study was significant in models evaluating incident kidney disease, the study term was no longer significant when mortality was included as an outcome (data not shown). In additional analyses, waist circumference alone was highly correlated with BMI and was not a significant predictor of incident kidney disease (data not shown).
Discussion
In a generalizable, community-based US population, waist to hip ratio but not body mass index was associated with development of reduced kidney function. Additionally, waist to hip ratio was associated with an increased risk of the composite outcome of reduced kidney function and mortality while increased BMI appeared protective for this outcome. Given epidemic rates of obesity and progressively increasing rates of chronic kidney disease and kidney failure, the ability to identify risk factors for developing kidney disease is critical to addressing this public health problem.
Obesity is associated with many of the factors that cause both kidney disease and kidney disease progression, including diabetes, hypertension, and cardiovascular disease. Most public health literature in the United States focuses on use of BMI to identify obesity and its sequelae, as BMI correlates with body fat in most individuals (12, 13). However, BMI has limitations - notably BMI does not distinguish between weight from muscle and fat, between visceral and subcutaneous fat, and between peripheral and central adiposity. Although alternate measures of obesity exist including WHR, the public health community’s focus remains on BMI as the primary marker of obesity. This likely reflects ease of measurement as well as predictive ability in younger and healthier individuals.
In defining obesity, several recent guidelines also incorporate waist circumference (14, 15), potentially a better marker of visceral fat in individuals with CKD (16). Although hip circumference is a less well-studied marker, one recent prospective evaluation of 24,508 residents of Norfolk, UK demonstrated that WHR was independently and more consistently predictive of coronary heart disease than waist circumference or BMI, and, at any given waist circumference, those with greater hip circumference had lower coronary heart disease rates than those with smaller hips (17). The benefits of increased hip circumference after controlling for central obesity may reflect the differing metabolic properties of peripheral versus central adiposity (18, 19).
In this study, we found an association between WHR ratio and incident CKD but not between BMI and incident CKD. This association was present despite adjustment for several potential mediators of the effects of obesity on kidney disease. Further we found that elevated BMI was protective for a composite outcome that included mortality; in contrast, we found that increased WHR was a risk factor for this same composite outcome. This finding may foreshadow the altered risk factor relationship seen in chronic disease states, including heart failure and kidney failure requiring dialysis, where higher BMI is associated with improved survival (20, 21).
Although WHR and BMI are often highly correlated, they may identify different body types. BMI provides information about body volume and mass, while waist circumference, optimally presented as WHR, provides information about body shape and fat distribution (22). BMI is a less specific measure less able to differentiate between visceral and subcutaneous fat as well as between central and peripheral fat. Critically, excess visceral fat more so than subcutaneous fat is associated with cardiovascular disease and cardiovascular disease risk factors (23).
Other factors may mediate the pathway between WHR and development of kidney disease; these include features of the metabolic syndrome (increased waist circumference, hyperglycemia, hypertension, hypercholesterolemia and atherosclerosis), inflammation and oxidative stress. In the current analysis, we adjusted for many metabolic risk factors at baseline; this fully attenuated the relationship between BMI and development of kidney disease while the relationship between WHR and kidney disease remained robust, suggesting that WHR identifies additional risk beyond that suggested by pre-existing diabetes, hypertension and dyslipidemia. Additionally other unmeasured factors associated more with visceral obesity than subcutaneous obesity, including leptin, plasminogen activator inhibitor-1, and angiotensinogen, may contribute to incident kidney disease (24). The differences in our study between WHR and BMI may reflect the fact that they assess different features of obesity and potentially different forms of fat (central obesity and visceral fat with WHR versus subcutaneous and total fat with BMI).
Several prior studies have used BMI to investigate the association of obesity and development of kidney disease (generally eGFR<60 mL/min/1.73m2). In the Physicians Health Study, each kg/m2 increase in baseline BMI assessed in 1982 was associated with a statistically significant 3% increased risk of kidney disease after fourteen years follow-up in adjusted analyses (25). Baseline kidney function was not measured in this cohort. Similarly in an analysis of the Hypertension Detection and Follow-Up Program which recruited 5,897 hypertensive adults in 1974, higher baseline BMI was significantly associated with incident CKD (26). Finally, evaluation of the Framingham Offspring Study, recruited from 1978-1982, found a statistically significant 23% increased risk per standard deviation increase in BMI in adjusted analyses after indirectly calibrating baseline and subsequent serum creatinine measurements (27). Critical differences between the current study and these studies include: 1) Requirement of a clinically significant change in kidney function to meet the definition of incident kidney disease; 2) Indirect calibration of creatinine to allow comparison between study periods; 3) Use of a community-based rather than selected population; and 4) Marked changes in the prevalence and nature of obesity in the US from 1970s and early 1980s when recruitment was ongoing for these studies versus the late 1980s and early 1990s, when the participants in the current study were recruited (28).
This study has several important strengths. Pooling the ARIC and CHS cohorts provides a large diverse population with more than 9 years of follow-up that allowed adjustment for critical risk factors and enhanced generalizability as evidenced by the similarity in BMI levels to those seen in NHANES III. Additionally, as the studies were designed to evaluate cardiovascular disease (CVD), both ARIC and CHS devoted considerable effort to identifying CVD and CVD risk factors at study enrollment.
There are also several limitations of this analysis, including use of single measurements of creatinine at each time point. However, as subjects were not acutely ill at the time of study evaluation, these values likely are consistent with chronic kidney function. Additionally, we set conservative definitions for incident kidney disease that required significant changes in serum creatinine levels and eGFR over time that exceed that expected by chance and laboratory error. A second limitation is the lack of albuminuria data, an important risk factor for development of kidney disease that may also be associated with obesity. Third, we have limited data available on socioeconomic status and rely on education level as a proxy for this potentially important risk factor. Finally, results are limited by the absence of gold standard for body fat assessment, although previous studies evaluating WHR and BMI concurrent with imaging have noted that WHR was highly correlated with visceral fat measurement on both DEXA and single slice CT scan (29).
In conclusion, WHR, but not BMI, is an independent risk factor for development of reduced kidney function in a diverse, community-based population. Additionally, WHR was associated with an increased risk of the composite outcome of kidney disease and mortality while increased BMI appeared protective for this outcome. Further research should assess mediators of this relationship and gear education toward measurement of the waist to hip ratio rather than body mass index.
Acknowledgements
The ARIC Study and CHS are conducted and supported by the National Heart, Lung and Blood Institute (NHLBI) in collaboration with the individual study investigators. This manuscript was not prepared in collaboration with the study investigators and does not necessarily reflect the opinions or views of the study investigators or the NHLBI.
Research Support: This research was supported by US National Institutes of Health grants K23 DK71636, R21 DK068310, and T32 DK007777 and Amgen Inc., Thousand Oaks, CA. Study sponsors were not involved in data analysis or interpretation of findings. Study sponsors were given the opportunity to review the manuscript prior to submission.
Footnotes
An abstract based on this manuscript was presented at the 2007 meeting of the American Society of Nephrology in San Francisco, CA on November 3, 2007.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of Chronic Kidney Disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 5.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 7.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DE, Tighiouart H, Stark PC, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167:1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health and Human Services. National Institutes of Health [Accessed July 17, 2007];National Heart Lung and Blood Institute Obesity Education Initiative. http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm.
- 13.Department of Health and Human Services. Centers for Disease Control and Prevention [Accessed July 17, 2007];Defining Overweight and Obesity. http://www.cdc.gov/nccdphp/dnpa/obesity/defining.htm.
- 14.World Health Organization [Accessed July 17, 2007];Global Database on Body Mass Index. http://www.who.int/bmi/index.jsp.
- 15.Daviglus ML, Liu K, Yan LL, et al. Relation of body mass index in young adulthood and middle age to Medicare expenditures in older age. JAMA. 2004;292:2743–2749. doi: 10.1001/jama.292.22.2743. [DOI] [PubMed] [Google Scholar]
- 16.Sanches FMR, Avesani CM, Kamimura MA, et al. Waist Circumference and Visceral Fat in CKD: A Cross-sectional Study. Am J Kidney Dis. 2008 doi: 10.1053/j.ajkd.2008.02.004. in press. [DOI] [PubMed] [Google Scholar]
- 17.Canoy D, Boekholdt SM, Wareham N, et al. Body Fat Distribution and Risk of Coronary Heart Disease in Men and Women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk Cohort. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 18.Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 19.Snijder MB, Dekker JM, Visser M, et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 24.Mathew B, Patel SB, Reams GP, Freeman RH, Spear RM, Villarreal D. Obesity-hypertension: emerging concepts in pathophysiology and treatment. Am J Med Sci. 2007;334:23–30. doi: 10.1097/MAJ.0b013e3180959e4e. [DOI] [PubMed] [Google Scholar]
- 25.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Adv Data. 2004;347:1–17. [PubMed] [Google Scholar]
- 29.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord. 1998;22:338–342. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]