Abstract
Objective. To meta-analyze published data about the diagnostic accuracy of fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) or PET/computed tomography (PET/CT) for primary tumor evaluation in patients with cholangiocarcinoma (CCa). Methods. A comprehensive literature search of studies published through December 31, 2013, was performed. Pooled sensitivity and specificity were calculated on a per patient based analysis. Subgroup analyses considering the device used (PET versus PET/CT) and the localization of the primary tumor (intrahepatic cholangiocarcinoma (IH-CCa), extrahepatic cholangiocarcinoma (EH-CCa), and hilar cholangiocarcinoma (H-CCa)) were carried out. Results. Twenty-three studies including 1232 patients were included in the meta-analysis. Pooled sensitivity and specificity of 18F-FDG-PET or PET/CT were 81% and 82%, respectively. Pooled sensitivity and specificity, respectively, were 80% and 89% for PET, 82% and 75% for PET/CT, 95% and 83% for IH-CCa, 84% and 95% for H-CCa, and 76% and 74% for EH-CCa. Conclusions. 18F-FDG-PET and PET/CT were demonstrated to be accurate diagnostic imaging methods for primary tumor evaluation in patients with CCa. These tools have a better diagnostic accuracy in patients with IH-CCa than in patients with EH-CCa. Further studies are needed to evaluate the accuracy of 18F-FDG-PET or PET/CT in patients with H-CCa.
1. Introduction
Cholangiocarcinoma (CCa) is a malignant tumor arising from the epithelium of the bile ducts and is usually classified by anatomical and clinical criteria into intrahepatic cholangiocarcinoma (IH-CCa), hilar cholangiocarcinoma (H-CCa), and extrahepatic cholangiocarcinoma (EH-CCa) [1]. CCa has a poor prognosis and surgical resection with appropriate lymph node dissection is advocated as the curative approach in some patients [2]. Consequently, accurate evaluation and staging are critical to provide indication to surgery and to avoid unnecessary surgical interventions [3].
Several diagnostic tools have been used in this setting, including ultrasonography (US), computed tomography (CT), magnetic resonance (MR), endoscopic retrograde cholangiopancreatography (ERCP), and percutaneous transhepatic cholangiography (PTC).
Fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and PET/CT have been proposed as noninvasive imaging methods to assess the disease extent in cancer patients [4]. Since 18F-FDG is a glucose analogue, this radiopharmaceutical may be very useful in detecting malignant lesions which usually present high glucose metabolism. Hybrid PET/CT device allows enhanced detection and characterization of neoplastic lesions, by combining the functional data obtained by PET with morphological data obtained by CT [4].
Several studies have assessed the diagnostic accuracy of 18F-FDG-PET or PET/CT in the evaluation of primary tumor in patients with CCa, reporting different values of sensitivity and specificity. The purpose of our study is to meta-analyze published data on the diagnostic accuracy of 18F-FDG-PET or PET/CT in the evaluation of primary tumor in patients with CCa, in order to provide more evidence-based data and to address further studies in this setting.
2. Materials and Methods
2.1. Search Strategy
A comprehensive computer literature search of PubMed/MEDLINE and Embase databases was carried out to find relevant published articles concerning the evaluation of primary tumor in patients with CCa. We used a search algorithm based on a combination of terms (“PET” or “positron emission tomography”) and (“cholangiocarcinoma” or “cholangiocellular” or “cholangio∗” or “biliar” or “biliary” or “bile” or “Klatskin”). Only articles in English language were considered. The search was performed from inception to December 31, 2013. To expand our search, references of the retrieved articles were also screened for additional studies.
2.2. Study Selection
Studies or subsets in studies investigating the role of 18F-FDG-PET or PET/CT in the evaluation of primary CCa were eligible for inclusion. Case reports, small case series, review articles, letters, editorials, and conference proceedings were excluded. The following inclusion criteria were applied to select studies for this meta-analysis:
original studies in which 18F-FDG-PET or PET/CT were performed in patients with CCa or suspicious CCa;
a sample size of at least ten patients with CCa or suspicious CCa;
sufficient data to reassess sensitivity and specificity of 18F-FDG-PET or PET/CT in detecting the primary tumor in patients with CCa;
no data overlap.
Three researchers (SA, DAP, and CC) independently reviewed titles and abstracts of the retrieved articles, applying the above-mentioned selection criteria. Articles were rejected if they were clearly ineligible. The same three researchers then independently evaluated the full-text version of the included articles to determine their eligibility for inclusion.
2.3. Data Extraction
Information about basic study (authors, year of publication, and country of origin), study design (prospective or retrospective), patients' characteristics (number of patients with biliary ducts lesions performing 18F-FDG-PET or PET/CT, mean age, and gender), and technical aspects (injected activity of 18F-FDG and time between injection and image acquisition) was collected.
Each study was analyzed to retrieve the number of true-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN) findings of 18F-FDG-PET or PET/CT in patients with CCa or suspicious CCa, according to the reference standard. Only studies providing such complete information were finally included in the meta-analysis.
2.4. Quality Assessment
The 2011 Oxford Center for Evidence-Based Medicine checklist for diagnostic studies was used for quality assessment of the included studies. This checklist has 5 major parts as follows: representative spectrum of the patients, consecutive patient recruitment, ascertainment of the gold standard regardless of the index test results, independent blind comparison between the gold standard and index test results, and enough explanation of the test to permit replication.
2.5. Statistical Analysis
Sensitivity and specificity of 18F-FDG-PET and PET/CT in the evaluation of primary CCa were obtained from the individual studies, on a per patient-based analysis. We considered as positive a biliary ducts lesion with increased uptake of 18F-FDG, according to the criteria reported by the different authors. When a positive lesion was histologically confirmed as malignant, this was considered a TP lesion, whereas a histologically confirmed benign lesion was considered as a FP lesion. We considered as negative a lesion with no uptake of 18F-FDG: when the lesion was histologically confirmed as malignant, this was considered as FN lesion, whereas a histologically confirmed benign lesion was considered as a TN lesion.
Sensitivity was determined according to the following formula: TP/(TP + FN); specificity was determined according to this following formula: TN/(TN + FP). Statistical pooling of the data was performed by means of a random effects model. Pooled data are presented with 95% confidence intervals (95% CI). Heterogeneity between studies was assessed by an I 2 index. A summary receiving operator characteristics (ROC) curve was obtained for selected studies and area under the curve (AUC) was calculated to assess the overall accuracy of 18F-FDG-PET and PET/CT.
Subsequently, subgroup analyses were also performed, calculating the pooled sensitivity and specificity of 18F-FDG-PET and PET/CT in three different groups of primary CCa (IH-CCa, EH-CCa, and H-CCa) and in two groups based on the different device used (PET or PET/CT).
For publication bias evaluation, funnel plots, Egger's regression intercept, and Duval and Tweedie's method were used [5].
Statistical analyses were performed using Meta-DiSc statistical software version 1.4.
3. Results
3.1. Literature Search
The comprehensive computer literature search from PubMed/MEDLINE and Embase databases revealed 449 articles. Reviewing titles and abstracts, 406 records were excluded as reviews, editorials or letters, case reports or case series, or no direct link with the main subject. Twenty articles were excluded due to absence of data to reassess the pooled sensitivity or specificity of 18F-FDG-PET or PET/CT in evaluating the primary tumor in patients with CCa or suspicious CCa. Finally, 23 articles including 1232 patients were selected and were eligible for the meta-analysis [1–3, 6–25]; no additional studies were found screening the references of these articles (Figure 1). The characteristics of the included studies are presented in Tables 1, 2, 3 and 4.
Figure 1.
Plot of the literature search.
Table 1.
Basic characteristics of the included studies.
| Authors | Year | Country | Study design | Patients performing 18F-FDG-PET or PET/CT | Mean age (years) |
Gender (% male) |
Site of primary tumour |
|---|---|---|---|---|---|---|---|
| Fritscher-Ravens et al. [6] | 2001 | Germany | Prospective | 15 | 58 | 60% | 15 H-CCa |
|
| |||||||
| Kluge et al. [7] | 2001 | Germany | Prospective | 54 | NR | 54% | NR |
|
| |||||||
| Kato et al. [8] | 2002 | Japan | NR | 30 | 68 | 70% | 30 EH-CCa |
|
| |||||||
| Kim et al. [9] | 2003 | Korea | Retrospective | 21 | 57 | 52% | 10 H -CCa and 11 IH-CCa |
|
| |||||||
| Anderson et al. [10] | 2004 | USA | NR | 36 | 63 | 55% | NR |
|
| |||||||
| Reinhardt et al. [11] | 2005 | Germany | NR | 20 | 63 | 50% | 20 H-CCa |
|
| |||||||
| Wakabayashi et al. [12] | 2005 | Japan | Retrospective | 30 | 71 | 50% | 5 IH-CCa and 25 EH-CCa |
|
| |||||||
| Petrowsky et al. [13] | 2006 | Switzerland | Prospective | 61 | 64 | 51% | 14 IH-CCa and 33 EH-CCa |
|
| |||||||
| Prytz et al. [14] | 2006 | Sweden | Prospective | 24 | 39 | 83% | NR |
|
| |||||||
| Nishiyama et al. [15] | 2007 | Japan | Retrospective | 37 | 70 | 59% | 29 EH-CCa |
|
| |||||||
| Corvera et al. [16] | 2008 | USA | Retrospective | 126 | 62 | 52% | 41 EH-CCa and 21 IH-CCa |
|
| |||||||
| Furukawa et al. [17] | 2008 | Japan | Prospective | 72 | 69 | 57% | 64 EH-CCa |
|
| |||||||
| Kim et al. [18] | 2008 | Korea | Prospective | 123 | 60 | 65% | 36 IH-CCa and 87 EH-CCa |
|
| |||||||
| Li et al. [19] | 2008 | Germany | Prospective | 17 | 62 | 65% | 17 H-CCa |
|
| |||||||
| Moon et al. [20] | 2008 | Korea | Retrospective | 54 | 59 | 63% | 23 IH-CCa, 12 H-CCa, and 11 EH-CCa |
|
| |||||||
| Lee et al. [21] | 2010 | Korea | Retrospective | 99 | 67 | 59% | 17 IH-CCa and 49 EH-CCa |
|
| |||||||
| Alkhawaldeh et al. [22] | 2011 | Germany | Retrospective | 65 | 63 | 60% | 34 H-CCa and 23 IH-CCa |
|
| |||||||
| Kitamura et al. [23] | 2011 | Japan | NR | 73 | 66 | 63% | 45 H-CCa and 28 EH-CCa |
|
| |||||||
| Ruys et al. [24] | 2011 | Netherlands | Retrospective | 30 | 62 | 47% | 26 H-CCa |
|
| |||||||
| Yamada et al. [25] | 2012 | Japan | Retrospective | 73 | 68 | 63% | 16 IH-CCa, 18 H-CCa, and 20 EH-CCa |
|
| |||||||
| Albazaz et al. [3] | 2013 | UK | Retrospective | 81 | 65 | 41% | 47 IH-CCa and 34 EH-CCa |
|
| |||||||
| Choi et al. [1] | 2013 | Korea | Retrospective | 39 | 64 | 72% | 34 EH-CCa |
|
| |||||||
| Lee et al. [2] | 2013 | Korea | Retrospective | 52 | 69 | 53% | 23 EH-CCa, 17 H-CCa, and 12 IH-CCa |
NR: not reported; H-CCa: hilar cholangiocarcinoma; EH-CCa: extrahepatic cholangiocarcinoma; IH-CCa: intrahepatic cholangiocarcinoma.
Table 2.
Technical characteristics of the included studies.
| Authors | Year | Device | 18F-FDG mean injected dose (MBq) | Time between 18F-FDG injection and image acquisition (min) | Image analysis | Other imaging methods performer |
|---|---|---|---|---|---|---|
| Fritscher-Ravens et al. [6] | 2001 | PET | Range: 320–400 | 60 | Visual and semiquantitative | CT and ERCP |
|
| ||||||
| Kluge et al. [7] | 2001 | PET | 370 | 50 | Visual and semiquantitative | US, CT, MR, and ERCP |
|
| ||||||
| Kato et al. [8] | 2002 | PET | NR | 60 | Visual and semiquantitative | CT |
|
| ||||||
| Kim et al. [9] | 2003 | PET | 370 | 60 | Visual and semiquantitative | CR, MR, ERCP, and PTC |
|
| ||||||
| Anderson et al. [10] | 2004 | PET | 370 | 60 | Visual | CT and MR |
|
| ||||||
| Reinhardt et al. [11] | 2005 | PET/CT | 369 | 101 | Visual and semiquantitative | ERCP |
|
| ||||||
| Wakabayashi et al. [12] | 2005 | PET | 185 | 60 | Visual | CT, ERCP, and PTC |
|
| ||||||
| Petrowsky et al. [13] | 2006 | PET/CT | 370 | 45 | Visual and semiquantitative | CT, ERCP, and PTC |
|
| ||||||
| Prytz et al. [14] | 2006 | PET | 300 | Dynamic 0–90 | Visual and quantitative | US, CT, MR, ERCP, and PTC |
|
| ||||||
| Nishiyama et al. [15] | 2007 | PET | 3/kg | 70 | Visual and semiquantitative | US, CT, and MR |
|
| ||||||
| Corvera et al. [16] | 2008 | PET | Range: 370–555 | NR | Visual and semiquantitative | US, CT, and MR |
|
| ||||||
| Furukawa et al. [17] | 2008 | PET | Range: 200–250 | 60 | Visual | US and CT |
|
| ||||||
| Kim et al. [18] | 2008 | PET/CT | 370 | 60 | Visual and semiquantitative | CR, CT, MR, ERCP, and PTC |
|
| ||||||
| Li et al. [19] | 2008 | PET/CT | 350 | 60 | Visual and semiquantitative | CT, MR, and ERCP |
|
| ||||||
| Moon et al. [20] | 2008 | PET | 370 | 60 | Visual and semiquantitative | CT |
|
| ||||||
| Lee et al. [21] | 2010 | PET/CT | Range: 370–555 | 60 | Visual and semiquantitative | CT and ERCP |
|
| ||||||
| Alkhawaldeh et al. [22] | 2011 | PET/CT | 2.52/kg | 100 | Visual and semiquantitative | ERCP |
|
| ||||||
| Kitamura et al. [23] | 2011 | PET/CT | 250 | 60 | Visual and semiquantitative | US, CT, MR, ERCP, PTC, and HS |
|
| ||||||
| Ruys et al. [24] | 2011 | PET | 296 | 50 | Visual and semiquantitative | CT, MR, ERCP, and PTC |
|
| ||||||
| Yamada et al. [25] | 2012 | PET | 4.5/kg | 60 | Visual and semiquantitative | CT, MR, and ERCP |
|
| ||||||
| Albazaz et al. [3] | 2013 | PET-CT | 400 | 60 | Visual and semiquantitative | CT and MR |
|
| ||||||
| Choi et al. [1] | 2013 | PET/CT | Range: 370–555 | 60 | Visual and semiquantitative | US, CT, and MR |
|
| ||||||
| Lee et al. [2] | 2013 | PET/CT | Range: 370–555 | 60 | Visual and semiquantitative | US, CT, MR, and ERCP |
NR: not reported; CT: computed tomography; ERCP: endoscopic retrograde cholangiopancreatography; MR: magnetic resonance; US: ultrasonography; PTC: percutaneous transhepatic cholangiography; HS: hepatobiliary scintigraphy.
Table 3.
Diagnostic accuracy data of 18F-FDG-PET or PET/CT on a per patient-based analysis.
| Author | Year | Overall | PET | PET/CT | IH-CCa | H-CCa | EH-CCa | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | TP | FP | FN | TN | TP | FP | FN | TN | TP | FP | FN | TN | TP | FP | FN | TN | TP | FP | FN | TN | ||
| Fritscher-Ravens et al. [6] | 2001 | 10 | 2 | 3 | 0 | 10 | 2 | 3 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 10 | 2 | 3 | 0 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Kluge et al. [7] | 2001 | 24 | 2 | 2 | 26 | 24 | 2 | 2 | 26 | NR | NR | NR | NR | NR | NR | NR | NR | 24 | 2 | 2 | 26 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Kato et al. [8] | 2002 | 18 | 0 | 12 | 0 | 18 | 0 | 12 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 18 | 0 | 12 | 0 |
|
| |||||||||||||||||||||||||
| Kim et al. [9] | 2003 | 20 | 0 | 1 | 0 | 20 | 0 | 1 | 0 | NR | NR | NR | NR | 11 | 0 | 0 | 0 | 9 | 0 | 1 | 0 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Anderson et al. [10] | 2004 | 19 | 1 | 12 | 4 | 19 | 1 | 12 | 4 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Reinhardt et al. [11] | 2005 | 19 | 2 | 2 | 7 | 19 | 2 | 2 | 7 | NR | NR | NR | NR | 2 | 0 | 0 | 3 | NR | NR | NR | NR | 12 | 2 | 1 | 2 |
|
| |||||||||||||||||||||||||
| Wakabayashi et al. [12] | 2005 | 12 | 0 | 0 | 8 | NR | NR | NR | NR | 12 | 0 | 0 | 8 | NR | NR | NR | NR | 12 | 0 | 0 | 8 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Petrowsky et al. [13] | 2006 | 3 | 1 | 1 | 19 | 3 | 1 | 1 | 19 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Prytz et al. [14] | 2006 | 31 | 3 | 16 | 5 | NR | NR | NR | NR | 31 | 3 | 16 | 5 | 13 | 1 | 1 | 4 | NR | NR | NR | NR | 18 | 2 | 15 | 1 |
|
| |||||||||||||||||||||||||
| Nishiyama et al. [15] | 2007 | 25 | 1 | 4 | 7 | 25 | 1 | 4 | 7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 25 | 1 | 4 | 7 |
|
| |||||||||||||||||||||||||
| Corvera et al. [16] | 2008 | 46 | 1 | 13 | 2 | 46 | 1 | 13 | 2 | NR | NR | NR | NR | 19 | 0 | 1 | 1 | NR | NR | NR | NR | 27 | 1 | 12 | 2 |
|
| |||||||||||||||||||||||||
| Furukawa et al. [17] | 2008 | 40 | 0 | 7 | 0 | 40 | 0 | 7 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 40 | 0 | 7 | 0 |
|
| |||||||||||||||||||||||||
| Kim et al. [18] | 2008 | 79 | 6 | 15 | 23 | NR | NR | NR | NR | 79 | 6 | 15 | 23 | 20 | 3 | 1 | 12 | NR | NR | NR | NR | 59 | 3 | 14 | 11 |
|
| |||||||||||||||||||||||||
| Li et al. [19] | 2008 | 41 | 1 | 5 | 7 | 41 | 1 | 5 | 7 | NR | NR | NR | NR | 21 | 1 | 2 | 4 | 10 | 0 | 2 | 1 | 10 | 0 | 1 | 2 |
|
| |||||||||||||||||||||||||
| Moon et al. [20] | 2008 | 10 | 0 | 7 | 0 | NR | NR | NR | NR | 10 | 0 | 7 | 0 | NR | NR | NR | NR | 10 | 0 | 7 | 0 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Lee et al. [21] | 2010 | 69 | 5 | 13 | 12 | NR | NR | NR | NR | 69 | 5 | 13 | 12 | 17 | 0 | 0 | 0 | NR | NR | NR | NR | 38 | 0 | 11 | 0 |
|
| |||||||||||||||||||||||||
| Alkhawaldeh et al. [22] | 2011 | 45 | 6 | 2 | 12 | NR | NR | NR | NR | 45 | 6 | 2 | 12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Kitamura et al. [23] | 2011 | 23 | 4 | 3 | 0 | NR | NR | NR | NR | 23 | 4 | 3 | 0 | NR | NR | NR | NR | 23 | 4 | 3 | 0 | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Ruys et al. [24] | 2011 | 50 | 0 | 23 | 0 | 50 | 0 | 23 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||||||||||||||||||||
| Yamada et al. [25] | 2012 | 47 | 4 | 7 | 0 | 47 | 4 | 7 | 0 | NR | NR | NR | NR | 16 | 3 | 0 | 0 | 14 | 0 | 4 | 0 | 17 | 1 | 3 | 0 |
|
| |||||||||||||||||||||||||
| Albazaz et al. [3] | 2013 | 48 | 1 | 14 | 0 | NR | NR | NR | NR | 48 | 1 | 14 | 0 | 36 | 0 | 3 | 0 | NR | NR | NR | NR | 12 | 1 | 11 | 0 |
|
| |||||||||||||||||||||||||
| Choi et al. [1] | 2013 | 27 | 1 | 7 | 4 | NR | NR | NR | NR | 27 | 1 | 7 | 4 | NR | NR | NR | NR | NR | NR | NR | NR | 27 | 1 | 7 | 4 |
|
| |||||||||||||||||||||||||
| Lee et al. [2] | 2013 | 51 | 0 | 10 | 0 | NR | NR | NR | NR | 51 | 0 | 10 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
NR: not reported; IH-CCa: intrahepatic cholangiocarcinoma; H-CCa: hilar cholangiocarcinoma; EH-CCa: extrahepatic cholangiocarcinoma; TP: true positive; FP: false positive; FN: false negative; TN: true negative.
Table 4.
Quality assessment of the included studies.
| First author/year | Spectrum of patients | Consecutive or random selection of patients | Reference standard | Application of reference standard regardless of indexed test | Enough explanation of the index test to ensure reproducibility | Independent blind comparison between index test and reference standard |
|---|---|---|---|---|---|---|
| Fritscher-Ravens, 2001 [6] | Patients with obstructive jaundice and hilar lesions | Yes | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Kluge, 2001 [7] | 26 patients with CCa, 8 patients with benign bile duct stenosis, and 20 controls | No | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Kato, 2002 [8] | Patients with bile duct cancer | N/A | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Kim, 2003 [9] | Patients with intrahepatic CCa | N/A | Histopathology or clinical and radiological findings | Yes | Yes | No blinding |
|
| ||||||
| Anderson, 2004 [10] | Patients suspected of CCa or gallbladder carcinoma | Yes | Histopathology or cytopathology | Yes | Yes | No information regarding blinding |
|
| ||||||
| Reinhardt, 2005 [11] | Patients with extrahepatic bile duct strictures on endoscopic retrograde cholangiography | Yes | Histopathology or cytopathology and imaging criteria and follow-up | No (patients with negative PET and cytology did not undergo surgery) | Yes | No |
|
| ||||||
| Wakabayashi, 2005 [12] | Patients with suspicious malignant biliary stricture | N/A | Histopathology (biopsy and surgical findings) | Yes | Yes | N/A |
|
| ||||||
| Petrowsky, 2006 [13] | Patients with suspected or proven CCa or gallbladder cancer | Yes | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Prytz, 2006 [14] | Patients with primary sclerosing cholangitis within 2 weeks after listing for liver transplantation and with no evidence of malignancy on CT, magnetic resonance imaging, or ultrasonography | Yes | Histology of explanted livers | Yes | Yes | Yes |
|
| ||||||
| Nishiyama, 2007 [15] | Patients with biliary stricture who underwent PET imaging | Yes | Histopathology or cytology | Yes | Yes | Yes |
|
| ||||||
| Corvera, 2008 [16] | Patients with clinical diagnosis of biliary tract cancers | N/A | Histopathology | Yes | Yes | No |
|
| ||||||
| Furukawa, 2008 [17] | Patients with suspected extrahepatic biliary cancers (bile duct dilatation and/or mass lesions detected by ultrasonography and CT) | N/A | Histopathology and follow-up | Yes | Yes | No (no blinding) |
|
| ||||||
| Kim, 2008 [18] | Patients with suspected CCa | Yes | Histopathology or follow-up | Yes | Yes | Yes |
|
| ||||||
| Li, 2008 [19] | Patients with clinically suspected or already established diagnosis of hilar CCa | N/A | Histopathology | Yes | Yes | N/A |
|
| ||||||
| Moon, 2008 [20] | Patients with suspected CCa | Yes | Histopathology, cytopathology, or follow-up | Yes | Yes | Yes |
|
| ||||||
| Lee, 2010 [21] | Patients with suspected CCa or gallbladder cancer | N/A | Histopathology or follow-up | Yes | Yes | Yes |
|
| ||||||
| Alkhawaldeh, 2011 [22] | Heterogeneous patients including patients with suspected CCa, patients with positive cytology for CCa, and patients with negative cytology | No | Histopathology or follow-up | Yes | Yes | N/A |
|
| ||||||
| Kitamura, 2011 [23] | Patients with extrahepatic bile duct cancer | Yes | Histopathology | Yes | Yes | No |
|
| ||||||
| Ruys, 2011 [24] | Patients highly suspected of hilar CCa | Yes | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Yamada, 2012 [25] | Patients with diagnosis of cancer of biliary tract | Yes | Histopathology | Yes | Yes | No |
|
| ||||||
| Albazaz, 2013 [3] | Patients with primary biliary tumors | Yes | Histopathology | Yes | Yes | Yes |
|
| ||||||
| Choi, 2013 [1] | Patients with suspected extrahepatic malignancy based on imaging studies | Yes | Histopathology or follow-up | Yes | Yes | Yes |
|
| ||||||
| Lee, 2013 [2] | Patients with confirmed biliary duct or gallbladder cancers | N/A | Histopathology or follow-up | Yes | Yes | N/A |
CCa: cholangiocarcinoma.
3.2. Qualitative Analysis (Systematic Review)
Using the database search, 23 original articles written over the past 12 years were selected [1–3, 6–25]. About the study design, 7 of these studies were prospective [6, 7, 13, 14, 17–19] and 12 were retrospective [1–3, 9, 12, 15, 16, 20–22, 24, 25] and in 4 articles this information was not provided [8, 10, 11, 23].
Ten studies used hybrid PET/CT [1–3, 11, 13, 18, 19, 21–23], whereas thirteen studies used PET only [6–10, 12, 14–17, 20, 24, 25]. Heterogeneous technical aspects between the included studies were found (Table 2). PET image analysis was performed by using qualitative criteria (visual analysis) in all the included studies [1–3, 6–25] and adjunctive semiquantitative criteria (based on the calculation of the standardized uptake value (SUV)) in 19 articles [1–3, 6–9, 11, 13, 15, 16, 18–25]. One study used quantitative criteria (based on blood sampling and the Gjedde-Patlak linearization procedure) [14].
The reference standard used to validate the 18F-FDG-PET or PET/CT findings in the included studies was quite different (Table 4). The results of the quality assessment of the studies included in this systematic review, according to the 2011 Oxford Center for Evidence-Based Medicine checklist for diagnostic studies, are shown in Table 4.
3.3. Quantitative Analysis (Meta-Analysis)
The diagnostic accuracy values of 18F-FDG-PET and PET/CT in the 23 studies included in the meta-analysis are presented in Figures 2–4. All the 23 studies had sufficient data to calculate the pooled sensitivity [1–3, 6–25], whereas only 13 studies [1, 7, 10–16, 18, 20–22] provided information about TN and FP lesions, thus allowing assessment of pooled specificity.
Figure 2.
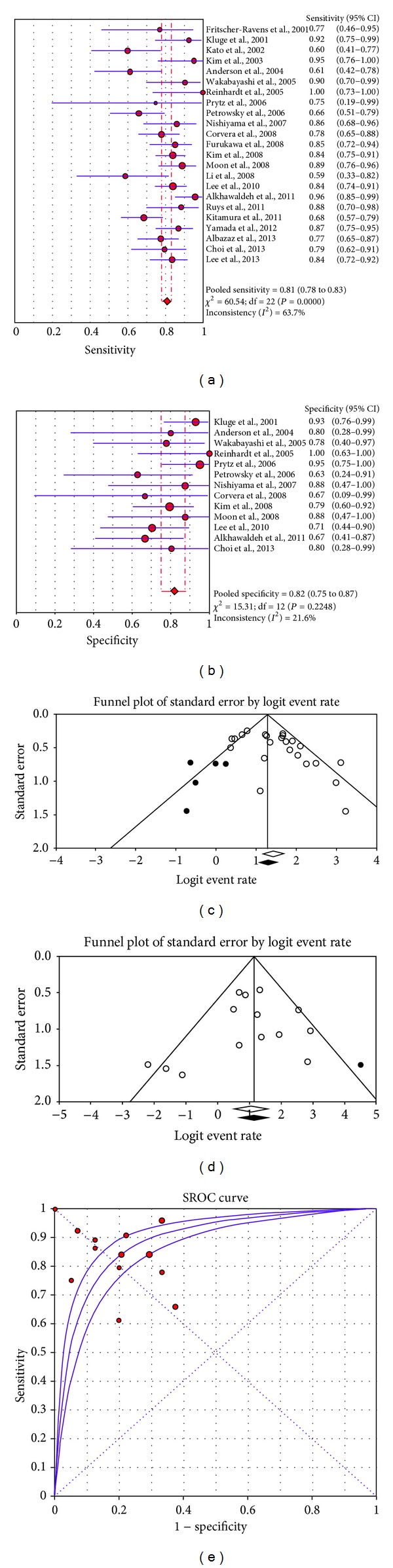
Plots of pooled sensitivity (a) and specificity (b), publication bias analysis for sensitivity (c) and specificity (d), and summary ROC curve (e) of 18F-FDG-PET or PET/CT in primary cholangiocarcinoma.
Figure 4.
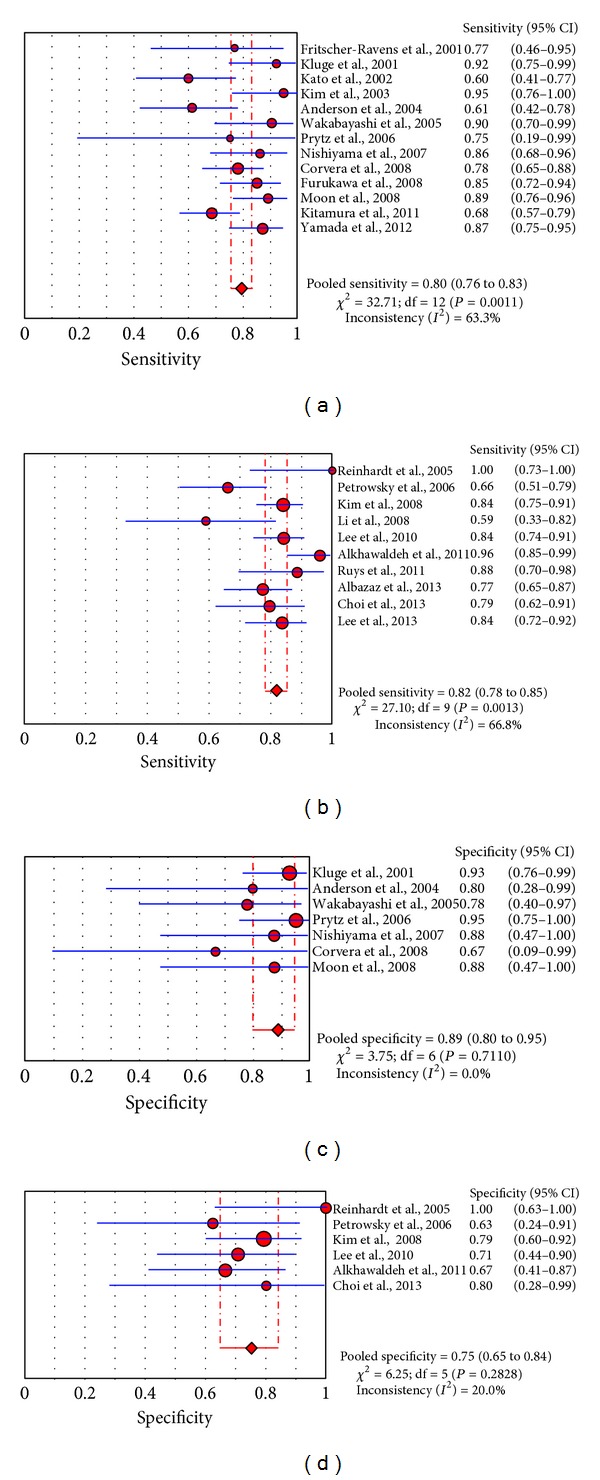
Plots of pooled sensitivity and specificity of 18F-FDG-PET ((a), (c)) or PET/CT ((b), (d)) in primary cholangiocarcinoma.
Sensitivity and specificity values of 18F-FDG-PET or PET/CT on a per patient-based analysis ranged from 59 to 100% and from 63 to 100%, with pooled estimates of 81% (95% CI: 78–83%) and 82% (95% CI: 75–87%), respectively. The area under the summary ROC curve was 0.89. The included studies showed statistical heterogeneity in their estimate of sensitivity (I 2: 63.7%).
Egger's regression intercepts for sensitivity and specificity pooling were 1.9 (95% CI: 0.3 to 3.5, P = 0.02) and −0.7 (95% CI: −2.4 to 0.9, P = 0.35), respectively. Applying Duval and Tweedie's method, the funnel plot of sensitivity and specificity reached symmetry and the adjusted sensitivity and specificity decreased 2.4% and increased 1.8%, respectively (Figure 2).
To reduce the heterogeneity, subgroup analyses considering the different device used (PET or PET/CT) were performed (Figure 4). In studies in which 18F-FDG-PET was used, values of sensitivity (thirteen eligible studies) and specificity (seven eligible studies) on a per patient-based analysis ranged from 60 to 95% and from 67 to 95%, respectively, with pooled estimates of 80% (95% CI: 76–83%) and 89% (95% CI: 80–95%), respectively. Statistical heterogeneity was found only in their estimate of sensitivity (I 2: 63%). The area under the ROC curve was 0.92.
In studies in which hybrid 18F-FDG-PET/CT was used, values of sensitivity (ten eligible studies) and specificity (six eligible studies) on a per patient-based analysis ranged from 59 to 100% and from 63 to 100%, respectively, with pooled estimates of 82% (95% CI: 78–85%) and 75% (95% CI: 65–84%), respectively. Statistical heterogeneity was found only in their estimate of sensitivity (I 2: 67%). The area under the ROC curve was 0.81.
Finally, subgroup analyses considering different anatomic sites of CCa (IH-CCa, EH-CCa, and H-CCa) were carried out (Figure 3). In patients with IH-CCa, values of sensitivity (nine eligible studies) and specificity (five eligible studies) on a per patient-based analysis ranged from 91 to 100% and from 80 to 100%, respectively, with pooled estimates of 95% (95% CI: 91–98%) and 83% (95% CI: 64–94%), respectively. No statistical heterogeneity was found, among the included studies, in both the estimate of sensitivity and the estimate of specificity (I 2: 0%). The area under the ROC curve was 0.95.
Figure 3.
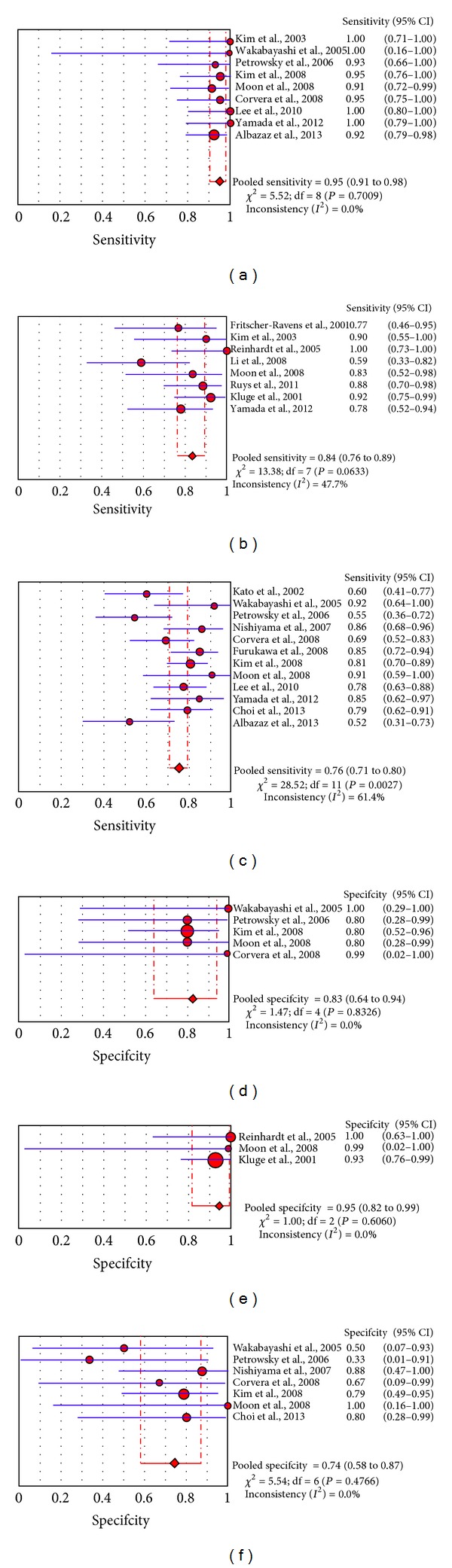
Plots of pooled sensitivity and specificity of 18F-FDG-PET or PET/CT in primary intrahepatic cholangiocarcinoma ((a), (d)), hilar cholangiocarcinoma ((b), (e)), and extrahepatic cholangiocarcinoma ((c), (f)).
In patients with EH-CCA, values of sensitivity (twelve eligible studies) and specificity (seven eligible studies) on a per patient-based analysis ranged from 52 to 92% and from 33 to 100%, respectively, with pooled estimates of 76% (95% CI: 71–80%) and 74% (95% CI: 58–87%), respectively. Statistical heterogeneity was found only in their estimate of sensitivity (I 2: 61%). The area under the ROC curve was 0.82.
In patients with H-CCA, values of sensitivity (eight eligible studies) and specificity (three eligible studies) on a per patient-based analysis ranged from 59 to 100% and from 93 to 100%, respectively, with pooled estimates of 84% (95% CI: 76–89%) and 95% (95% CI: 82–99%), respectively. No significant statistical heterogeneity was found in their estimate of sensitivity (I 2: 48%) and specificity (I 2: 0%). The area under the ROC curve was 0.98.
4. Discussion
To the best of our knowledge, this meta-analysis is the first to evaluate the diagnostic accuracy of 18F-FDG-PET or PET/CT in the evaluation of primary tumor in patients with CCa [26]. Several studies have used 18F-FDG-PET or PET/CT in this setting reporting different values of sensitivity and specificity. However, many of these studies have limited power, analyzing only relatively small numbers of patients. In order to derive more robust estimates of the diagnostic accuracy of 18F-FDG-PET or PET/CT in this setting we pooled published studies. A systematic review process was adopted in ascertaining studies, thereby avoiding selection bias.
Pooled results of our meta-analysis indicate that 18F-FDG-PET or PET/CT have a good sensitivity (81%) and specificity (82%) in the evaluation of primary tumor in patients with CCa. Furthermore, the value of the AUC (0.89) demonstrates that 18F-FDG-PET or PET/CT are accurate diagnostic methods in this setting. Considering patients with all anatomical localizations of primary CCa, independently of the device used (PET or PET/CT), significant heterogeneity between the studies in their estimate of sensitivity was found (I 2: 63.7%). In order to reduce possible source of heterogeneity, subgroup analyses considering different device used (PET or PET/CT) and patients with different anatomical localizations (IH-, H-, and EH-CCa) were performed.
These subgroup analyses provide differences in the diagnostic accuracy data for various anatomical localizations. 18F-FDG-PET and PET/CT seem to be more sensitive and specific in the evaluation of primary tumor in patients with IH-CCA than in patients with H-CCA and EH-CCA.
In particular 18F-FDG-PET and PET/CT have a moderate diagnostic accuracy in evaluating primary EH-CCa (sensitivity of 76% and specificity of 74%). In this setting, sensitivity and specificity of 18F-FDG-PET and PET/CT may be affected by FN (due to the confounding anatomical localization of extrahepatic bile ducts) and FP (due to inflammation of extrahepatic bile ducts). Larger use of hybrid PET/CT and, consequently, further studies about the role of PET/CT in evaluation of primary tumour in patients with EH-CCA may improve these results.
Conversely, the diagnostic accuracy of 18F-FDG-PET and PET/CT in primary IH-CCA (sensitivity of 95% and specificity of 83%) seems to be better than in the other anatomical localizations of primary CCa. Possible explanations are the easier individuation of illness in the liver parenchyma and the small number of FP cases (intrahepatic noncancerous disease positive with 18F-FDG-PET). Further studies are needed to evaluate if different histological types of IH-CCA (nodular or mass-forming type, infiltrating type, and intraluminal type) could cause different diagnostic accuracy of 18F-FDG-PET and PET/CT in this setting.
Finally, the diagnostic accuracy of 18F-FDG-PET and PET/CT in evaluating primary H-CCa is good (sensitivity of 84% and specificity of 95%). Nevertheless, we cannot exclude that the low number of the included studies in this subgroup analysis may have influenced the results. FP findings (due to the presence of 18F-FDG-avid lymph nodes in the hepatic hilum) and FN results (due to the difficult anatomical localization of the hepatic hilum) should be considered. More studies are needed to further evaluate sensitivity and specificity of 18F-FDG-PET and PET/CT in primary H-CCa.
However, performing these subgroup analyses has been useful in demonstrating that the anatomical localization of primary tumor (IH-CCa, EH-CCa, or H-CCA) is a source of heterogeneity among the studies. In fact, no significant heterogeneity was found in the subgroup analyses performed, except in the calculation of pooled sensitivity of 18F-FDG-PET or PET/CT in primary EH-CCA.
Pooled sensitivity is similar in the subgroup analyses regarding different device used (80% for PET and 82% for PET/CT, resp.). Nevertheless, heterogeneity was found in these groups, in particular for the calculation of pooled sensitivity, suggesting that, beyond the device used, other factors (such as the anatomical localization of the primary CCa) seem to be a stronger source of heterogeneity. PET alone seems to be more specific than PET/CT (89% and 75%, resp.). A possible explanation of these surprising findings could be the higher number of patients with primary EH-CCa included in the studies which performed PET/CT compared to those which performed PET only.
Finally, regarding the diagnostic workup of patients with CCa, 18F-FDG-PET and PET/CT may have little diagnostic advantage over traditional imaging modalities in detecting the primary CCA [3]. 18F-FDG-PET and PET/CT can be complementary to CT and MR in the diagnosing and staging of CCA [20]. Since 18F-FDG-PET imaging is a whole-body scanning technique, it allows detection of unsuspected metastatic lymph nodes or distant spread that may lead to major changes in the surgical management of patients with biliary tract cancer [25]. Nevertheless, the diagnostic performance of 18FDG-PET or PET/CT in detecting metastatic lymph nodes or distant spread was not object of our analysis.
This study has several limitations. Different anatomical classifications of CCa were used by several studies. For example, it is likely that some H-CCa were classified as EH-CCa by some studies. Other possible limitations of our meta-analysis could be the heterogeneity between the included studies (nevertheless subgroup analyses were performed to reduce the heterogeneity) and the possible publication bias. We assessed publication bias in our meta-analysis using qualitative and quantitative methods (Egger's regression and Duval and Tweedie's method). Funnel plots showed the importance of possible publication bias in particular for the estimation of pooled sensitivity (Figure 2).
Overall, 18F-FDG-PET and PET/CT were demonstrated to be accurate noninvasive tools in the evaluation of primary tumors in patients with CCa. Furthermore, more studies in patients with H-CCa and cost-effectiveness analyses of the role of 18F-FDG-PET or PET/CT in this setting are needed.
5. Conclusions
18F-FDG-PET and PET/CT were demonstrated to be accurate diagnostic imaging methods in the evaluation of primary tumors in patients with CCa. These tools seem to have a better diagnostic accuracy in the evaluation of primary IH-CCa compared to EH-CCa. Further studies are needed to evaluate the accuracy of 18F-FDG-PET and PET/CT in assessing primary H-CCa.
Acknowledgment
This manuscript was written by using ABREOC funding from the Oncology Institute of Southern Switzerland.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Choi EK, IeR Y, Kim SH, et al. The clinical value of dual-time point 18F-FDG PET/CT for differentiating extrahepatic cholangiocarcinoma from benign disease. Clinical Nuclear Medicine. 2013;38:e106–e111. doi: 10.1097/RLU.0b013e318266f402. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Kim HJ, Yim SH, et al. Primary tumor maximum standardized uptake value measured on 18F-fluorodeoxyglucose positron emission tomography-computed tomography is a prognostic value for survival in bile duct and gallbladder cancer. The Korean Journal of Gastroenterology. 2013;62:227–233. doi: 10.4166/kjg.2013.62.4.227. [DOI] [PubMed] [Google Scholar]
- 3.Albazaz R, Patel CN, Chowdhury FU, Scarsbrook AF. Clinical impact of FDG PET-CT on management decisions for patients with primary biliary tumours. Insights Imaging. 2013;4:691–700. doi: 10.1007/s13244-013-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treglia G, Cason E, Fagioli G. Recent applications of nuclear medicine in diagnostics (first part) Italian Journal of Medicine. 2010;4(2):84–91. [Google Scholar]
- 5.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritscher-Ravens A, Bohuslavizki KH, Broering DC, et al. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nuclear Medicine Communications. 2001;22(12):1277–1285. doi: 10.1097/00006231-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kluge R, Schmidt F, Caca K, et al. Positron emission tomography with [18F]fluoro-2-deoxyd-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33(5):1029–1035. doi: 10.1053/jhep.2001.23912. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Tsukamoto E, Kuge Y, et al. Clinical role of 18F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. European Journal of Nuclear Medicine. 2002;29(8):1047–1054. doi: 10.1007/s00259-002-0852-z. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y-J, Yun M, Lee WJ, Kim KS, Lee JD. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30(11):1467–1472. doi: 10.1007/s00259-003-1297-8. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. Journal of Gastrointestinal Surgery. 2004;8(1):90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt MJ, Strunk H, Gerhardt T, et al. Detection of Klatskin’s tumor in extrahepatic bile duct strictures using delayed 18F-FDG PET/CT: preliminary results for 22 patient studies. Journal of Nuclear Medicine. 2005;46(7):1158–1163. [PubMed] [Google Scholar]
- 12.Wakabayashi H, Akamoto S, Yachida S, et al. Significance of fluorodeoxyglucose PET imaging in the diagnosis of malignancies in patients with biliary stricture. European Journal of Surgical Oncology. 2005;31(10):1175–1179. doi: 10.1016/j.ejso.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Petrowsky H, Wildbrett P, Husarik DB, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. Journal of Hepatology. 2006;45(1):43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Prytz H, Keiding S, Björnsson E, et al. Dynamic FDG-PET is useful for detection of cholangiocarcinoma in patients with PSC listed for liver transplantation. Hepatology. 2006;44(6):1572–1580. doi: 10.1002/hep.21433. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama Y, Yamamoto Y, Kimura N, et al. Comparison of early and delayed FDG PET for evaluation of biliary stricture. Nuclear Medicine Communications. 2007;28(12):914–919. doi: 10.1097/MNM.0b013e3282f1ac85. [DOI] [PubMed] [Google Scholar]
- 16.Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. Journal of the American College of Surgeons. 2008;206(1):57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa H, Ikuma H, Asakura-Yokoe K, Uesaka K. Preoperative staging of biliary carcinoma using 18F-fluorodeoxyglucose PET: prospective comparison with PET+CT, MDCT and histopathology. European Radiology. 2008;18(12):2841–2847. doi: 10.1007/s00330-008-1062-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Kim M-H, Lee TY, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. American Journal of Gastroenterology. 2008;103(5):1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Kuehl H, Grabellus F, et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. Journal of Surgical Oncology. 2008;98(6):438–443. doi: 10.1002/jso.21136. [DOI] [PubMed] [Google Scholar]
- 20.Moon CM, Bang S, Chung JB, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. Journal of Gastroenterology and Hepatology. 2008;23(5):759–765. doi: 10.1111/j.1440-1746.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Kim HJ, Park JH, et al. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. Journal of Gastroenterology. 2010;45(5):560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 22.Alkhawaldeh K, Faltten S, Biersack H-J, Ezziddin S. The value of F-18 FDG PET in patients with primary sclerosing cholangitis and cholangiocarcinoma using visual and semiquantitative analysis. Clinical Nuclear Medicine. 2011;36(10):879–883. doi: 10.1097/RLU.0b013e3182291a64. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura K, Hatano E, Higashi T, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with extrahepatic bile duct cancer. Journal of Hepato-Biliary-Pancreatic Sciences. 2011;18(1):39–46. doi: 10.1007/s00534-010-0293-1. [DOI] [PubMed] [Google Scholar]
- 24.Ruys AT, Bennink RJ, Van Westreenen HL, et al. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB. 2011;13(4):256–262. doi: 10.1111/j.1477-2574.2010.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada I, Ajiki T, Ueno K, et al. Feasibility of (18)F-fluorodeoxyglucose positron-emission tomography for preoperative evaluation of biliary tract cancer. Anticancer Research. 2012;32:5105–5110. [PubMed] [Google Scholar]
- 26.Treglia G, Sadeghi R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: the state of the art. Clinical and Translational Imaging. 2013;1:73–75. [Google Scholar]


