Abstract
Background
Several studies have described increased oxidative stress (OxS) parameters and imbalance of antioxidant enzymes in Bipolar Disorder (BD) but few is know about the impact of treatment at these targets. However, no study has evaluated OxS parameters in unmedicated early stage BD and their association with lithium treatment in bipolar depression.
Methods
Patients with BD I or II (n = 29) in a depressive episode were treated for 6 weeks with lithium. Plasma samples were collected at baseline and endpoint, and were also compared to age-matched controls (n = 28). The thiobarbituric acid reactive substances (TBARS), and the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were measured.
Results
Subjects with BD depression at baseline presented a significant increase in CAT (p = 0.005) and GPx (p < 0.001) levels, with lower SOD/CAT ratio (p = 0.001) and no changes on SOD or TBARS compared to healthy controls. Regarding therapeutics, lithium only induced a decrease in TBARS (p = 0.023) and SOD (p = 0.029) levels, especially in BDII. Finally, TBARS levels were significantly lower at endpoint in lithium responders compared to non-responders (p = 0.018) with no difference in any biomarker regarding remission.
Conclusion
The present findings suggest a reactive increase in antioxidant enzymes levels during depressive episodes in early stage BD with minimal prior treatment. Also, decreased lipid peroxidation (TBARS) levels were observed, associated with lithium’s clinical efficacy. Overall, these results reinforce the role for altered oxidative stress in the pathophysiology of BD and the presence of antioxidant effects of lithium in the prevention of illness progression and clinical efficacy.
Keywords: Oxidative stress, Lithium, Bipolar Disorder, Depression, Neuroprotection
1. Introduction
It has been proposed a progressive course of Bipolar Disorder (BD) associated with longer illness duration, cognitive decline, decreased functioning and impaired cellular resilience leading to deleterious consequences on signal transduction and synaptic plasticity (Machado-Vieira et al., 2013). Thus, intervention in the early stage of BD may be a valuable tool to improve the course of the illness and provide a better prognosis (Berk et al., 2013).
Increased Oxidative Stress (OxS) generates deleterious consequences on signal transduction, synaptic plasticity, and cellular resilience, especially by inducing lipid peroxidation in membranes, proteins and DNA (Grintzalis et al., 2013; Mahadik et al., 2001; Soeiro-de-Souza et al., 2013). DNA damage, which can be induced by oxidative stress, has been found to be associated with the severity of depressive symptoms in BD (Andreazza et al., 2007b).
Thiobarbituric acid reactive substances (TBARS) levels are a direct index of cell lipid peroxidation whereas antioxidant system involves coordinated effects of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Reddy et al., 1991).
Imbalance in antioxidant enzymes has been reported in several studies of BD and is consistently associated with increased OxS (Andreazza et al., 2008). Only one study evaluated OxS parameters in BD patients with short duration of illness and found increased activity of antioxidant system in mania (Machado-Vieira et al., 2007). No study, however, has evaluated OxS in recent-onset BD patients in unmedicated depressive episodes.
Also, consistent evidences support the role of a subtle mitochondrial compromise in BD (Clay et al., 2011; Manji et al., 2012). Initially, magnetic resonance spectroscopy studies showed increased lactate levels in BD, suggesting a metabolic shift (reviewed in Stork and Renshaw, 2005). Post-mortem studies using brains of BD patients have shown decreased expression of mitochondrial electron transport chain genes (Konradi et al., 2004; Sun et al., 2006). Mitochondrial dysfunction increases production of reactive oxygen species, leading to enhanced OxS. OxS parameters have been found to be increased in BD post-mortem brains (Andreazza et al., 2010) and also in the peripheral blood of subjects with BD (Andreazza et al., 2008). Only one study evaluated subjects with BD during a depressive episode in a smaller sample, and found a slight increase in CAT levels compared to controls (Andreazza et al., 2007a).
Evidence suggests that the presence of OxS might be associated with the consistently found hyperactivation of the glutamatergic and dopaminergic systems in BD (Berk et al., 2011). Glutamatergic hyperactivity leads to increased calcium influx (Plein and Berk, 2001) which increases OxS (Shao et al., 2005). On the other hand, enhanced OxS has been suggested to increase glutamate (Lovell et al., 2000; Volterra et al., 1994). The excessive dopamine production increases OxS due to the production of reactive oxygen species in dopamine metabolism (Miyazaki and Asanuma, 2008). Moreover, the opposite mechanism also happens when OxS induces dopamine uptake, thus increasing dopamine activity (Kim and Andreazza, 2012) in a vicious cycle.
Other systems associated with BD are γ-Aminobutyric acid (Brambilla et al., 2003) and serotonergic systems (Fountoulakis et al., 2012), which are found to show decreased activity. Similar to other systems, OxS is associated with decreased γ-Aminobutyric acid release (Palmeira et al., 1993). Increased metabolism of serotonin, which decreases serotonergic function, is found to be associated with increased oxidative stress (Bianchi et al., 2005; Nocito et al., 2007). Consistent with these findings, evidences suggest that treatment with antidepressants can decrease OxS (Bilici et al., 2001).
Lithium is recommended as a first-line treatment for bipolar depression (Haeberle et al., 2012; Yatham et al., 2013) and it has shown several neuroprotective and neurotrophic actions (Machado-Vieira et al., 2009). Lithium has been shown to increase brain-derived neurotrophic factor (BDNF) (de Sousa et al., 2011), regulate intracellular Ca+2 (Wasserman et al., 2004), activate CREB (Ozaki and Chuang, 1997), increase Akt (Yazlovitskaya et al., 2006), and inhibit apoptotic caspase-3 (Ghribi et al., 2002). Similarly, lithium has been shown to protect against glutamatergic excitotoxicity (Nonaka et al., 1998). This agent has shown to decrease OxS in preclinical models (Schäfer et al., 2004), in bipolar mania (Machado-Vieira et al., 2007) and healthy volunteers (Khairova et al., 2012). Nonetheless, the effects of lithium on OxS parameters specifically in bipolar depression have never been studied.
The present study evaluated OxS parameters (TBARS, SOD, CAT and GPx) in unmedicated bipolar depression versus controls as well as the potential antioxidant effects of lithium in a therapeutically relevant paradigm. Our hypothesis was that subjects in a bipolar depression episode would present increased OxS (TBARS) and imbalance of antioxidant enzymes during depressive episodes and that lithium would lower OxS levels and enhance antioxidant enzymes levels.
2. Methods
2.1. Subjects
Subjects were evaluated between August 2010 and June 2012 at the Institute of Psychiatry, University of Sao Paulo, Brazil. Twenty-nine patients, 21 (72.4%) women, with 28.4 (±5.5) years of age and diagnosis of BD I (38%) or BD II (62%) in a depressive episode were included, as diagnosed by the Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID) (First et al., 1995). Patients who had a score ≥18 on the 21-item Hamilton Depression Scale (HAM-D) (HAMILTON, 1960) were eligible for the study. Also, 26 (89.6%) patients were drug-free for at least 6 weeks prior to their enrollment and 21 (72.4%) subjects were treatment-naïve at baseline. Exclusion criteria included presence of chronic medical illness, comorbid substance abuse or dependence in the past year, rapid cycling in the past 12 months, previous head trauma, current major axis I psychiatric disorder, subjects submitted to electro-convulsive therapy and current significant abnormal laboratory tests.
The comparison group was constituted by 28 age-matched healthy controls (within 3 years of difference of BD patients); these 16 men (57.1%) and 12 women (42.8%) (age = 28.0 ± 7.2) were recruited through advertisement in the local community. Controls were excluded if they had lifetime history of any mental disorder (by SCID), including substance abuse or dependence, or if they had any disease with central nervous system involvement or any first-degree relative with a mental disorder. This study was approved by the local institutional review board, and all participants provided written informed consent before study entry.
2.2. Study design
Patients had blood samples collected at baseline and at endpoint (week 6), while healthy controls had only one-point sample collection. At baseline, patients were started on open-label lithium carbonate at 450 mg/day, with flexible doses increase according to clinical improvement, controlling plasma lithium levels to ensure compliance and avoid toxicity (<1.2 mEq/L).
Most patients were on lithium monotherapy, although hypnotic (benzodiazepine or zolpidem) use as needed was allowed and 4 patients were also in use of antipsychotics or mood stabilizer. Psychometric assessments were made at baseline and on week 1, week 2, week 4, and week 6 (endpoint). Assessment of symptoms was performed with the HAM-D, Young Mania Rating Scale (YMRS) (Young et al., 1978), and Clinical Global Impression. Clinical response was defined as a decrease of 50% or more in the HAM-D at endpoint and remission as HAM-D < 8 and YMRS < 8 at endpoint.
2.3. Assays
Blood samples were collected from 8:00 to 10:00 AM using vacutainer tubes. All subjects were in 8-h fasting. Samples were centrifuged at 20 °C and 1620 ≥ g for 15 min. Plasma was obtained, frozen, and stored at −80 °C. Given the complexity of the study, not all the patients and controls had samples available to be included in all analyses. All samples were assessed in duplicate. TBARS levels (malondialdehyde – thiobarbituric acid adduct) and SOD, CAT, and GPx activities were determined using spectrophotometry according to commercially available kits from Cayman Chemical Company®. Since SOD and CAT act sequentially, the results are also expressed as SOD/CAT ratio. CAT and GPx levels are presented as nM/min/mL, SOD as U/mL and TBARS as nM/mL.
2.4. Statistics
Student’s t test and Mann–Whitney test were used for intragroup comparisons with normal and non-normal distributions of variables, respectively. Changes in OxS measures and enzyme activities before and after lithium treatment in the BD group were compared using paired student’s t test and Wilcoxon signed ranks test. Kruskal–Wallis and ANOVA were used to compare two subgroups of patients with controls. Significance level was set at <0.05 (two-tailed). Statistical analysis was performed using the SPSS 14.0 and last observation carried forward was used in one patient who discontinued treatment.
3. Results
3.1. Clinical and demographical data
Demographic and clinical data are summarized in Table 1; patients and controls showed similar age, but a trend for different gender distribution (p = 0.05). Patients had a significant decrease in depressive symptoms measured by HAM-D from baseline (22.5 ± 3.5) to endpoint (7.3 ± 5.9) (z = −4.68, p < 0.001). Twenty-five (86.2%) patients responded to treatment and 18 (62.1%) achieved symptomatic remission at week 6. Mean duration of illness was 3.0 years (±1.6).
Table 1.
Demographic and clinical characteristics of bipolar disorder patients and healthy controls.
| Controls (n = 28) | Bipolar (n = 29) | p | |
|---|---|---|---|
| Gender | |||
| Male/Female, n (%) | 16 (57.1)/12 (42.9) | 8 (27.6)/21 (72.4) | 0.05*a |
| Age, years | 28.0 (±7.2) | 28.4 (±5.5) | 0.60b |
| Bipolar Disorder type | |||
| Type I/Type II, n (%) | 11 (37.9)/18 (62.1) | ||
| Mood Phase | |||
| Depressive/mixed | 27 (93.1)/2 (6.9) | ||
| Duration of illness, years | 3.0 (±1.6) | ||
| Drug-naïve, n (%) | 21 (72.4) | ||
| Drug-free, n (%) | 26 (89.6) | ||
| History of psychosis, n (%) | 4 (13.8) | ||
| HAM-D | |||
| Baseline/endpoint | 22.6 (±3.6)/7.3 (±5.9) | ||
| YMRS | |||
| Baseline/endpoint | 6.1 (±5.6)/3.8 (±8.6) | ||
| Response, n (%) | 25 (86.2) | ||
| Remission, n (%) | 18 (62.1) | ||
| Dropout, n (%) | 1 (3.4) | ||
| Endpoint serum lithium, mEq/L |
0.49 (±0.20) |
HAM-D – Hamilton Depression Scale, YMRS – Young Mania Rating Scale.
Significantly different.
Chi-square,
Student’s t test.
3.2. Antioxidant enzymes are imbalanced in drug-free bipolar depression compared to controls
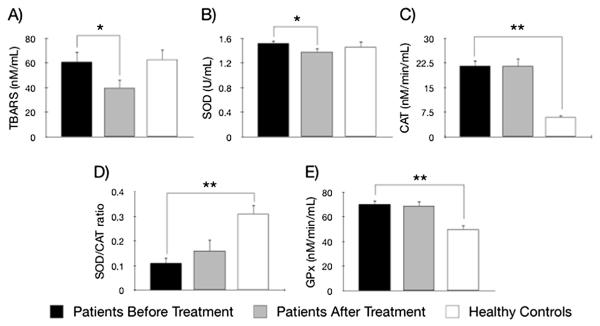
TBARS levels in BD patients at baseline (n = 29) and controls (n = 22) were not different (p = 0.95) (Fig. 1A) (Table 2). Baseline SOD levels in BD patients (n = 25) and controls (n = 28) were similar (p = 0.56) (Fig. 1B). CAT was increased in BD patients (n = 29) in comparison to controls (n = 22) (p = 0.005) (Fig. 1C). SOD/CAT ratio (n = 25) in bipolar depression = was decreased compared to controls (n = 22) (p = 0.001) (Fig. 1D). Finally, baseline GPx in subjects with BD (n = 25) was increased in comparison to controls (n = 27) (t = 4.19, p < 0.001) (Fig. 1E).
Fig. 1.
OxS parameters in patients with bipolar disorder in a depressive episode before (black bar) and after lithium treatment (grey bar) compared to healthy controls (white bar): A) TBARS– Thiobarbituric Acid Reactive Substances; B) SOD – Superoxide Dismutase; C) CAT – Catalase; D) SOD/CAT ratio, and E) GPx – Glutathione Peroxidase; *p < 0.05, **p < 0.01.
Table 2.
OxS parameters in bipolar disorder patients in a depressive episode before and after lithium treatment compared to healthy controls.
| Before treatment (n = 29) | After treatment (n = 28) | Healthy controls (n = 22) | Patients vs. controls p | Before vs. after treatment p | |
|---|---|---|---|---|---|
| TBARS (nM/mL) | 60.77 ± 45.14 | 37.88 ± 35.85 | 62.74 ± 37.58 | 0.95 | 0.023* |
| SOD (U/mL) | 1.52 ± 0.23 | 1.38 ± 0.30 | 1.46 ± 0.45 | 0.56 | 0.029* |
| CAT (nM/min/mL) | 21.59 ± 9.49 | 22.17 ± 12.70 | 5.96 ± 2.67 | 0.005** | 0.85 |
| SOD/CAT ratio | 0.11 ± 0.11 | 0.15 ± 0.22 | 0.31 ± 0.17 | 0.001** | 0.48 |
| GPx (nM/min/mL) | 70.32 ± 15.62 | 68.76 ± 19.53 | 49.91 ± 19.10 | <0.001** | 0.64 |
TBARS – Thiobarbituric Acid Reactive Substances, SOD – Superoxide Dismutase, CAT – Catalase, GPx – Glutathione Peroxidase,
p < 0.05,
p < 0.01.
Since the BD and control groups had a trend for unbalance in gender, we compared OxS parameters of males versus females in patients and then in controls to assure that the gender difference did not influence the results. After analyses (data not shown), the effects of gender difference in the sample did not influence in the results.
3.3. Lithium treatment decreases TBARS and SOD in bipolar depression
There was a significant decrease in TBARS from baseline to endpoint (n = 28, p = 0.023) (Fig. 1A) (Table 2). SOD activity decreased after lithium treatment (n = 24, p = 0.029) (Fig. 1B), while CAT levels did not show difference (n = 28, p = 0.85) (Fig. 1C). SOD/CAT ratio at endpoint showed no difference from baseline (n = 24, p = 0.48) (Fig. 1D). GPx levels were not different from baseline after lithium treatment (n = 24, t = 0.47, p = 0.64) (Fig. 1E).
3.4. TBARS at endpoint was decreased in responders compared to non-responders at endpoint
At endpoint, TBARS was decreased in responders (31.4 ± 32.9) compared to non-responders (76.7 ≥ 30.2) (p = 0.018) (Fig. 2A), although endpoint TBARS values from remitters (n = 18, 30.5 ± 32.7) and non-remitters (n = 10, 51.2 ± 39.1) showed significant difference (p = 0.14). There was no association between any other biological measure with response or remission (data not shown). Finally, lithium levels showed no association with any marker at endpoint (data not shown).
Fig. 2.
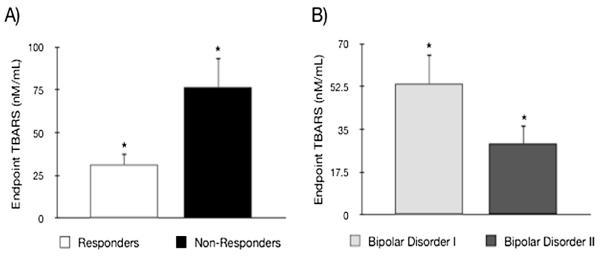
A) Endpoint thiobarbituric acid reactive substances (TBARS) levels in responders compared with non-responders to lithium treatment. B) Endpoint TBARS levels in Bipolar Disorder I compared to Bipolar Disorder II; *p < 0.05.
3.5. BD II but not BD I patients showed decrease in TBARS after lithium treatment
A selective decrease of TBARS in BD II (58.63 ± 46.23 nM/mL) to endpoint (29.14 ± 31.86 nM/mL) was observed (n = 18, p = 0.039), whereas in BD I, TBARS showed no alteration from baseline (67.86 ± 46.06 nM/mL) to endpoint (53.62 ± 38.87 nM/mL) (n = 10, t = 0.71, p = 0.50). Endpoint TBARS in BD II was significantly decreased compared to endpoint TBARS in BD I (p = 0.04) (Fig. 2B). Other OxS parameters were not associated with BD subtype (data not shown).
4. Discussion
The present findings showed an imbalance of antioxidant enzymes in bipolar depression, with increased CAT and GPx and decreased SOD/CAT ratio in recent-onset drug-free subjects compared to healthy controls. Also, lithium significantly decreased plasma TBARS and SOD activity after 6 weeks of lithium treatment; importantly, TBARS levels at endpoint were associated with clinical response. TBARS decrease was only relevant in subjects with BD II. A number of different factors might contribute to the heterogeneity of results observed in the literature of OxS enzymes in BD (Kuloglu et al., 2002; Montero et al., 2012). Nevertheless, it has been suggested that enzymes imbalance is more associated with overall changes on OxS parameters than a specific alteration affecting a single protein (Andrades et al., 2005; Andreazza et al., 2007a).
We found increased CAT and GPx, and decreased SOD/CAT ratio in bipolar depression, which is consistent with imbalance and increased OxS found in other studies. Previously, our group found increased CAT activity in BD patients during drug naïve mania (Machado-Vieira et al., 2007). Only one study evaluated BD patients during a depressive episode (Andreazza et al., 2007a), but subjects were under diverse treatments and compared with other mood states. Other analyses comprised patients without depression or samples including symptomatic and asymptomatic patients and found decreased CAT levels (Andreazza et al., 2007a; Ozcan et al., 2004; Raffa et al., 2012; Ranjekar et al., 2003) or unaltered (Fontoura et al., 2012) in BD versus healthy subjects. Also, our finding of increased GPx levels in bipolar depression is in line with Andreazza et al. (2007a), while other studies did not find a significant difference in different mood states (Abdalla et al., 1986; Andreazza et al., 2009; Kuloglu et al., 2002; Raffa et al., 2012; Ranjekar et al., 2003). In spite of the fact that imbalance of anti-oxidant enzymes is an indicative of OxS present across mania, depression, and euthymia, BD studies do not suggest a specific antioxidant enzyme alteration associated with any mood phase.
Although the mechanisms underlying CAT and GPx balance are unclear, increases in CAT and GPx found here in early stage BD are compatible with a compensatory mechanism, proposed to be present in early stages of BD (Berk et al., 2013). Loss of neurotrophic support, increased oxidative stress and inflammation are associated with illness progression in BD (Berk et al., 2013). Oxidative stress and loss of neurotrophic support play key roles in the development of several neuropsychiatric disorders, including BD. Oxidative stress can down-regulate neurotrophic factors, while neurotrophic factors promote the expression of antioxidant proteins, also limiting inflammation.
The increases in CAT and GPx found in our study might explain the unaltered TBARS values found in our BD patients at baseline, in contrast with increased TBARS in most BD studies (Andreazza et al., 2008). Increases in CAT were also found in early stage BD during mania (Machado-Vieira et al., 2007). Importantly, CAT has shown efficacy against dopamine-induced OxS in mitochondria (isolated from) rat brain (Berman and Hastings, 1999) and in one study in SH-SY5Y neuroblastoma human cells (Lai and Yu, 1997) although not in another study performed in the same cells (Emdadul Haque et al., 2003). Moreover, an elevation in GPx activity was the response to oxygen reactive species exposure in mice (Esposito et al., 1999). Glutathione, which antioxidant reactions are catalyzed by the enzyme GPx, was protective from dopamine-induced OxS in vitro (Kuhn et al., 1999), in SH-SY5Y neuroblastoma human cells (Emdadul Haque et al., 2003; Kuhn et al., 1999), and in rat striatum (Hastings et al., 1996). Regarding the glutamatergic system, glutathione decreased glutamatergic activity through reducing glutamate binding to glutamate receptors (Ogita et al., 1986; Varga et al., 1997). Also, our results showing decreased SOD/CAT ratio in BD patients relative to controls might be seen as compensatory, since decreased SOD/CAT ratio as well as increased CAT and GPx favors scavenge of the reactive oxygen species H2O2 (Gsell et al., 1995; Khairova et al., 2012). The role of CAT and GPx as markers of early stage bipolar depression warrants further investigation.
Regarding the effects of lithium on OxS parameters, it was observed a clinically relevant decrease in lipid peroxidation (TBARS) levels. Our findings are in line with previous studies showing lower TBARS levels in lithium treated BD after mania (Machado-Vieira et al., 2007) and euthymia (Banerjee et al., 2012). In the study of Banerjee et al. (2012), lithium’s antioxidant effect was correlated with the activity of Na+-K+-ATPase enzyme, pointing to a possible antioxidant mechanism of the drug. Thus, our results provide further support to the idea that lithium possesses an antioxidant effect.
Importantly, the response to lithium treatment was associated with decrease in TBARS levels. This finding reinforces the presence of an association of central and peripheral levels of OxS markers, which also was previously suggested by studies in acute neurological disorders such as stroke (Polidori et al., 1998). The decrease in TBARS levels in responders compared to non-responders warrants further research to confirm TBARS as a marker of treatment efficacy.
Interestingly, TBARS decreased after lithium treatment only in subjects with BD II. Although BD I and BD II are associated with different clinical course and response to treatment, neurobiological differences between the two types of BD remain understudied (D’Addario et al., 2012; Huang et al., 2012). Lithium treatment also decreased SOD activity in our sample. A decrease in SOD was also observed in healthy subjects taking lithium (Khairova et al., 2012), reinforcing that its antioxidant profile may involve SOD activity, independently of disorder and treatment outcome. Since SOD catalyzes a dismutation reaction that converts superoxide in hydrogen peroxide (Liochev and Fridovich, 1999), SOD decrease could potentially lower hydrogen peroxide formation.
The study is limited by the lack of a randomized double-blind design, also lacking a second point blood collection for controls, gender unbalance between BD and control groups, and the relatively small sample size. Also, the absence of patients studied during mania and euthymia in the sample may limit the generalization of the findings to all phases of BD, thus not addressing the specificity of our findings for bipolar depression. The younger age, short length of illness and minimal prior treatment (most drug-free/naïve patients) are major strengths of this investigation and also a novelty in OxS research in BD during depression.
Overall, the present findings suggest a compensatory elevation in antioxidant enzymes CAT and GPx during depressive episodes in early stage of BD, with a decrease in lipid peroxidation (TBARS) associated with lithium’s antidepressant actions. The present results reinforce the role for altered oxidative stress in the pathophysiology of BD and potential clinically relevant antioxidant effects of lithium in the prevention of illness progression.
Acknowledgments
This study was sponsored by Sao Paulo Research Foundation (Fapesp, Brazil). The Laboratory of Neuroscience is supported by the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Footnotes
Conflict of interest CAZ is listed as co-inventor on a patent for the use of ketamine in major depression and have assigned their patent rights on ketamine to the US government. The other authors report no conflict of interest.
Contributors All authors contributed to manuscript writing or data analysis, and agreed to submit the final version for publication.
Role of the funding source Sao Paulo Research Foundation (Fapesp) funded the study, but had no role in study design or analysis.
References
- Abdalla DS, Monteiro HP, Oliveira JA, Bechara EJ. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem. 1986;32:805–7. [PubMed] [Google Scholar]
- Andrades M, Ritter C, Moreira JC, Dal-Pizzol F. Oxidative parameters differences during non-lethal and lethal sepsis development. J Surg Res. 2005;125:68–72. doi: 10.1016/j.jss.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LM, Nardin P, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007a;41:523–9. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007b;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Gonçalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–71. [PMC free article] [PubMed] [Google Scholar]
- Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–8. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Banerjee U, Dasgupta A, Rout JK, Singh OP. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:56–61. doi: 10.1016/j.pnpbp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, et al. Stage managing bipolar disorder. Bipolar Disord. 2013 Jun 20; doi: 10.1111/bdi.12099. http://dx.doi.org/10.1111/bdi.12099. [Epub ahead of print] [DOI] [PubMed]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem. 1999;73:1127–37. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–37. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–24. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario C, Dell’Osso B, Palazzo MC, Benatti B, Lietti L, Cattaneo E, et al. Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacol. 2012;37:1647–55. doi: 10.1038/npp.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa RT, van de Bilt MT, Diniz BS, Ladeira RB, Portela LV, Souza DO, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494:54–6. doi: 10.1016/j.neulet.2011.02.054. [DOI] [PubMed] [Google Scholar]
- Emdadul Haque M, Asanuma M, Higashi Y, Miyazaki I, Tanaka K, Ogawa N. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim Biophys Acta. 2003;1619:39–52. doi: 10.1016/s0304-4165(02)00440-3. [DOI] [PubMed] [Google Scholar]
- Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–5. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. Biometrics Research Department, New York Psychiatric Institute; New York: 1995. Patient ed. [Google Scholar]
- Fontoura PC, Pinto VL, Matsuura C, Resende A e C, de Bem GF, Ferraz MR, et al. Defective nitric oxide-cyclic guanosine monophosphate signaling in patients with bipolar disorder: a potential role for platelet dysfunction. Psychosom Med. 2012;74:873–7. doi: 10.1097/PSY.0b013e3182689460. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Kelsoe JR, Akiskal H. Receptor targets for antidepressant therapy in bipolar disorder: an overview. J Affect Disord. 2012;138:222–38. doi: 10.1016/j.jad.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Herman MM, Spaulding NK, Savory J. Lithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activation. J Neurochem. 2002;82:137–45. doi: 10.1046/j.1471-4159.2002.00957.x. [DOI] [PubMed] [Google Scholar]
- Grintzalis K, Zisimopoulos D, Grune T, Weber D, Georgiou CD. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic Biol Med. 2013;59:27–35. doi: 10.1016/j.freeradbiomed.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Gsell W, Conrad R, Hickethier M, Sofic E, Frölich L, Wichart I, et al. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J Neurochem. 1995;64:1216–23. doi: 10.1046/j.1471-4159.1995.64031216.x. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle A, Greil W, Russmann S, Grohmann R. Mono- and combination drug therapies in hospitalized patients with bipolar depression. Data from the European drug surveillance program AMSP. BMC Psychiatry. 2012 Sep 21;12:153. doi: 10.1186/1471-244X-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chang YH, Lee SY, Chen SL, Chen SH, Chu CH, et al. The interaction between BDNF and DRD2 in bipolar II disorder but not in bipolar I disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:501–7. doi: 10.1002/ajmg.b.32055. [DOI] [PubMed] [Google Scholar]
- Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, et al. Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep. 2012;5:680–2. doi: 10.3892/mmr.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Andreazza AC. The relationship between oxidative stress and post-translational modification of the dopamine transporter in bipolar disorder. Expert Rev Neurother. 2012;12:849–59. doi: 10.1586/ern.12.64. [DOI] [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE, Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J Neurochem. 1999;73:1309–17. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- Lai CT, Yu PH. Dopamine- and L-beta-3,4-dihydroxyphenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol. 1997;53:363–72. doi: 10.1016/s0006-2952(96)00731-9. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. The relative importance of HO* and ONOO– in mediating the toxicity of O*–. Free Radic Biol Med. 1999;26:777–8. doi: 10.1016/s0891-5849(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radic Biol Med. 2000;29:714–20. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, da Silva Vargas R, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett. 2007;421:33–6. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl. 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J Biol Psychiatry. 2013 Sep 2; doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik SP, Evans D, Lal H. Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:463–93. doi: 10.1016/s0278-5846(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama. 2008;62:141–50. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev. 2012;13:441–55. doi: 10.1111/j.1467-789X.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterol. 2007;133:608–18. doi: 10.1053/j.gastro.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–7. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita K, Kitago T, Nakamuta H, Fukuda Y, Koida M, Ogawa Y, et al. Glutathione-induced inhibition of Na+-independent and -dependent bindings of L-[3H] glutamate in rat brain. Life Sci. 1986;39:2411–8. doi: 10.1016/0024-3205(86)90482-0. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem. 1997;69:2336–44. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Palmeira CM, Santos MS, Carvalho AP, Oliveira CR. Membrane lipid peroxidation induces changes in gamma-[3H]aminobutyric acid transport and calcium up-take by synaptosomes. Brain Res. 1993;609:117–23. doi: 10.1016/0006-8993(93)90863-i. [DOI] [PubMed] [Google Scholar]
- Plein H, Berk M. The platelet as a peripheral marker in psychiatric illness. Hum Psychopharmacol. 2001;16:229–36. doi: 10.1002/hup.251. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Frei B, Cherubini A, Nelles G, Rordorf G, Keaney JF, et al. Increased plasma levels of lipid hydroperoxides in patients with ischemic stroke. Free Radic Biol Med. 1998;25:561–7. doi: 10.1016/s0891-5849(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:371–5. doi: 10.1016/j.pnpbp.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Reddy R, Sahebarao MP, Mukherjee S, Murthy JN. Enzymes of the antioxidant defense system in chronic schizophrenic patients. Biol Psychiatry. 1991;30:409–12. doi: 10.1016/0006-3223(91)90298-z. [DOI] [PubMed] [Google Scholar]
- Schäfer M, Goodenough S, Moosmann B, Behl C. Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res. 2004;1005:84–9. doi: 10.1016/j.brainres.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–84. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol. 2013;16:1505–12. doi: 10.1017/S1461145713000047. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–19. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- Varga V, Jenei Z, Janáky R, Saransaari P, Oja SS. Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. Neurochem Res. 1997;22:1165–71. doi: 10.1023/a:1027377605054. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol Pharmacol. 1994;46:986–92. [PubMed] [Google Scholar]
- Wasserman MJ, Corson TW, Sibony D, Cooke RG, Parikh SV, Pennefather PS, et al. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacol. 2004;29:759–69. doi: 10.1038/sj.npp.1300400. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients withbipolar disorder: update 2013. Bipolar Disord. 2013 Feb;15(1):1–44. doi: 10.1111/bdi.12025. [DOI] [PubMed] [Google Scholar]
- Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–86. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]