Abstract
Purpose
The purpose of the present study was to identify abnormal areas of regional synchronization in patients with mesial temporal lobe epilepsy and hippocampus sclerosis (mTLE-HS) compared to healthy controls, by applying a relatively novel method, the Regional Homogeneity (ReHo) method to resting state fMRI(RS-fMRI) data.
Methods
Eyes closed RS-fMRI data were acquired from 10 mTLE-HS patients (4 right-side, 6 left-side) and 15 age and gender matched healthy subjects, and were analyzed by using ReHo. For group analysis, 4 right-side MTLE-HS patients’ functional images were flipped, so that a homogeneous left MTLE-HS group with 10 cases were made.
Key Findings
Compared to the healthy control group, patients showed significantly increased ReHo in ipsilateral parahippocampal gyrus, midbrain, insula, corpus callosum, bilateral sensorimotor cortex and fronto-parietal subcortical structures, while decreased ReHo was mainly observed in default model network (DMN) (including precuneous and posterior cingulate gyrus, bilateral inferior lateral parietal and mesial prefrontal cortex) and cerebellum in patients relative to the control group.
Significance
This study identified that ReHo pattern in mTLE-HS patients was altered compared to healthy controls. We consider decreased ReHo in DMN to be responsible for wide functional impairments in cognitive processes. We propose that the increased ReHo in specific regions may compose a network which might be responsible for seizure genesis and propagation.
Keywords: Regional Homogeneity, Resting state, fMRI, Epilepsy, Hippocampal sclerosis
1 Introduction
Epilepsy is a common neural disorder, characterized by hyper-synchronous neuronal activity as shown from electrophysiological recordings (Ortega, et al. 2008, Schevon, et al. 2007). Specific regions in the temporal cortex have been observed to undergo increased synchronization in patients with temporal lobe epilepsy, which is believed to be involved in the generation of interictal activity (Ortega, et al. 2008). Intracranial electroencephalograph (EEG) studies have demonstrated increased local coherence in mesial temporal lobe epilepsy (mTLE) (Ponten, et al. 2007). MTLE is the most common type of focal epilepsy in adults, which is frequently caused by hippocampal sclerosis (HS) (Berg 2008).
Currently, EEG is the most commonly used and basal approach for detecting epileptic activity. Although EEG has high temporal resolution and sensitivity, it lacks spatial resolution and is not sensitive to activity borne deep within the brain. With better spatial resolutions, non-invasive blood oxygenation level dependent functional magnetic resonance image (BOLD-fMRI) method has been widely used as an effective technique for epilepsy investigation (Detre 2006). Recently, simultaneous EEG-fMRI has been used in epilepsy study as it employs the advantages of both EEG and fMRI (Di Bonaventura, et al. 2006). In this method, simultaneous EEG provides time points of interictal epileptoform data for fMRI data analysis to explore activation and deactivation in epilepsy. However, the usage of simultaneous EEG-fMRI is limited, because of practical issues, such as high cost, complicated EEG data analysis, and time consuming preparation.
Resting-state fMRI (RS-fMRI), which was first reported by Biswal in 1995 (Biswal, et al. 1995), is widely used in brain research and its BOLD signal is believed to reflect spontaneous neuronal synchronization and endogenous neurophysiological process of the human brain (Fox and Raichle 2007). Several data-driven methods have been developed for RS-fMRI data analysis. For instance, independent component analysis (ICA) can separate the resting state network into subset of networks (Beckmann, et al. 2005, Smith, et al. 2009). Morgan used 2-dimensional temporal clustering analysis (2d-TCA) to investigate the alteration of BOLD signal in temporal lobe epilepsy and showed its potential capability to localize the seizure (Morgan, et al. 2007, Morgan, et al. 2010). The limitations of this method include extraneous regions that may not be involved in epilepsy, leading to uncertainty in the results (Khatamian, et al. 2011).
Another novel data-driven method, regional homogeneity (ReHo), explores regional brain activity during rest by examining the degree of regional coherence of fMRI time courses, and is able to measure the synchronization of activity in different brain regions (Zang, et al. 2004). This method has been successfully used to investigate the functional modulations in the resting state in the patients with attention-deficit hyperactivity disorder (ADHD), Alzheimer’s disease (AD), autism spectrum disorders(ASD), and Parkinson’s disease (PD), (Cao, et al. 2006, He, et al. 2007, Paakki, et al. 2010, Wu, et al. 2009). It has also been used in generalized tonic-clonic seizures in adults and non-lesion temporal lobe epilepsy in pediatric population, and successfully detected abnormal epileptic synchronization (Mankinen, et al. 2011, Zhong, et al. 2011). However, to the best of our knowledge, no study has observed the alteration of synchrony in mTLE adult patients with HS using ReHo method. The purpose of the present study is to identify the abnormal pattern of regional synchronization in mTLE-HS patients compared to healthy controls, by applying the ReHo method to resting state fMRI data, and to characterize the underlying potential network differences.
2 Materials and methods
2.1 Participants
2.1.1 Patients
Ten right-handed patients (age = 34.4 ± 10.6 years) observed to have unilateral mesial temporal lobe epilepsy with hippocampal sclerosis (mTLE-HS) participated in the present study. Of all the patients, 4 had right-sided mTLE (4 females), while 6 had left-sided mTLE (4 females). All patients were from University of Wisconsin Madison Hospital and were scanned using fMRI between August 2010 and August 2011. According to the classification of the International League Against Epilepsy (Berg, et al. 2010), the diagnosis of mTLE-HS was based on clinical, electroencephalographic and MRI findings. All patients underwent a comprehensive clinical evaluation with the following inclusion criteria:
All had one or more typical symptoms of mTLE. All patients had complex partial seizures. In addition some patients also had simple partial seizures and/or secondary generalized tonic-clonic seizures.
MRI manifestations of the hippocampal sclerosis (HS), unilateral hippocampal atrophy on T1 image with associated hyperintensity on T2 fluid attenuated inverted recovery image. There was no identifiable structural MRI abnormality other than the hippocampal sclerosis (HS) in the patients’ brain.
EEG findings, predominantly left or right-sided interictal epileptic discharges shown by scalp EEG.
2.1.2 Controls
Fifteen right-handed healthy subjects were recruited in this study, that were equivalent in age and gender (age = 35.5 ± 14.6 years, 12 female) to our seizure patient group. All were healthy and free of any neurological or psychiatric disorders at the time of the study.
This study was approved by the Institutional Review Board of University of Wisconsin Madison and informed consent was obtained from each participant.
2.2 Data acquisition
The participants lay supine with the head snugly fixed by straps and foam pads to minimize head movement. During resting-state scanning, participants were instructed to keep as motionless as possible with eyes closed, not to think of anything in particular and not to fall asleep. Images were acquired using a 3.0-Tesla scanner (GE MRI 750, Milwaukee, USA) in University of Wisconsin Hospital and Clinics, Madison. The resting-state functional data was acquired using an echo-planar imaging sequence with the following parameters: 28 axial slices, TR=2000 ms, TE=30 ms, flip angle=90°, thickness/gap=4.0/0.0 mm, FOV=24cm×24cm, matrix= 64×64, 150 volumes. A high resolution T1-weighted anatomical image was acquired in an axial orientation using a spoiled gradient-recalled sequence covering the whole brain.
2.3 Data processing
2.3.1 Data preprocessing
The first 10 volumes of each subject’s rest data was discarded to allow longitudinal magnetization to reach a steady state and for participants to get used to the scanning environment. Preprocessing of the fMRI datasets included standard slice timing, realignment, normalization (voxel size [3, 3, 3]), smoothing (FWHM [4, 4, 4]), archived by using DPARSF based on SPM8 and REST (Chao-Gan and Yu-Feng 2010, Song, et al. 2011) [http://www.restfmri.net, http://www.fil.ion.ucl.ac.uk/spm/]. Participants with head motion larger than 3 mm or 3° in any of the 6 parameters (x, y, z, pitch, roll, yaw) were excluded.
Regional Homogeneity measurement
ReHo analysis was performed for each subject by calculating Kendall coefficient of concordance (KCC) of the time series of a given voxel with those of its nearest neighbors in all directions on a voxel-wise basis (here, 26 voxels). The KCC is calculated in a voxel-wise way as follows (Zang, et al. 2004):
where W is KCC among timeseries of given voxels, ranging from 0 to 1; Ri is the sum rank of the Rth time point; where R̄ = ((n + 1)K)/2 is the mean of the Ri’s; K is the number of time series within a measured cluster and n is the number of ranks (our study had 150 total volumes in which 10 were discarded, making n = 140). This procedure was automatically implemented by DPARSF.
2.3.2 Group analyses of ReHo
All images from the 4 right mTLE were flipped (using the spm_flip utility from SPM2) to be left mTLE allowing for observing results from group ReHo changes to be explained as either ipsilateral or contralateral to left mTLE. The advantages of the approach includes both maximizing sample size and examining unilateral changes with smaller sample size (left side mTLE, n=10) (Blumenfeld, et al. 2009).
2.3.2.1 One-sample t-test
To observe which brain regions have significantly higher ReHo value than global mean in each group, one-sided and one-sample t-tests were performed to generate the T maps in both patients and healthy control groups separately.
2.3.2.2 Two-sample t-test
Voxelwise two sample t-tests were employed to compare the ReHo results between patients and controls, using the statistical analysis tool in REST software based on SPM8.
For both one-sample and two-sample t-test, a cluster of greater than 10 voxels with p value less than 0.001(corrected) were considered significant.
3 Results
3.1 Within group
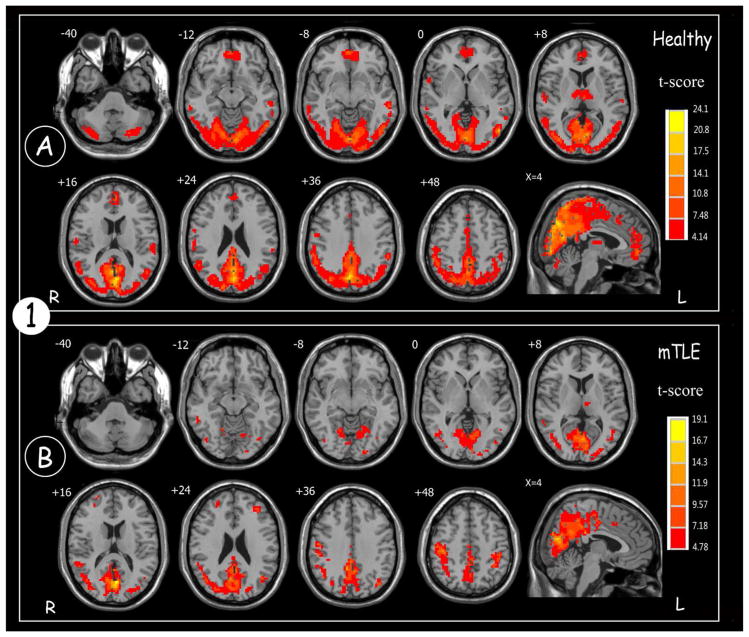
Results from one-sample t-test showed a specific spatial pattern and intensity of ReHo activation for both healthy controls and patients with mTLE, as shown in Figure 1. In the healthy control group, significantly increased ReHo activations were mainly found in the default model network (DMN) (including precuneus, posterior cingulate gyrus (PCC), bilateral inferior lateral parietal and mesial prefrontal cortex (MPFC)), bilateral visual cortex and cerebellum.
Figure 1.
Results of ReHo map across the (A) healthy controls and (B) patients with mTLE (One sample t-test; p<0.001, voxels >10, with FWE correction). Color scale indicates increased ReHo value.
In the mTLE patient group, increased ReHo was mainly observed in the DMN and the visual cortex. However, compared to the healthy controls, patients showed a decrease in size and activation intensity in these regions. The most prominent difference observed was that in the patient group ReHo activation was significantly decreased in the cerebellar region and no significant activation was seen in the MPFC.
3.2 Between groups
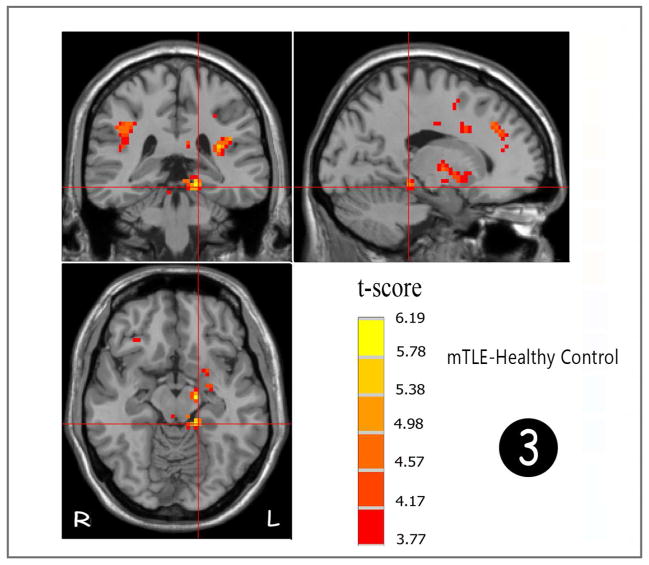
A two-sample t-test was performed to examine differences between patients with mTLE and healthy controls as can be seen in Figure 2 and Tables 1&2. Patients showed robust and increased ReHo in comparison to patients in the ipsilateral (left side) parahippocampal gyrus (fig 3), midbrain, insula (mainly anterior division), rolandic operculum, putamen, and corpus callosum. Increased ReHo were also seen in bilateral parietal and frontal subcortical white matter and bilateral pre-and postcentral gyri. Significantly decreased ReHo was mainly seen in DMN (prominently in PCC, precuneus, MPFC) and cerebellum (prominently in right side).
Figure 2.
Statistic t-map showing the difference between the mTLE group and healthy control (two sample t-test, p<0.001, voxel>10). Warm colors indicate mTLE > healthy control, while cool colors indicate healthy > mTLE.
Table 1.
Significant Increased ReHo Cluster Between Patients with mTLE and Healthy Control (Patients-HC), p<0.001, voxel>10
| Brain region | H | Peak MNI coordinate | Cluster Voxels | Peak t Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| ParaHippocampal | L | −15 | −36 | −12 | 47 | 6.19 |
| Midbrain | L | −15 | −15 | −12 | 69 | 6.02 |
| Insula, Putamen, Middle Frontal Gyrus | L | −27 | 27 | 30 | 410 | 5.91 |
| Rolandic Operculum | L | −33 | −33 | 18 | 76 | 5.87 |
| Corpus Callosum Splenium | R | 3 | −30 | 18 | 27 | 578 |
| Frontal Sub-Gyral White matter | R | 27 | 33 | 27 | 106 | 5.57 |
| Sub-Pos-Precentral Gyral White matter | L | −30 | −33 | 42 | 35 | 5.50 |
| Pallidum | L | −20 | −6 | 0 | 20 | 5.49 |
| Frontal Sub-Gyral White matter | R | 27 | −15 | 36 | 35 | 5.39 |
| Sub-Frontal/Parietal White matter | R | 42 | −45 | 27 | 236 | 5.37 |
| Paracentral_Lobule/Supp_Motor_Area | L | −9 | −18 | 66 | 17 | 5.06 |
| Middle and Superior Temporal Gyrus | R | 39 | 3 | −36 | 14 | 5.04 |
| Postcentral Gyrus | L | −33 | −30 | 44 | 22 | 5.43 |
| Precentral Gyrus | R | 30 | −12 | 57 | 13 | 4.69 |
| Postcentral Gyrus | R | 27 | −30 | 66 | 16 | 4.61 |
| Precentral Gyrus | L | −31 | −14 | 46 | 18 | 4.53 |
Abbreviations: H=Hemisphere; mTLE=mesial temporal lobe epilepsy;
Table 2.
Significant Decreased ReHo Cluster Between Patients with mTLE and Healthy Control (Patients-HC), p<0.001, voxel>10
| Brian Region | H | Peak MNI Coordinate | Cluster Voxels | Peak Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| MPFC | R/L | 3 | 48 | −9 | 262 | −8.14 |
| Precuneus, PCC | R/L | 3 | −72 | 36 | 125 | −7.51 |
| Inferior Occipital Gyrus | L | −51 | −72 | −6 | 23 | −6.22 |
| Cuneus | L | −3 | −75 | 15 | 17 | −6.05 |
| Cerebellum Posterior Lobe | R | 36 | −66 | −24 | 120 | −5.97 |
| Cuneus | R | 15 | −100 | 6 | 16 | −5.91 |
| Precuneus | L | −3 | −66 | 69 | 28 | −5.74 |
| Cerebellum Posterior Lobe | R | 48 | −51 | −54 | 31 | −5.53 |
| Cerebellum Posterior Lobe | L | −39 | −72 | −39 | 26 | −5.11 |
| Inferior Temporal Gyrus | R | 66 | −45 | −18 | 11 | −4.76 |
Abbreviations: H=Hemisphere; mTLE=mesial temporal lobe epilepsy; MPFC=mesial prefrontal cortex; PCC =posterior cingulate cortex
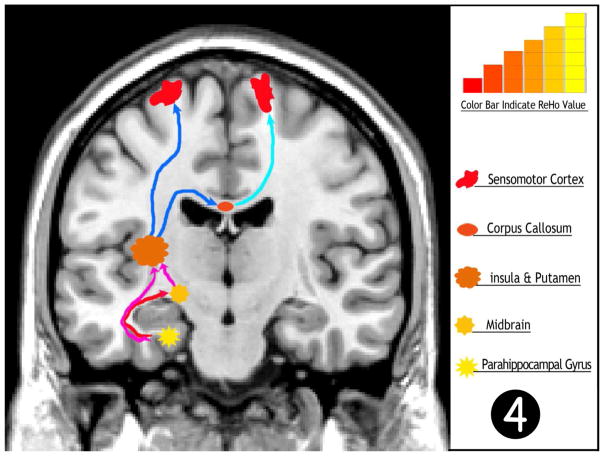
Figure 3.
Statistic t-map show the strongest activation of ReHo in parahippocampus (two sample t-test, p<0.001, voxel>10). Warm color indicates increased ReHo in mTLE
4 Discussion
The present study demonstrated the alterations of regional synchronization in patients with mTLE-HS using ReHo analysis during the interictal period. The main findings are as follows: within group analysis, the results show differences in the spatial pattern and intensity of ReHo activation in the two groups; compared to the healthy control group, ReHo increased significantly in patients in the ipsilateral (left side) parahippocampal gyrus, midbrain, insula, corpus callosum, bilateral sensorimotor cortex and fronto-parietal subcortical structure, while decreased ReHo was observed in DMN and cerebellum in patients group. These findings are further discussed below.
A consensus has been reached, based on recent studies, that low frequency fluctuations of BOLD signal in resting state reflect spontaneous fluctuations in brain physiology and metabolism at baseline (Fox and Raichle 2007). So the measurement of resting state BOLD fMRI is considered as an imaging marker of brain function without the usage of external tasks. ReHo, which measures the synchrony of different brain regions, is one of such indices of RS-fMRI, which could be considered as an imaging marker of brain function.
4.1 Increased ReHo
Accumulating evidence, from both animal model and human studies, have confirmed that there is an increased synchronization in the epileptogenic zone during seizures and interictal state (Bettus, et al. 2008, David, et al. 2008). Intracranial EEG studies have also shown increased regional synchronization of electrophysiological signals during interictal mesial temporal lobe seizures (Ortega, et al. 2008, Ponten, et al. 2007). In our study, the most robust increase in ReHo activation was observed to be in the parahippocampus, suggesting this region has the strongest synchronization allowing us to infer the parahippocampus as an epileptogenic zone in the mTLE. Interestingly, Morgan et al used 2d-TCA to analyze RS-fMRI data of the same type of patients and also found strong activation in the parahippocampus, similar to the observed activity in our study (Morgan, et al. 2010). The above findings suggest that the ReHo method may have the potential to detect the epileptogenic zone.
In the present study the second area of robust activation of ReHo was observed in the midbrain. Increased CBF was noted in midbrain in temporal epilepsy patients with hippocampal sclerosis more than 90 s after onset of temporal lobe complex seizures in a study utilizing single photon emission computed tomography (SPECT) (Blumenfeld, et al. 2004). Our study is consistent with this result, which would suggest that midbrain is an important node in the mTLE network.
Results also showed increased ReHo in the ipsilateral insula (mainly anterior division). Ipsilateral insula was found to be active also in a fmri study of epilepsy patients (Morgan, et al. 2010). Moreover positron emission tomography (PET) study also showed the ipsilateral insula involved in mTLE (Bouilleret, et al. 2002). The insula is a complex structure, which has been implicated in several higher order cognitive functions such as in saliency, switching, attention and control network, including regions that subserve motor and somatosensory functions (Menon and Uddin 2010). Evidence from these studies suggests that that the insular cortex plays an essential role in seizures propagation in temporal lobe epilepsy patients.
A significant increased ReHo cluster was found in the corpus callosum. Corpus callosum connects the two hemispheres of the brain which allows for interhemispheric communication, but also contributes to the spread of seizure impulses from one side of the brain to the other. A corpus callosotomy interrupts the spread of seizures from one hemisphere to the other hemisphere, and eventually helps improve patient’s quality of life (Cukiert, et al. 2009, Liang, et al. 2010). ReHo shows increased synchrony in corpus callosum, which also suggests that it is part of the epileptogenic network.
Both parietal and frontal subcortical white matter and ipsilateral putamen are involved with increased ReHo. The emerging evidences suggest that subcortical structures may play a critical role in the propagation and behavioral manifestations of human epileptogenic seizures (Norden and Blumenfeld 2002). Spencer summarized that subcortical structures are key to the manifestation of partial seizures, supporting the contention that specific subcortical regions are part of specific epileptogenic networks (Spencer 2002). It is generally accepted that the thalamus is an important transfer station for seizure propagation, especially in generalized epilepsy (Tyvaert, et al. 2009). However, both our study and Mankinen’s studies did not find significant increased ReHo in bilateral thalami in temporal lobe epilepsy compared to healthy controls (Mankinen, et al. 2011). Interestingly, Wang et al revealed increased ReHo in bilateral thalami in generalized tonic-clonic seizures (Wang, et al. 2011). The difference in our result and Wang et al. may be due to the different type of epilepsy patients. The lack of increased ReHo in this region in our study and Mankinen’s study suggests that in patients with mTLE, the thalamus may not be an important node for this type of seizure propagation. Our study showed increased ReHo in bilateral pre- and post central gyri. Electrophysiological evidence from animal models of epilepsy demonstrate increased synchronization in these regions, supporting our findings (David, et al. 2008).
Compared to the healthy control, result of one sample t test showed reduced area of ReHo in visual cortex in mTLE group. Interestingly, Yan et al found reduction of ReHo in visual cortex in older adult group comparison with young adults (Yan, et al. 2011). Reductions of ReHo have been reported in older adult subjects and patients with Parkinson’s and Alzheimer’s disease (He, et al. 2007, Wu, et al. 2009, Wu, et al. 2007). This suggests that reduced synchronized activity in the visual cortex may be a common change across the life-span and in the disease state.
4.2 Decreased ReHo
Results from the one sample t-test in our study demonstrated the altered spatial and intensity pattern of the DMN in mTLE patients. Differing from the typical DMN pattern in the healthy controls, absence of increased ReHo in MPFC, and predominantly decreased intensity in PCC and precuneus were the main characteristics in the mTLE patients. Moreover, group comparisons with two sample t-test confirmed decreased ReHo in the DMN and cerebellum.
Deactivation or suspension of the DMN activity in epilepsy has been reported by previous studies (Archer, et al. 2003, Gotman, et al. 2005). In contrast, Mankinen et al reported significantly increased ReHo in the PCC in pediatric patients with nonlesion TLE (Mankinen, et al. 2011). However, Archer et al. reported deactivation in PCC during generalized spike and slow-wave discharges (Archer, et al. 2003). It is widely accepted that exogenous or endogenous stimulation of the brain will interrupt the resting state and cause the deactivation and suspension of the DMN. Based on the above theory, the interictal activity could be considered as internal stimulation, which may decrease or interrupt the DMN. The contrary result between our study and Mankinen’s study may be differences in patient profiles, with adult patients with mTLE-HS in our study and pediatric patient with nonlesion temporal lobe epilepsy in Mankinen’s study; however further studies are required to confirm the current findings.
It has been repeatedly shown that the DMN is one of the most important networks of resting-state network, which maintains the baseline brain activities related to self-awareness, episodic memory and interactive modulation between internal mental activities and external tasks (Buckner, et al. 2008, Fox, et al. 2005). MPFC has been associated with cognitive operations and emotional processes. PCC and precuneus are recognized as the most salient nodes in DMN (Jiao, et al. 2011). Reduced BOLD signal (as measured by ReHo) in DMN suggests reduced neural activity in this region. So, we speculated that decreased ReHo in the DMN of mTLE patients may be due to the long-term injurious effects of epileptic activity, which may eventually cause DMN functional impairments.
Bilateral posterior cerebellum showed decreased ReHo in mTLE group compared to the healthy control group, prominently in the contralateral side of the temporal lesion (right side). Mankinen et al (Mankinen, et al. 2011) also found decreased ReHo in cerebellum, but in cerebellar culmen not in the posterior lobe. In contrast, Zhong et al revealed increased ReHo in bilateral cerebellum in generalized tonic-clonic seizure during interictal seizures (Zhong, et al. 2011). This significant difference may be due to the different types of seizures. Van Paesschen et al also found hypoperfusion in contralateral posterior cerebellum lobe by using SPECT (Van Paesschen, et al. 2003), which is consistent with our finding. This phenomenon was called ‘crossed cerebellar diaschisis’, and was considered as an indication of disconnection of the glutamatergic corticopontocerebellar tracts (Nelissen, et al. 2006). Since one of the main functions of cerebellum is motor coordination, it is possible that reduced ReHo could be a sign of decreased motor cooordination.
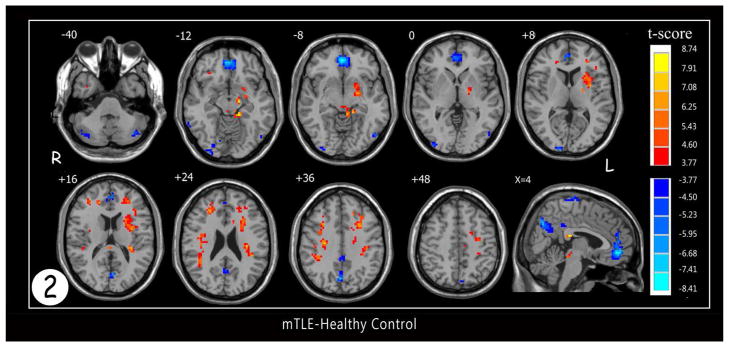
In summary, these findings provide additional evidence to support epileptogenic network theories from a new perspective. The alterations of ReHo in mTLE could be considered as part of an organized network. We propose that abnormal increased ReHo (reflecting increased spontaneous activity) in the hippocampus, midbrain, insula, and fronto-parietal subcortical structure comprise a network, which might be responsible for the seizure genesis and propagation (Fig 4). The abnormal decreased ReHo activation in the visual cortex, cerebellum, and DMN, may be a part of a network of regions which are functional impaired, such as vision, motor coordination, and cognition respectively, seen in these patients.
Fig 4.
Hypothesized epileptogenic network of seizure propagation for mTLE-HS based on ReHo value in this study. The decreasing order of significant ReHo values noted from parahippocampal gyrus to midbrain to insula/putamen to corpus callosum to frontal white matter (ipsilateral and contralateral) to sensorimotor cortex as shown in Table 1 suggests a directionality in terms of seizure origination and propagation through this network.
5 Limitations
There are several limitations of this study. First, the sample size is modest, which can reduce sensitivity and accuracy of our results, so further work is needed to confirm our findings. Second, in order to increase the sample size and the homogeneity of the group, we flipped the activation maps for 4 right sided mTLE patients. Blumenfeld used the same method to study cortical and subcortical networks in secondarily generalized tonic–clonic seizure patients (Blumenfeld, et al. 2009). Although the possibility is minimal, flipping the activation maps may lead to some uncertainty in the results. Third, we did not have neuropsychological evaluations in these patients for correlation analysis of behavior with brain activity. Finally, the lack of simultaneous EEG in this study limits our ability to confirm our ReHo result of increased ReHo regions in descending order representing a pathway for epileptoform discharge propagation.
6 Conclusions
We used ReHo, one of the indices of the RS-fMRI, which measures the synchrony of local brain region, to study the mTLE-HS patients by comparing them to healthy controls. Altered (both increased and decreased) regional homogeneity in the resting state networks of mTLE-HS patients were found. Decreased ReHo in several areas were found which may correspond to wide functional impairments found in these patients. We propose that the increased ReHo regions that were found may compose a network which is responsible for seizure genesis and propagation. This method may have the potential ability of detecting epileptogenic zone or network in seizure patients.
Supplementary Material
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The project described was supported by Award Number R25GM083252 from the National Institute Of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or the National Institutes of Health
We acknowledge Benjamin J. Swan for his help in exporting and converting the functional image data of some subjects.
Footnotes
Conflict of interest
We declare that we have no conflict of interest.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT. The natural history of mesial temporal lobe epilepsy. Curr Opin Neurol. 2008;21:173–178. doi: 10.1097/WCO.0b013e3282f36ccd. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Jr, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol. 2002;51:202–208. doi: 10.1002/ana.10087. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukiert A, Burattini JA, Mariani PP, Cukiert CM, Argentoni-Baldochi M, Baise-Zung C, Forster CR, Mello VA. Outcome after extended callosal section in patients with primary idiopathic generalized epilepsy. Epilepsia. 2009;50:1377–1380. doi: 10.1111/j.1528-1167.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23:808–815. doi: 10.1002/jmri.20585. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura C, Vaudano AE, Carni M, Pantano P, Nucciarelli V, Garreffa G, Maraviglia B, Prencipe M, Bozzao L, Manfredi M, Giallonardo AT. EEG/fMRI study of ictal and interictal epileptic activity: methodological issues and future perspectives in clinical practice. Epilepsia. 2006;47(Suppl 5):52–58. doi: 10.1111/j.1528-1167.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Jiao Q, Lu G, Zhang Z, Zhong Y, Wang Z, Guo Y, Li K, Ding M, Liu Y. Granger causal influence predicts BOLD activity levels in the default mode network. Hum Brain Mapp. 2011;32:154–161. doi: 10.1002/hbm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatamian YB, Fahoum F, Gotman J. Limits of 2D-TCA in detecting BOLD responses to epileptic activity. Epilepsy Res. 2011 doi: 10.1016/j.eplepsyres.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Li A, Zhao M, Jiang H, Meng X, Sun Y. Anterior temporal lobectomy combined with anterior corpus callosotomy in patients with temporal lobe epilepsy and mental retardation. Seizure. 2010;19:330–334. doi: 10.1016/j.seizure.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, Nikkinen J, Starck T, Remes J, Rantala H, Zang YF, Kiviniemi V. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res. 2011;1373:221–229. doi: 10.1016/j.brainres.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. Cluster analysis detection of functional MRI activity in temporal lobe epilepsy. Epilepsy Res. 2007;76:22–33. doi: 10.1016/j.eplepsyres.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, Dupont P. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006;32:684–695. doi: 10.1016/j.neuroimage.2006.04.185. [DOI] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Ortega GJ, Menendez de la Prida L, Sola RG, Pastor J. Synchronization clusters of interictal activity in the lateral temporal cortex of epileptic patients: intraoperative electrocorticographic analysis. Epilepsia. 2008;49:269–280. doi: 10.1111/j.1528-1167.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila ML, Zang Y, Kiviniemi V. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol. 2007;118:918–927. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann G, Jr, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage. 2007;35:140–148. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Tyvaert L, Chassagnon S, Sadikot A, LeVan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018–2022. doi: 10.1212/WNL.0b013e3181c55d02. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lu G, Zhang Z, Zhong Y, Jiao Q, Tan Q, Tian L, Chen G, Liao W, Li K, Liu Y. Altered resting state networks in epileptic patients with generalized tonic-clonic seizures. Brain Res. 2011;1374:134–141. doi: 10.1016/j.brainres.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett. 2007;423:189–193. doi: 10.1016/j.neulet.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Yan L, Zhuo Y, Wang B, Wang DJ. Loss of Coherence of Low Frequency Fluctuations of BOLD FMRI in Visual Cortex of Healthy Aged Subjects. Open Neuroimag J. 2011;5:105–111. doi: 10.2174/1874440001105010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Lu G, Zhang Z, Jiao Q, Li K, Liu Y. Altered regional synchronization in epileptic patients with generalized tonic-clonic seizures. Epilepsy Res. 2011;97:83–91. doi: 10.1016/j.eplepsyres.2011.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.