Abstract
De novo malignancies represent an increasing concern in the transplant population, particularly as long-term graft and patient survival improves. EBV-associated B-cell lymphoma in the setting of PTLD is the leading malignancy in children following solid organ transplantation. Therapeutic strategies can be categorized as pharmacologic, biologic, and cell-based but the variable efficacy of these approaches and the complexity of PTLD suggest that new treatment options are warranted. Here, we review current therapeutic strategies for treatment of PTLD. We also describe the life cycle of EBV, addressing the viral mechanisms that contribute to the genesis and persistence of EBV+ B-cell lymphomas. Specifically, we focus on the oncogenic signaling pathways activated by the EBV LMP1 and LMP2a to understand the underlying mechanisms and mediators of lymphomagenesis with the goal of identifying novel, rational therapeutic targets for the treatment of EBV-associated malignancies.
Keywords: Epstein–Barr virus, post-transplant lymphoproliferative disorder, B cell
EBV is a gammaherpes virus that has infected >95% of the world’s population (1). In healthy individuals, EBV infection is usually asymptomatic and the virus establishes a latent infection, residing in memory B cells for the lifetime of the host. However, when the immune system is debilitated as in immunosuppressed transplant recipients, opportunistic EBV-associated B-cell lymphomas can arise (2). These lymphomas are the most serious manifestation of PTLD (3). The incidence of EBV-associated PTLD is known to vary widely with the organ transplanted and the serologic status of the recipient (4). Nevertheless, EBV-associated B-cell lymphomas are the most common cancer in children that receive solid organ grafts, representing well over half of the de novo, post-transplant malignancies. Children are at particular risk for PTLD, in part, because they are often immunologically naïve at the time of transplant and can acquire a primary infection when receiving a graft from a seropositive donor in the setting of immunosuppression (5). Although uncommon, PTLD may also arise from T cells; however, this review will be focused on EBV-induced PTLD, which accounts for 93% of lymphoproliferative disorders in pediatric organ transplant recipients.
The current standard therapeutic approaches for the treatment of EBV-associated PTLD include reduction or elimination of immunosuppression, chemotherapy, radiotherapy, the use of anti-B-cell antibodies such as rituximab, and surgical resection when feasible. Determining the optimal treatment for individual patients, however, remains difficult and requires extensive interaction between transplant specialists and oncologists. The recent emergence of cellular-based immunotherapy holds promise for alternate approaches and underscores the need for new strategies to manage children with EBV-associated B-cell lymphomas.
Current therapeutics for treatment of EBV+ PTLD
The spectrum of EBV infection in transplant recipients is wide, from a primary infection (mononucleosis-like syndrome) to full-blown monomorphic monoclonal lymphoma. Thus, the treatment varies according to the stage of the disease. Furthermore, it is usually individualized and varies greatly according to the treating physician’s preference. In a Canadian study that included 33 transplant centers covering the period from 1988 to 1997, reduction of immunosuppression was used in 71%, antiviral agents in 52%, immunoglobulin in 22%, surgical resection in 20%, radiation therapy in 14%, and IFN-α in 12% (6). In this retrospective study the types of organ transplants were: renal (14/33, 42.4%), liver (7/33, 21.2%), heart (6/33, 18.2%), lung (3/33, 9%), pancreas (2/33, 6%), and intestinal (1/33, 3%). The survival after PTLD at two yr was 51.0% (6). In a similar study from Spanish centers, the treatment strategies were somewhat different: reduction of immunosuppression in 91%, chemotherapy in 59%, rituximab in 33%, and antiviral agents in 13% of the patients (7).
For EBV-induced PTLD, reduction of immunosuppression appears to be effective in 23–50% of cases (8). The difference in response seems to be related to degree of reduction (or withdrawal) of immunosuppression. For example, in pediatric heart transplantation, reduction of immunosuppression may result in acute rejection leading to arrythmias, cardiogenic shock, and death. Thus, physicians are less willing to significantly reduce the intensity of the immunosuppression. In contrast, reduction or withdrawal of immunosuppression in liver transplant recipients is readily carried out as acute rejection of the liver is seldom a life-threatening complication. In kidney transplantation, the immunosuppression should be reduced; however, it should be discontinued in those patients with aggressive PTLD refractory to treatment. The patient’s life should not be put at risk by trying to save the kidney (9, 10). The response also depends on the time of the onset of disease. Patients with early onset of the disease experience a better outcome with the reduction of the immunosuppression than those who present with late onset PTLD, particularly in patients with risk factors such as high LDH, organ dysfunction, and multiorgan involvement (11). In a series of 42 adult recipients of solid organ transplants, 89% without any of these risk factors had a good response with the reduction of immunosuppression, compared to none of seven patients with two or three risk factors (11).
The length of time of reduction of immunosuppression remains unknown. In our own institution, we maintain our PTLD liver transplant recipients with low or no immunosuppression until the patients experience an episode of rejection. An interesting observation is that many of these patients did not experience rejection and thus immunosuppression was not restarted (12). How the reduction of immunosuppression is carried out also varies according to the type of organ transplant as well as the transplant team’s preference. Some will discontinue or reduce the dose of calcineurin inhibitors and keep the patients on low-dose steroids. The risk of this treatment is potential graft loss from rejection, but such complication seems to be infrequent. Others may substitute the calcineurin inhibitors for sirolimus (mTOR inhibitor) with the hope that this treatment will abrogate the proliferation of B cells as sirolimus is known to have antiproliferative properties in vitro (13). However, caution must be undertaken with this approach as PTLD has been observed (and the incidence may be increased) in clinical trials of patients treated with sirolimus in combination with other immunosuppressive agents (14).
The utilization of antiviral therapy for the treatment of PTLD is common practice, but whether or not antiviral therapy is effective on latent or transformed forms of EBV remains unresolved (8). It does decrease viral shedding in early onset of EBV infections such as plasmacytic hyperplasia and mononucleosis-like syndromes. The efficacy of acyclovir or gancyclovir for the treatment of PTLD comes mostly from anecdotal reports. There is no consensus if one is more effective than the other. Foscarnet has also been shown to be effective in individual case reports (15), but because of its potential toxicity, most protocols include acyclovir or gancyclovir. In summary, there is no evidence that antiviral therapy is effective in the latent phase of PTLD, although they may be effective in the lytic phase of EBV infection (8).
Intravenous immunoglobulin is often included in treatment protocols for PTLD in transplant recipients. Its efficacy is unknown as it is often used in combination with reduction of immunosuppression and/or antiviral therapies (16). As mentioned previously, the initial treatment for patients with monoclonal malignant lymphoma is to reduce or discontinue the immunosuppression. If there is no response, anti-B-cell antibodies, chemotherapy, and/or radiation and occasional surgical resection may be necessary for control of the disease.
Treatment with chemotherapy will include protocols often used for the treatment of lymphoma such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). There are modifications of this protocol by using lower doses or different chemotherapy agents (17-19). The complexity of managing transplant patients on chemotherapy (because of its potential toxicity) requires the participation in tandem of highly specialized oncologic and transplant teams as many of these patients have some degree of end organ failure (renal dysfunction, for example) from the prolonged treatment with nephrotoxic drugs such as cyclosporine or tacrolimus. PTLD involvement of the CNS system also poses a significant challenge as many of the chemotherapy drugs do not cross the brain barrier. Reported treatments for PTLD involving the CNS include the use of antivirals, immunotherapy, radiation, and chemotherapy, but the prognosis is poor (20). High-dose-MTX has been found to be effective in patients with PTLD involvement of the CNS. At our institution, we used a combination of high-dose MTX along with intrathecal MTX successfully in a child who had underwent a combined liver and intestinal transplantation complicated by PTLD involvement of the brain (21).
Immunotherapy, particularly the anti-CD20 antibody, rituximab, seems to be receiving wide acceptance as first line of treatment for those patients who do not respond to reduction or discontinuation of immunosuppression. In a prospective study of 43 patients with previously untreated B-cell PTLD, the overall response rate to rituximab was 40% and the survival rate at one yr was 67% (22). In a different retrospective study of 19 patients, the administration of rituximab was associated with a 68% response rate of the patients (23). Recently, rituximab has been used in combination with low-dose chemotherapy in those patients who did not respond to reduction of immunosuppression and the results were encouraging (24). Bortezomib, a protease inhibitor, was successfully used in an adult patient who developed PTLD-multiple myeloma after kidney transplantation (25). PTLD-multiple myeloma accounts for 4% of PTLD observed in transplant recipients. To our knowledge, bortezomib has not been used for the treatment of PTLD in pediatric transplant recipients. Likewise, anti-CD21 and anti-CD24 murine antibodies were investigated in a series of 58 patients (31 of them recipients of solid organ transplants) with PTLD and the response and relapse rate was 61% and 8%, respectively (26). The overall response rate of chemotherapy for the treatment of malignant PTLD is about 70% (27).
The experience with the utilization of IFN-α for the treatment of PTLD is rather limited and amounts to individual institutional reports. One of the studies included a series of 16 patients with disseminated PTLD. Of the patients who received at least three wk of IFN-α therapy, eight of 14 experienced remission. No relapse was noted among the eight who responded, but two developed a new neoplastic clone (28). The potential risk of IFN-α in transplant recipients is the potential risk of the drug promoting steroid resistant rejection. This has been observed in kidney transplant recipients with co-existent hepatitis C infection who were treated with IFN-α as an attempt at eradicating the hepatitis C virus.
There are other modalities of treatment that were studied in the past or are currently undergoing investigation: adoptive immunotherapy using lymphokine-activated killer cells, infusions of donor leukocytes or EBV–specific cytotoxic T cell lines, administration of photosensitizing agents such as methoxalen, induction of the latent viral thymidine kinase gene in the tumor cells followed by treatment with gancyclovir and the mTOR inhibitor, rapamycin (29-32). Although promising, such therapies continue to remain in the realm of experimentation. In summary, a range of therapeutic approaches has been utilized for patients diagnosed with EBV+ PTLD, but their efficacy remains to be established.
EBV: Insights from the normal life cycle
One approach to identifying new treatment strategies is to understand how EBV infects and persists in normal human B cells and how this, in turn, can result in the development of EBV-associated malignancies. Along these lines, the life cycle of EBV highlights the ability of the virus to co-opt normal B-cell function to enter into the memory B-cell reservoir, where infected cells can escape immunosurveillance by EBV-specific CTLs. The life cycle of EBV very closely mimics the stages of B-cell differentiation, from antigen activation via the BCR through the GC into the memory B-cell reservoir (33).
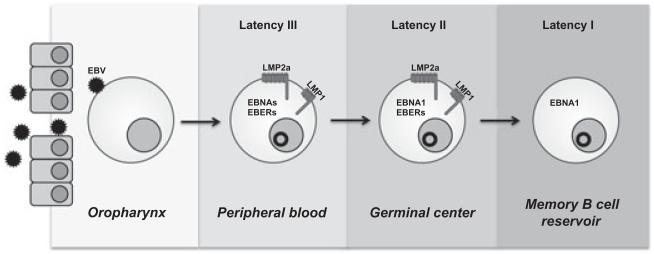
Models have emerged to explain how expression of particular viral genes promotes viral persistence through the B-cell differentiation process (34) (Fig. 1). In particular, the EBV LMP1 and LMP2a play critical roles in driving infected cells into the memory B-cell compartment. During B-cell differentiation, B cells require signals both from the antigen engagement of the BCR and from engagement of CD40 with CD154 on activated T helper (TH) cells or FDCs to proceed to the memory compartment; without receiving either signal during this phase, activated B cells undergo apoptosis. LMP1 and LMP2a may provide signals independent of the antigen-driven interactions with TH cells or FDCs that are required for B-cell differentiation. The EBV protein LMP2a, a functional mimic of the BCR, simultaneously blocks signals through the BCR while providing the tonic signals required for B-cell survival (35-37). EBV also expresses a functional CD40 mimic, LMP1, which delivers these potent survival signals in the absence of T cell help (38). Unlike their B-cell counterparts, both viral proteins provide constitutive signals, independent of antigen, through self-aggregation. Signals from these two viral proteins – LMP1 and LMP2a – likely act as the mediators for the survival of infected B cells into the long-lived, peripheral memory B-cell pool. Additionally, recirculating tonsillar memory B cells can express LMP1 and LMP2a, potentially assisting in ensuring the long-term survival of this latently infected memory B-cell pool (39).
Fig. 1.
Viral gene expression during the life cycle of EBV. EBV preferentially establishes an infection in naïve, tonsillar B cells. While a minority of infected cells remains permissive for the production of infectious viral particles, a latent infection is established in the majority of infected cells. Infected cells displaying this form of latency (latency III) express the EBV nuclear antigens (EBNAs) 1, 2, 3A, 3B, 3C, LP, LMP1 and LMP2a, and EBV small RNAs (EBERs 1, 2). Expression of a type III latency has been proposed to promote activation of the naïve B cell to become a proliferating lymphoblast, similar to B-cell activation by antigen. Infected cells then transition through a latency II, which is characterized by the loss of EBNA 2, 3A, 3B, 3C, and LP expression. During latency II, LMP1 (the CD40 homolog of EBV) and LMP2a (the BCR homolog of EBV) have been proposed to provide signals independent of the antigen-driven interactions with TH cells or FDCs that are required for B-cell differentiation through the GC and into the memory B-cell compartment. Finally, EBV persists for the lifetime of the host in the peripheral memory B-cell reservoir by switching to a type I latency. In this program, latently infected cells escape immunosurveillance by EBV-specific cytotoxic T lymphocytes by not expressing any latent genes. To ensure passage of the viral episome during cell division, the poorly immunogenic EBNA1 is periodically expressed in latently infected memory B cells.
Signal mimicry: LMP1 and LMP2a
LMP1 is the primary oncogene of EBV, as its expression is sufficient to transform rodent fibroblasts and it is essential for EBV-induced B-cell transformation in vitro (40, 41). LMP1 functionally resembles the tumor necrosis factor receptor (TNFR) superfamily member CD40 (42, 43). Studies of LMP1 signaling in vivo have revealed that the constitutive signaling of LMP1 abrogates the requirement for EBV-infected B cells to form a GC before entering the memory compartment (44).
LMP1 signaling induces expression of cell-surface adhesion molecules and activation antigens (41). LMP1 also upregulates anti-apoptotic proteins, including Bcl-2, A20, and cFLIP, that protect infected cells against apoptosis (45-47). Additionally, LMP1 induces secretion of the autocrine growth factor IL-10 (48). Much of the oncogenicity of LMP1 can be ascribed to its constitutive activation of multiple cellular signaling pathways that promote cellular growth and activation. Interactions of cellular TRAF and TNFR-associated death domains (TRADD) adaptor proteins with the LMP1 C-terminal tail signaling domains, carboxy-terminal activating region 1 and 2 (CTAR1 or CTAR2), initiate signal transduction through a variety of pathways including the p38, Erk, and JNK MAPK and NF-jB pathways (42, 49-52). Recent studies have also implicated LMP1 in the activation of the PI3K/Akt signaling pathway; activation of PI3K/Akt has been shown to depend primarily upon the CTAR1 domain of LMP1 (48, 53, 54).
Like LMP1, LMP2a is a functional, constitutively active mimic of a B-cell activation signal, in this case the BCR. In transgenic mice, B-cell restricted expression of LMP2a in lieu of the immunoglobulin heavy chain abrogates normal B-cell development, driving Ig-negative cell colonization in peripheral lymphoid organs (55). While not required for B-cell transformation in vitro, LMP2a expression can transform epithelial cells and enhance their adhesion and motility (56). LMP2a expression can, however, rescue survival of GC B cells with crippling mutations in the BCR (57). This suggests that LMP2a can contribute to the transformation of primary B cells (58, 59). LMP2a expression in B cells sequesters the key tyrosine kinases, Lyn and Syk, away from the BCR, blocking normal BCR signaling (35, 59). Self-aggregation of LMP2a (60) delivers a constitutive, BCR-like signal to latently infected cells through Syk, Lyn, Btk, BLNK, PI3K/Akt, and other signaling mediators. These signals, coordinated by Syk activation, function to maintain viral latency, sustain survival, and induce expression of a range of genes involved in cell-cycle induction, inhibition of apoptosis, and suppression of cell-mediated immunity (35-37, 57, 61-64).
Therapeutics in development that target pathways activated by LMP1 and LMP2a
Emerging therapeutics for the treatment of PTLD fall into two general categories: cellular immunotherapies and strategies targeting EBV-specific mechanisms of lymphomagenesis. Cellular immunotherapies include the infusion of autologous or allogeneic EBV-specific CTLs (65); while well tolerated, their efficacy in the presence of immunosuppressive drugs has been mixed. Recent studies by our laboratory and others have elucidated cellular signaling pathways initiated by LMP1 and LMP2a in infected B cells, and thereby has revealed new opportunities for treatment of PTLD. For the purposes of this review, we focus specifically on the small molecule inhibitors in clinical development for other malignancies or diseases that target molecules relevant to the survival signals driven by the EBV proteins LMP1 and LMP2a (Fig. 2).
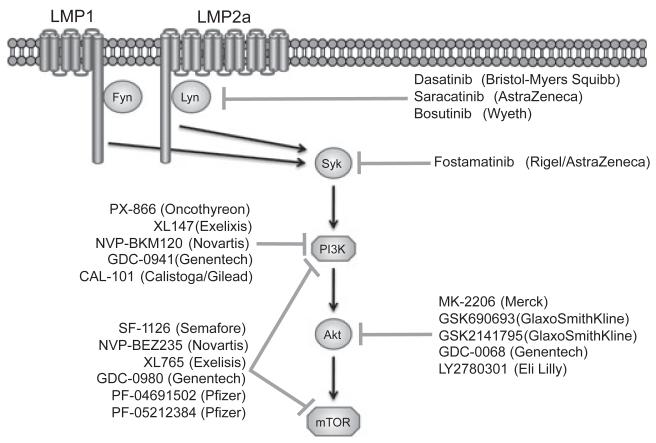
Fig. 2.
Therapeutics in development that target survival pathways activated by LMP1 and LMP2a. Both LMP1 and LMP2a activate members of the SFK (Fyn and Lyn, respectively) upstream of Syk activation. Syk then activates the PI3K/Akt/mTor signaling pathway. Small molecule inhibitors of these signaling molecules that are currently in preclinical or clinical development in other malignancies are listed next to their described target.
Syk
Ligand-independent, or tonic, signals from the BCR are required for the survival of all benign (66-69) and most malignant (70, 71) B cells. These tonic signals are executed primarily through the activation of Syk tyrosine kinase, which coordinates the activation of a myriad of downstream pathways. The EBV viral homolog of the BCR, LMP2a, is expressed in PTLD and activates Syk. LMP2a expression can rescue B-cell development and survival in mice unable to generate a functional BCR (36) only when LMP2a is able to bind and activate Syk (64), highlighting the importance of Syk activation by LMP2a for B-cell survival.
Pharmacological or genetic targeting of Syk has been pursued as a therapeutic strategy for the treatment of B-cell lymphomas. Currently, the only Syk inhibitor in clinical trails, according to clinicaltrials.gov, is fostamatinib (Rigel/Astra-Zeneca). We have shown that the Syk inhibitor fostamatinib reduces proliferation and induces apoptosis of EBV+ B-cell lymphomas lines derived from patients with PTLD in vitro (72). Fostamatinib also reduces the proliferation and induces the apoptosis of lymphoma cells, including those from DLBCL and B-cell CLL, in vitro (73-76). In murine models of NHL and CLL inhibition of Syk induces tumor regression or remission (77, 78). Finally, in phase I/II clinical trials, fostamatinib was efficacious in patients with DLBCL, FL, SLL, CLL, and MCL (79).
In contrast, however, current data also suggest that Syk can act in a tumor suppressive fashion in breast cancer and other epithelial cancers. For example, in breast cancer, a direct examination of the role of Syk revealed loss of Syk expression results in more aggressive, metastatic tumors and correlates with poorer clinical prognosis (80, 81). However, unbiased gene expression profiles do not include Syk in gene signatures associated with predictors or poor prognostic outcomes of breast cancer (82-84). Intriguingly, fostamatinib has been linked to lymphoma peripheralization. In the phase I/II clinical trial for fostamatinib, a transient increase in circulating lymphocytes observed in SLL and CLL patients (79). Thus, it is plausible that Syk is involved in multiple cellular functions such that additional studies are warranted to fully understand the consequences of inhibiting Syk for treatment of EBV+ PTLD.
SFK
Upstream of Syk activation are the SFK, a large family of kinases including Lyn, Fyn, Lck, Hck, Fgr, Blk, Yrk, Yes, and c-Src. Similar to the BCR, LMP2a activates the Src family kinase Lyn upstream of Syk (85). Additionally, we have observed that LMP1 can also activate Fyn, another member of the Src family of kinases (O. Hatton, S.L. Lambert, S.M. Krams, O.M. Martinez, unpublished data). Displaying robust anti-proliferative and antitumor activity in both solid and hematologic tumors, the orally available c-Src inhibitor dasatinib (Bristol Myers-Squibb) variably inhibits the other SFKs (86, 87). Saracatinib (AstraZeneca) is a pan-SFK inhibitor, which has been shown to have an antitumor effect in pancreatic cancer and prostate cancer xenografts (88, 89). Bosutinib (Wyeth), another c-Src inhibitor with activity against other SFKs, has shown modest activity in colon and pancreatic cancer xenografts (90, 91). All three inhibitors have entered phase I or II clinical trials, both as monotherapies and in combination with other agents, with preliminary data suggesting that these agents are well tolerated at clinically relevant doses (92). Based on our observations with Syk, however, SFK may be involved in the activation of numerous pathways that would preclude achieving a specific effect on survival without undesirable side effects. Additionally, the Src kinases inhibitors that are currently in clinical development display a wide range of off-target effects, including inhibiting ABL and c-kit (92). However, more studies will be needed to address the possibility of Src family kinase inhibitors in the treatment of PTLD.
PI3K/Akt
Downstream of Syk activation, the PI3K/Akt, and Erk MAPK signaling pathways have been implicated as executors of the Syk survival signal in B-cell lymphomas. PI3K/Akt signaling is of particular interest, as PI3K/Akt dysregulation is one of the most frequent occurrences in human cancers, including B-cell lymphomas (93, 94). PI3K/Akt signaling plays a central role in cell growth, proliferation, and metabolism (95). Both LMP1 and LMP2a activate the PI3K/Akt pathway, and inhibition of this pathway in vitro by the dual PI3K/mTOR inhibitor LY294002 induces apoptosis of B cells from LMP2a-transgenic mice (63), as well as EBV+ PTLD-derived B-cell lines (72). Finally, both PI3K and Akt lie upstream of mTOR and we have previously shown that rapamycin, an mTOR inhibitor, markedly suppresses the growth of EBV+ PTLD-derived B-cell lines in vitro and in a xenogeneic model of PTLD (13).
Given its involvement in a diverse number of malignant pathologies, it is not surprising that there are a considerable number of inhibitors for the PI3K/Akt/mTOR pathway in clinical development (96, 97). These inhibitors come in one of three flavors: PI3K inhibitors, Akt inhibitors, and dual PI3K/mTor inhibitors. The PI3K inhibitors in clinical development include PX-866 (Oncothyreon), XL147 (Exelixis), NVP-BKM120 (Novartis), GDC-0941 (Genentech), and CAL-101 (Calistoga/Gilead). PX-866 was successful in treatment of tumor xenografts with PI3K-activating mutations (98), and resulted in stable disease in 25% of patients in the phase I clinical trial (99). XL147 monotherapy produced durable disease control in 6 of 39 treated patients, according to preliminary reports (100). In addition to reported preclinical anti-tumor activity (101, 102), partial responses in two breast cancer patients have been reported in preliminary reports from the preclinical studies with NVP-BKM120 (103). One partial response in a breast cancer patient has been reported from the phase I trials of GDC-0941 (104). CAL-101 is the first small molecule inhibitor specific for the PI3K p110d isoform, which is predominantly expressed in leukocytes. In vitro, CAL-101 has been efficacious in inducing apoptosis of B-cell malignancies, including CLL and B-cell acute lymphoblastic leukemia (105, 106). In preliminary results from the phase I study, partial responses have been seen in 33% of patients with CLL, 57% of patients with indolent NHL, and 67% of patients with MCL (107). CAL-101 is currently entering phase II studies as a monotherapy for indolent NHL and in combination with rituximab for CLL in elderly patients.
In preclinical studies with lung and ovarian cancer lines, the Akt inhibitor MK-2206 (Merck) has been efficacious both in combination with cytotoxic agents and the targeted therapies erlotinib and lapatinib (108). According to preliminarily results, MK-2206 established stable disease in six of 19 patients in its phase I clinical trials, with tumor shrinkage observed in up to 23% of patients, in advanced solid tumors (109). Phase II trials in advanced endometrial cancer, colorectal cancer, and relapsed Hodgkin’s lymphoma and NHL are underway (clinicaltrials.gov). Additionally, MK-2206 is also being evaluated in combination with the MEK/Erk inhibitor AZD6244 (AstraZeneca); however, MEK/Erk inhibition has not been efficacious in inducing apoptosis of B cells from LMP2a-transgenic mice (63), as well as from our EBV+ PTLD-derived B-cell lines (72). Other Akt inhibitors in the early stages of clinical development include GSK690693 and GSK2141795 (GlaxoSmithKline), GDC-0068 (Genentech), and LY2780301 (Eli Lilly).
Owing to the structural similarity of the mTOR and the p110 subunit of PI3K, dual PI3K/mTOR inhibitors have also been developed. SF-1126 (Semafore) has been efficacious in preclinical solid tumor xenograft models of prostate cancer and glioblastoma (110). Preliminary results from the clinical trial have shown the establishment of stable disease in 19 of 38 patients with solid tumors, as well as partial responses in combination with rituximab in patients with B-cell lymphomas (CLL or DLBCL) (111). NVP-BEZ235 (Novartis) was efficacious in achieving tumor stasis or regression in preclinical solid tumor xenografts of breast cancer, prostate cancer, and glioblastoma (112, 113). Preliminary reports from the clinical trials show partial responses in two patients, with 14 of 51 patients achieving stable disease for four months or greater (114). Finally, preclinical models utilizing XL765 (Exelisis) have shown shrinkage of GBM xenografts, both as a monotherapy and in combination with TMZ (115). In the phase I monotherapy study, preliminary results in patients with solid tumors include the achievement of stable disease in 12 of 83 enrolled patients (116). Both Genentech (GCD-0980) and Pfizer (PF-04691502 and PF-05212384) also have dual PI3K/mTOR inhibitors entering clinical trials; however, these are in earlier stages of development than those discussed above.
In conclusion, EBV+ PTLD remains an important problem in solid organ transplantation. An improved understanding of viral mechanisms of transformation and persistence, coupled with the development and testing of inhibitors of the Akt/PI3K/mTOR pathway for other human malignancies, may reveal new opportunities for treatment of PTLD.
Abbreviations
- BCR
B-cell receptor
- CLL
chronic lymphocytic leukemia
- CTL
cytotoxic T lymphocyte
- DLBCL
diffuse large-B-cell lymphoma
- EBNA
EBV nuclear antigen
- EBV
Epstein–Barr virus
- FDC
follicular dendritic cell
- FL
follicular lymphoma
- GC
germinal center
- MTX
methotrexate
- IFN-α
interferon-α
- LMP1
latent membrane protein 1
- LMP2a
latent membrane protein 2a
- MCL
mantle cell lymphoma
- NHL
non-Hodgkin’s lymphoma
- PTLD
post-transplant lymphoproliferative disorder
- SFK
Src family kinases
- SLL
small lymphocytic leukemia
- TNFR
tumor necrosis factor receptor
- TRADD
TNFR-associated death domains
- TRAF
TNF receptor-associated factor
References
- 1.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Snow AL, Martinez OM. Epstein-Barr virus: Evasive maneuvers in the development of PTLD. Am J Transplant. 2007;7:271–277. doi: 10.1111/j.1600-6143.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- 3.Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323:1767–1769. doi: 10.1056/NEJM199012203232510. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 5.Ho M. Risk factors and pathogenesis of posttransplant lymphoproliferative disorders. Transplant Proc. 1995;27:38–40. [PubMed] [Google Scholar]
- 6.Allen U, Hébert D, Moore D, et al. Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplant recipients, 1988-97: A Canadian multi-centre experience. Pediatr Transplant. 2001;5:198–203. doi: 10.1034/j.1399-3046.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Barca E, Domingo-Domenech E, Gomez-Codina J. Firstline treatment with rituximab improves survival of patients with posttransplant lymphoproliferative disease (PTLD) [abstract] Blood. 2004;104:39a. [Google Scholar]
- 8.Paya CV, Fung JJ, Nalesnik MA, et al. Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International Consensus Development Meeting. Transplantation. 1999;68:1517–1525. doi: 10.1097/00007890-199911270-00015. [DOI] [PubMed] [Google Scholar]
- 9.McDonald R, Smith J, Ho M. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant. 2008;8:984–989. doi: 10.1111/j.1600-6143.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Martz KL, Stablein DM, et al. Improved survival with recent post-transplant lymphoproliferative disorder (PTLD) in children with kidney transplants. Am J Transplant. 2011;11:751–758. doi: 10.1111/j.1600-6143.2011.03470.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsai DE, Hardy CL, Tomaszewski JE, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: Analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076–1088. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz M, Desai DM, Cox KL, et al. Complete immunosuppressive withdrawal as a uniform approach to post-transplant lymphoproliferative disease in pediatric liver transplantation. Pediatr Transplant. 2004;8:267–272. doi: 10.1111/j.1399-3046.2004.00129.x. [DOI] [PubMed] [Google Scholar]
- 13.Nepomuceno RR, Balatoni CE, Natkunam Y, et al. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472–4480. [PubMed] [Google Scholar]
- 14.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: Results from five multicenter studies. Clin Transplant. 2004;18:446–449. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 15.Oertel SH, Ruhnke MS, Anagnostopoulos I, et al. Treatment of Epstein-Barr virus-induced posttransplantation lymphoproliferative disorder with foscarnet alone in an adult after simultaneous heart and renal transplantation. Transplantation. 1999;67:765–767. doi: 10.1097/00007890-199903150-00023. [DOI] [PubMed] [Google Scholar]
- 16.Schaar CG, Van Der Pijl JW, Van HoekB, et al. Successful outcome with a “quintuple approach” of posttransplant lymphoproliferative disorder. Transplantation. 2001;71:47–52. doi: 10.1097/00007890-200101150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Smets F, Vajro P, Cornu G, et al. Indications and results of chemotherapy in children with posttransplant lymphoproliferative disease after liver transplantation. Transplantation. 2000;69:982–984. doi: 10.1097/00007890-200003150-00053. [DOI] [PubMed] [Google Scholar]
- 18.Gross TG, Bucuvalas JC, Park JR, et al. Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol. 2005;23:6481–6488. doi: 10.1200/JCO.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen LJ, Mullen GM, Carr TJ, et al. Aggressive treatment for postcardiac transplant lymphoproliferation. Blood. 1995;86:3333–3340. [PubMed] [Google Scholar]
- 20.Snanoudj R, Durrbach A, Leblond V, et al. Primary brain lymphomas after kidney transplantation: Presentation and outcome. Transplantation. 2003;76:930–937. doi: 10.1097/01.TP.0000079253.06061.52. [DOI] [PubMed] [Google Scholar]
- 21.Twist C, Kjelson L, McKenney A, et al. Treatment of recurrent post-transplant lymphoproliferative disorder (PTLD) of the central mervous system (CNS) with high-dose methotrexate (HD-MTX) Medimond International Proceedings. 2009:111. [Google Scholar]
- 22.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: Results of a prospective multicenter phase 2 study. Blood. 2006;107:3053–3057. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 23.Elstrom RL, Andreadis C, Aqui NA, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569–576. doi: 10.1111/j.1600-6143.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Fricker FJ, González-Peralta RP, et al. Post-transplant lymphoproliferative disorder in children: Recent outcomes and response to dual rituximab/low-dose chemotherapy combination. Pediatr Transplant. 2010;14:896–902. doi: 10.1111/j.1399-3046.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 25.Solak Y, Atalay H, Anil M, et al. Cost of paid transplantation abroad: Possible donor-origin early multiple myeloma in a renal transplant recipient treated using bortezomib. Transplant Proc. 2010;42:2813–2815. doi: 10.1016/j.transproceed.2010.05.164. [DOI] [PubMed] [Google Scholar]
- 26.Benkerrou M, Jais JP, Leblond V, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: Prognostic factors and long-term outcome. Blood. 1998;92:3137–3147. [PubMed] [Google Scholar]
- 27.Trappe R, Riess H, Babel N, et al. Salvage chemotherapy for refractory and relapsed posttransplant lymphoproliferative disorders (PTLD) after treatment with single-agent rituximab. Transplantation. 2007;83:912–918. doi: 10.1097/01.tp.0000258647.50947.78. [DOI] [PubMed] [Google Scholar]
- 28.Davis CL, Wood BL, Sabath DE, et al. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66:1770–1779. doi: 10.1097/00007890-199812270-00035. [DOI] [PubMed] [Google Scholar]
- 29.Nalesnik MA, Rao AS, Furukawa H, et al. Autologous lymphokine-activated killer cell therapy of Epstein-Barr virus-positive and -negative lymphoproliferative disorders arising in organ transplant recipients. Transplantation. 1997;63:1200–1205. doi: 10.1097/00007890-199705150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Brewin J, Mancao C, Straathof K, et al. Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood. 2009;114:4792–4803. doi: 10.1182/blood-2009-07-228387. [DOI] [PubMed] [Google Scholar]
- 31.Schoch OD, Boehler A, Speich R, et al. Extracorporeal photochemotherapy for Epstein-Barr virus-associated lymphoma after lung transplantation. Transplantation. 1999;68:1056–1058. doi: 10.1097/00007890-199910150-00027. [DOI] [PubMed] [Google Scholar]
- 32.Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA. 2000;97:4285–4290. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorley-Lawson DA. Epstein-Barr virus: Exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 34.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 35.Miller CL, Burkhardt AL, Lee JH, et al. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell RG, Wilson JB, Anderson SJ, et al. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 37.Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 38.Kilger E, Kieser A, Baumann M, et al. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci USA. 2000;97:12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 41.Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosialos G, Birkenbach M, Yalamanchili R, et al. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 43.Gires O, Zimber-Strobl U, Gonnella R, et al. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 45.Henderson S, Rowe M, Gregory C, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 46.Laherty CD, Hu HM, Opipari AW, et al. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 47.Snow AL, Lambert SL, Natkunam Y, et al. EBV can protect latently infected B cell lymphomas from death receptor-induced apoptosis. J Immunol. 2006;177:3283–3293. doi: 10.4049/jimmunol.177.5.3283. [DOI] [PubMed] [Google Scholar]
- 48.Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol. 2007;179:8225–8234. doi: 10.4049/jimmunol.179.12.8225. [DOI] [PubMed] [Google Scholar]
- 49.Devergne O, Hatzivassiliou E, Izumi KM, et al. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: Role in NF-kappaB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eliopoulos AG, Blake SM, Floettmann JE, et al. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gires O, Kohlhuber F, Kilger E, et al. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 1999;18:3064–3073. doi: 10.1093/emboj/18.11.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell T, Sugden B. Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson CW, Tramountanis G, Eliopoulos AG, et al. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694–3704. doi: 10.1074/jbc.M209840200. [DOI] [PubMed] [Google Scholar]
- 54.Mainou BA, Everly DNJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene. 2005;24:6917–6924. doi: 10.1038/sj.onc.1208846. [DOI] [PubMed] [Google Scholar]
- 55.Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood. 2007;110:3715–3721. doi: 10.1182/blood-2007-05-090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of {beta}-catenin signaling. J Virol. 2005;79:2375–2382. doi: 10.1128/JVI.79.4.2375-2382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 60.Matskova L, Ernberg I, Pawson T, et al. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J Virol. 2001;75:10941–10949. doi: 10.1128/JVI.75.22.10941-10949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longnecker R, Miller CL. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- 62.Miller CL, Lee JH, Kieff E, et al. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619–8628. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- 64.Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–9124. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merlo A, Turrini R, Dolcetti R, et al. Adoptive cell therapy against EBV-related malignancies: A survey of clinical results. Expert Opin Biol Ther. 2008;8:1265–1294. doi: 10.1517/14712598.8.9.1265. [DOI] [PubMed] [Google Scholar]
- 66.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 67.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 68.Kraus M, Alimzhanov MB, Rajewsky N, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Meffre E, Nussenzweig MC. Deletion of immunoglobulin beta in developing B cells leads to cell death. Proc Natl Acad Sci USA. 2002;99:11334–11339. doi: 10.1073/pnas.172369999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 71.Gururajan M, Jennings CD, Bondada S. Cutting edge: Constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006;176:5715–5719. doi: 10.4049/jimmunol.176.10.5715. [DOI] [PubMed] [Google Scholar]
- 72.Hatton O, Phillips LK, Vaysberg M, et al. Syk activation of phosphatidylinositol 3-kinase/Akt prevents HtrA2-dependent loss of X-linked inhibitor of apoptosis protein (XIAP) to promote survival of Epstein-Barr virus+ (EBV+) B cell lymphomas. J Biol Chem. 2011;286:37368–37378. doi: 10.1074/jbc.M111.255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchner M, Fuchs S, Prinz G, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 75.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 76.Quiroga MP, Balakrishnan K, Kurtova AV, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: Specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young RM, Hardy IR, Clarke RL, et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113:2508–2516. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suljagic M, Longo PG, Bennardo S, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the El-TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116:4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 79.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coopman PJ, Do MT, Barth M, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 81.Coopman PJ, Mueller SC. The Syk tyrosine kinase: A new negative regulator in tumor growth and progression. Cancer Lett. 2006;241:159–173. doi: 10.1016/j.canlet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Finetti P, Cervera N, Charafe-Jauffret E, et al. Sixteen-kinase gene expression identifies luminal breast cancers with poor prognosis. Cancer Res. 2008;68:767–776. doi: 10.1158/0008-5472.CAN-07-5516. [DOI] [PubMed] [Google Scholar]
- 83.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 84.Veer LV, Dai H, Van De Vijver M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 85.Fruehling S, Swart R, Dolwick KM, et al. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosinekinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 87.Chang Q, Jorgensen C, Pawson T, et al. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajeshkumar NV, Tan AC, De Oliveira E, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15:4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 89.Yang JC, Ok J-H, Busby JE, et al. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 2009;69:151–160. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golas JM, Lucas J, Etienne C, et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005;65:5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- 91.Messersmith WA, Rajeshkumar NV, Tan AC, et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol Cancer Ther. 2009;8:1484–1493. doi: 10.1158/1535-7163.MCT-09-0075. [DOI] [PubMed] [Google Scholar]
- 92.Aleshin A, Finn RS. SRC: A century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 94.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 95.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway – beyond rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ihle NT, Lemos R, Wipf P, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimeno A, Herbst RS, Falchook GS, et al. Final results from a phase I, dose-escalation study of PX-866, an irreversible, pan-isoform inhibitor of PI3 kinase. J Clin Oncol. 2010;28(15 Suppl) Abstract 3089. [Google Scholar]
- 100.Shapiro G, Kwak E, Baselga J, et al. Phase I dose-escalation study of XL147, a PI3K inhibitor administered orally to patients with solid tumors. J Clin Oncol. 2009;27(146 Suppl) Abstract 3500. [Google Scholar]
- 101.Maira M, Menezes D, Pecchi S, et al. NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials, shows significant antitumor activity in xenograft and primary tumor models; Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC; Philadelphia, PA: AACR. 2010; Apr 17-21, 2010. Abstract 4497. [Google Scholar]
- 102.Voliva CF, Pecchi S, Burger M, et al. Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials; Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC; Philadelphia, PA: AACR. 2010; Apr 17-21, 2010. Abstract 4498. [Google Scholar]
- 103.Baselga J, De Jonge MJ, Rodon J, et al. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(15 Suppl) doi: 10.1200/JCO.2011.36.1360. Abstract 3003. [DOI] [PubMed] [Google Scholar]
- 104.Von Hoff DD, Lorusso P, Tibes R, et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol. 2010;28(15 Suppl) Abstract 2541. [Google Scholar]
- 105.Herman SEM, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-d inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lannutti BJ, Meadows SA, Herman SEM, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furman RR, Byrd JC, Flinn IW, et al. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;28(15 Suppl) Abstract 3032. [Google Scholar]
- 108.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 109.Yap TA, Patnaik A, Fearen I, et al. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity. J Clin Oncol. 2010;28(15 Suppl) Abstract 3009. [Google Scholar]
- 110.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 111.Mahadevan D, Chiorean EG, Harris W, et al. Phase I study of the multikinase prodrug SF1126 in solid tumors and B-cell malignancies. J Clin Oncol. 2011;29 Abstract 3015. [Google Scholar]
- 112.Maira S-M, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 113.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 114.Burris H, Rodon J, Sharma S, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(15 Suppl) Abstract 3005. [Google Scholar]
- 115.Prasad G, Sottero T, Yang X, et al. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro Oncol. 2011;13:384–392. doi: 10.1093/neuonc/noq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brana I, Lorusso P, Baselga J, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28(15 Suppl) Abstract 3030. [Google Scholar]