Abstract
BACKGROUND
Elevated NF-κB activity has been previously demonstrated in prostate cancer cell lines as hormone-independent or metastatic characteristics develop. We look at the effects of piperlongumine (PL), a biologically active alkaloid/amide present in piper longum plant, on the NF-κB pathway in androgen-independent prostate cancer cells.
METHODS
NF-κB activity was evaluated using Luciferase reporter assays and Western blot analysis of p50 and p65 nuclear translocation. IL-6, IL-8, and MMP-9 levels were assessed using ELISA. Cellular adhesion and invasiveness properties of prostate cancer cells treated with PL were also assessed.
RESULTS
NF-κB DNA-binding activity was directly down-regulated with increasing concentrations of PL, along with decreased nuclear translocation of p50 and p65 subunits. Expression of IL-6, IL-8, MMP-9, and ICAM-1 was attenuated, and a decrease of cell-to-matrix adhesion and invasiveness properties of prostate cancer cells were observed.
CONCLUSIONS
PL-mediated inhibition of NF-κB activity decreases aggressive growth characteristics of prostate cancer cells in vitro.
Keywords: piperlongumine, NF-κB, cytokines, IL-6, IL-8, MMP-9, apoptosis, adhesion, ROS, prostate cancer
INTRODUCTION
Prostate cancer is a heterogeneous disease, with 239,000 new cases projected to be diagnosed, resulting in nearly 30,000 deaths, in the United States in 2013 [1]. Approximately 95% of patients present with localized or regional disease at diagnosis, with 5-year survival approaching 100%. For those with metastatic disease, however, 5-year relative survival is poor, estimated at only 28%. A recent wave in development of novel agents for management of castration-resistant prostate cancer resulted in FDA approval of multiple new options for advanced disease [2]. These agents are expensive, with significant impact on healthcare access and cost. The arena of chemoprevention remains to be explored. A large NIH sponsored trial evaluating effects of Selenium and Vitamin E failed to demonstrate a positive impact on prostate cancer risk [3]. 5-α Reductase inhibitors demonstrated promising results, however, a concern for increase in higher-grade cancers was raised in this setting [4-7]. A need exists for a naturally occurring compound with a favorable toxicity profile and efficacy to address prostate cancer needs.
Piperlongumine (PL) is an alkaloid amide found naturally in Piper Longum plant isolates and was previously reported to have insecticidal and antibacterial properties [8,9]. Prior studies suggested cancer-cell-selective anti-tumorigenic effects in vitro and in murine xenograft models [10,11]. Toxicity profile of PL appears to be favorable [10]. PL use in modulation of prostate cancer via depletion of the androgen receptor has been reported [12]. Effects of PL have been implicated in ROS-dependent apoptotic pathways, mediated by hydrogen peroxide and nitric oxide induction [10]. Cell cycle arrest in G2/M phase, activation of caspase-3, and decrease in Bcl-2, inhibitor of intrinsic apoptotic pathway, were also observed in PL-treated PC-3 prostate cancer cells [13]. Effects of PL on Nuclear Factor-kappa B (NF-κB) have been previously evaluated only with respect to atherosclerotic plaque formation and on vascular smooth muscle cell proliferation [14]. Effects of PL on NF-κB in prostate cancer have not been explored.
NF-κB is a nuclear transcriptional factor and was previously shown to participate in progression of prostatic malignancies via regulation of gene expression involved in angiogenesis, proliferation, apoptosis, invasion, and metastasis [15-19]. The Rel/NF-κB family of eukaryotic transcription factors is comprised of several structurally related proteins that form both homo- and hetero-dimers. The most common Rel/NF-κB dimer in mammals contains p50-RelA (p65). The activity of NF-κB is regulated by interaction with inhibitory IκBα, which blocks the ability of NF-κB to enter the nucleus and bind to DNA. Upon activation, IκBα is phosphorylated and marked for ubiquitination and degradation by the proteosome-dependent pathway. This process allows for translocation of active NF-κB complexes into the nucleus [20,21]. Constitutive activation of NF-κB was associated with prostate cancer progression toward androgen-independent phenotype [22].
Several pathways may activate NF-κB. One cascade is activated by binding TNF-α to TNFR1 or TNFR2 receptor [23,24], which after trimerization recruits TRAF2, RIP, and NIK [25]. NIK then phosphorylates and activates IκB kinase (IKK) [26,27], which subsequently targets IκBα for ubiquitination and degradation by the proteosome. Another cascade is also activated via TNFR1 receptor, resulting in activation of phosphatidylinositol 3-kinase (PI3K) and its downstream target, Akt kinase, which mediate TNF-α-promoted NF-κB activation by phosphorylation of threonine 23 in IKKα [28].
Activation of NF-κB target genes has been shown to participate in the initiation and progression of many cancers, including prostate cancer. Importantly, NF-κB-mediated up-regulation of interleukin (IL)-6, IL-8, matrix metalloproteinase (MMP)-9 and intracellular adhesion molecule-1 (ICAM-1), which are major pro-metastatic molecules, has been associated with negative prognostic features in various cancer types [29-31]. NF-κB activation promotes expression of the above genes, making NF-κB an attractive target for anti-tumorigenic agents [32-35]. Suppression of NF-κB in human prostate cancer cells has been shown to reduce their tumorigenic and metastatic potential in nude mice by down-regulating IL-8 and MMP-9 [33]. In vivo, decrease in these cytokines correlates with fewer lymph node metastases and has been suggested to hold prognostic value [33,36].
Multiple stimuli may activate NF-κB, including TNF-α, and patients with elevated TNF-α are known to have higher mortality rates [37]. The present report evaluates effects of PL on NF-κB activity and pro-metastatic profile of prostate cancer cells.
MATERIALS AND METHODS
Cells and Materials
PC-3, DU-145, and LNCaP human prostate cancer cell lines were obtained from ATCC (Rockville, MD) and maintained in RPMI 1640 medium (Bio-Whittaker, Walkersville, MD) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), gentamicin (50 mg/L), sodium pyruvate (1 mM), and non-essential amino acids (0.1 mM). Experiments were performed under conditions indicated in figure legends.
Antibodies and Reagents
Antibodies to RelA (NF-κB p65), NF-κB p50, DNA Topoisomearse I (Topo I), and IκBα were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies to β-actin, ph-Akt, ph-GSK3β, ph-p70S6K, and ph-4EBP1 were obtained from Cell Signaling Technology (Beverly, MA). Calcein-AM and TNF-α were obtained from Sigma (St. Louis, MO). Fibronectin and Akt inhibitor IV were obtained from Calbiochem (San Diego, CA).
Analysis of Cancer Cells Proliferation
Prostate cancer cells were incubated with various PL concentrations for 48 hr. Cell proliferation was analyzed by CellTitier Blue assay (Promega, Madison, WI). Effective doses (ED) were calculated using XLift©, Microsoft Excel® add-in.
Western Blot Analysis
Whole cell lysates were prepared as described previously [38]. Samples were subjected to SDS–PAGE, followed by standard immunoblot analysis. The membranes were then developed with Super-Signal West Pico Chemiluminescent substrate (Thermo Scientific, Rockford, IL).
Measurement of NF-κB Activity Using Luciferase Reporter Assay
Cells were transfected with NF-κB-luc (Stratagene, La Jolla, CA) and pRL-TK (Promega) plasmids. Twenty-four hours after transfection, cells were treated with various concentrations of PL for 6 hr in fresh medium. Four hours after stimulation of NF-κB pathway by TNF-α, samples were assayed for firefly and renilla luciferase activities using Dual-Glo Luciferase assay System (Promega) and normalized as instructed by the manufacturer.
Measurement of IL-6, IL-8, and MMP-9 Proteins
Cells were pre-incubated with 10 μM of PL for 6 hr, followed by incubation with 40 ng/mL of TNF-α for additional 16 hr. IL-6, IL-8, and MMP-9 levels in cell culture supernatants were determined using ELISA kits (R&D Systems, Minneapolis, MN).
Measurement of ICAM-1 Expression Via Immunocytometry
Cells were pre-incubated with indicated concentrations of PL for 6 hr, followed by incubation with 40 ng/ml of TNF-α for additional 16 hr. Levels of ICAM-1 surface expression were determined by staining cells with FITC-conjugated anti-ICAM-1 antibodies (R&D Systems, Minneapolis, MN) as recommended by the supplier, and analyzed by flow cytometry utilizing FACScan (Becton Dickinson, Franklin Lakes, NJ). Normal mouse IgG-FITC (Santa Cruz Biotechnology, Inc.) was utilized as a negative control.
Analysis of Cancer Cells Invasiveness
Invasiveness was determined using BD Falcon HTS FluoroBlok system (BD Biosciences, Bedford, MA) in triplicate for each condition. PC-3 cells (2.5 × 103) were seeded in the upper compartment of the FluoroBlok chamber in the absence of serum, with or without the indicated concentrations of PL. Serum-containing medium in the lower compartment served as a chemo-attractant. Cells were incubated at 37°C for 16 hr and then stained with 2 μM of Calcein-AM. An intervening membrane was present to block fluorescence from labeled cells present in the top chamber of the insert system. Fluorescence from labeled cells present in the lower chamber of the insert system was detected using a microplate fluorimeter with excitation and emission wavelengths of 485 and 530 nm, respectively. Images were captured using fluorescence microscope equipped with a digital camera.
Adhesion Assay
Cells were stained with Calcein AM (2 μM), preincubated with indicated concentrations of PL for 16 hr and plated in triplicates onto 96-well plates (2.5 × 103 cells/well) pre-coated with fibronectin (50 mg/ml). Cells were allowed to attach at 37°C for 15 min. Wells were washed with PBS twice and fluorescence was detected using a microplate reader with excitation and emission wavelengths of 485 and 530 nm, respectively.
RESULTS
Cell Proliferation Is Attenuated by PL
PC-3 and DU-145 prostate cancer cell lines were exposed to varying concentrations of PL and ED were calculated. Figure 1 demonstrates dose dependence between PL concentration and cell proliferation. ED20, ED50, ED70, and ED90 concentrations were determined to be 2.6, 4.9, 7.2, and 13.5 μM for PC-3 cells, and 2.0, 3.4, 5.1, and 8.7 μM for DU-145 cells, respectively. Similar effectiveness was previously demonstrated on LNCaP androgen-dependent prostate cancer cell line [12].
Fig. 1.
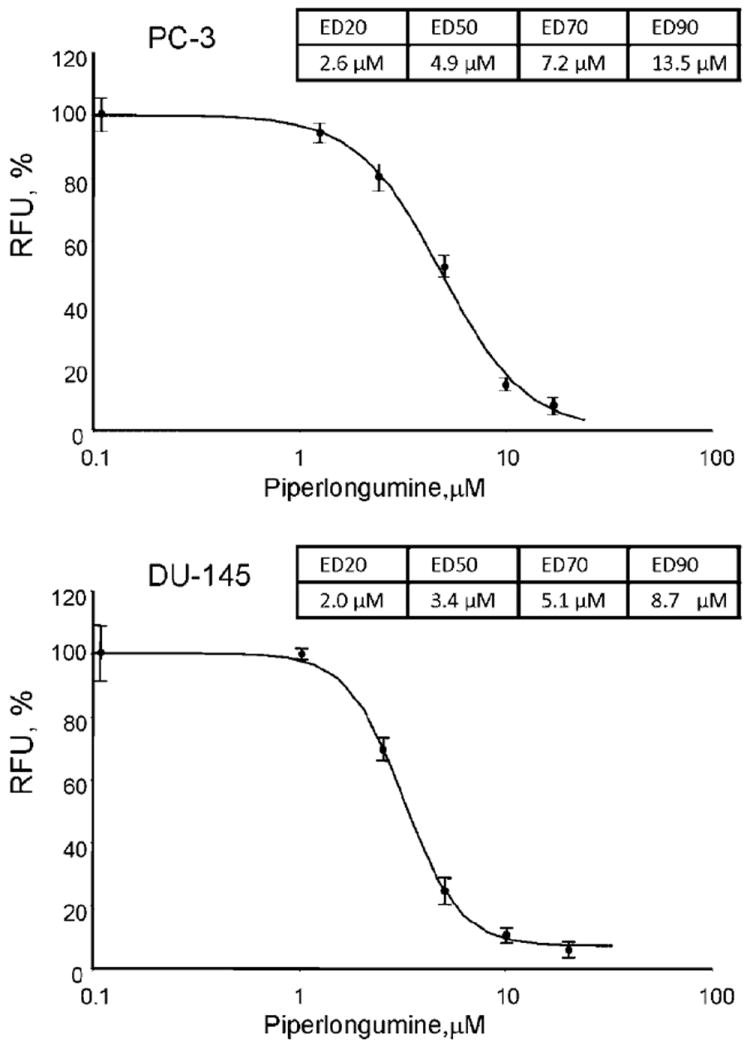
The effect of Piperlongumine (PL) on proliferation of human prostate cancer cell lines (PC-3 and DU-145). Cells were treated with indicated concentrations of PL for 48 hr. Cellular proliferation was assessed using the Cell Titer Blue assay.
PL Inhibits Transcriptional Activity of NF-κB in Prostate Cancer Cells
Basal and TNF-α-induced levels of NF-κB activity were determined by luciferase reporter assay as described in the Materials and Methods section. Three different human prostate cancer cell lines, PC-3, DU-145, and LNCaP, were analyzed to confirm that PL effect on NF-κB activity is not cell line specific. Figure 2 demonstrates that PL decreased basal NF-κB transcription in a dose-dependent fashion in both castrate-resistant (PC-3 and DU-145) and androgen-dependent (LNCaP) prostate cancer cell lines. Additionally, PL was able to significantly decrease TNF-α-induced stimulation, achieving levels below those seen in non-stimulated CRPC cells. As such, PL appears to be a potent suppressor of NF-κB functional activity. Of the tested cell lines only DU-145 expresses a functional PTEN protein [39-41], yielding the smallest decrease of TNF-α-induced activation of NF-κB (Fig. 2). These findings are consistent with previously published data which demonstrate that PTEN inhibits TNF-α-induced NF-κB activity [42,43].
Fig. 2.
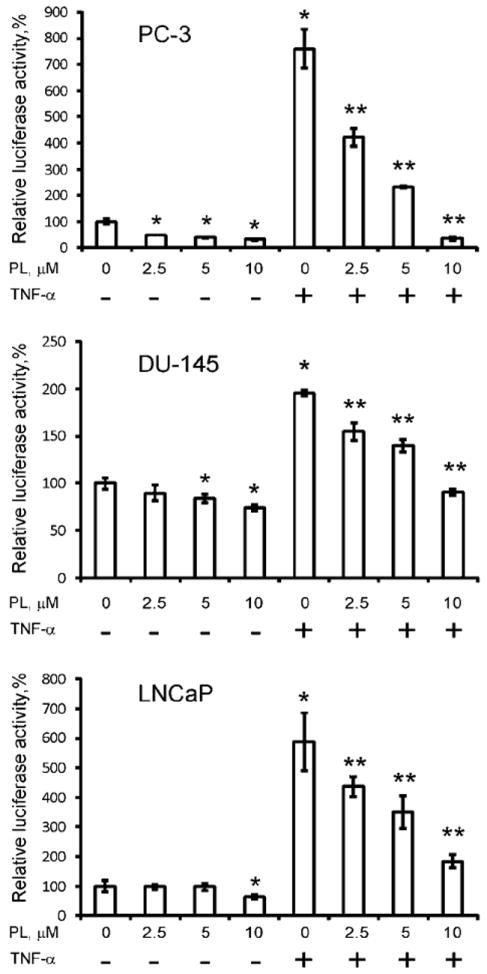
Levels of NF-κB activity were determined by Luciferase Reporter Assay as described in Materials and Methods section. PC-3, DU-145, and LNCaP cells were pre-incubated with indicated concentrations of PL for 6 hr prior to TNF-α (40 ng/ml) stimulation. Statistical analysis was performed by one-way ANOVA. Statistically significant (P < 0.05) compared with cells cultured in medium alone (*) or TNF-α-induced cells (**).
PL Inhibits NF-κB Nuclear Translocation
It has been established that NF-κB plays a critical role in cancer proliferation, invasion and progression and its up-regulation has been associated with castrate-resistant prostate cancer profile [44]. We examined the effect of PL on TNF-α-induced nuclear accumulation of RelA/p65 and p50 NF-κB and the degradation of the inhibitory protein IκBα. We observed impaired degradation of IκBα in the cytoplasmic fraction of PC-3 and DU-145 cells pre-treated for 4 hr with 10 μM of PL, which corresponded with significantly attenuated translocation and decreased nuclear accumulation of p50 and p65 (Fig. 3A and B). Prostate cancer cells treated with 40 ng/ml of TNF-α for 15 min were used as a positive control for IκBα degradation. Pre-incubating the cells with 10 μM of the proteasome inhibitor MG-132 for 4 hr completely blocked IκBα degradation and nuclear translocation of p50 and p65 (Fig. 3A and B).
Fig. 3.
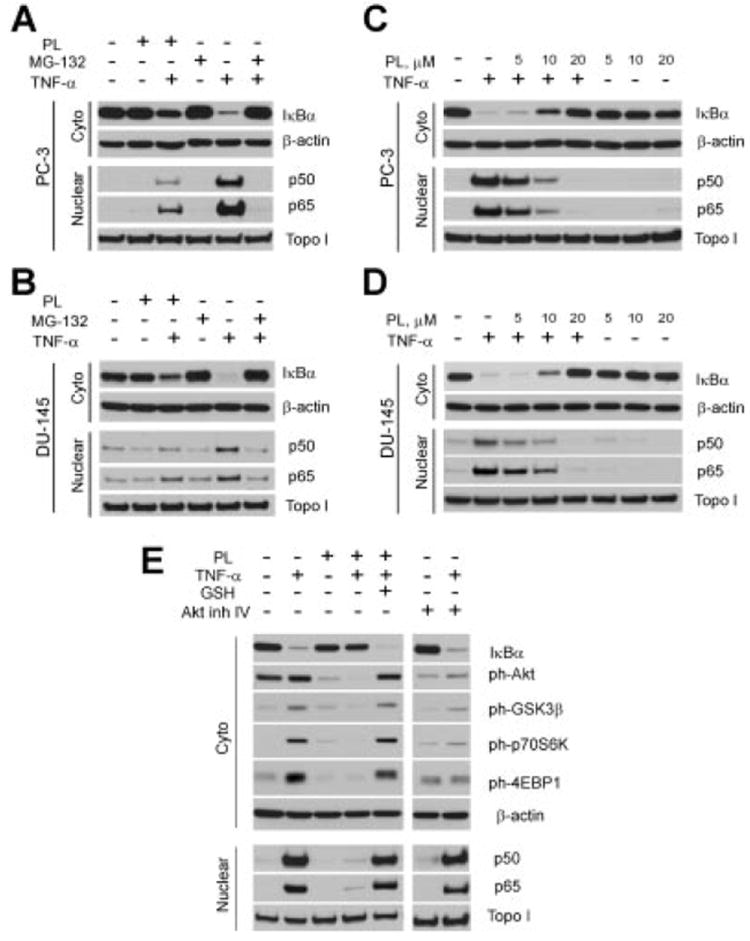
PL attenuates degradation of IκBα and blocks nuclear translocation of p50 and p65 in prostate cancer cells primarily via Akt-independent pathway. PL blocks NF-κB translocation in PC-3 (A), and DU-145 cells (B). PL effects on NF-κB translocation in dose-dependent fashion in PC-3 (C), and DU-145 (D) prostate cancer cell lines. E: PL (10 μM, overnight) effectively blocks both Akt and NF-κB pathways. Levels of the nuclear and cytoplasmic proteins were determined by Western blot analysis with specific antibodies. Expression of Topo I (nuclear extract) and β-actin (cytoplasmic extract) were used to control equal protein loading. Representative data from one of three experiments.
PC-3 and DU-145 cells were also incubated with different concentrations of PL. The findings presented in Figure 3C and D demonstrate dose-dependent NF-κB inactivation by PL. Nuclear translocation of p50 and p65 was significantly decreased at PL concentration of 10 μM and completely blocked at 20 μM over 4hr or at 10 μM overnight (Fig. 3C–E). As shown previously by Raj et al. [10], PL exerts its effects via ROS production in the cells. Co-administering 5 μM reduced glutathione (GSH) with PL completely reversed its inhibitory effect on NF-κB activation (Fig. 3E).
In addition to attenuating NF-κB translocation, PL also appeared to attenuate ph-Akt and its downstream targets. Recent publications confirm that TNF-α-induced NF-κB activation can be mediated via an Akt-dependent pathway [45]. Unlike PC-3 cell line, DU-145 cells do express a functional PTEN protein and therefore lack constitutive Akt activity [40] and TNF-α-induced Akt activity [43]. Our data demonstrate that PL successfully inhibited TNF-α-induced NF-κB translocation in DU-145 cells (Fig. 3B and D). Taking into consideration these observations we hypothesize that inhibitory effect of PL on NF-κB activity is decoupled from its effect on the Akt pathway. To better understand the role of the Akt pathway in activation of NF-κB we investigated the ability of Akt inhibitor IV to block NF-κB translocation in response to TNF-α stimulation in PC-3 cells. Indeed, TNF-α stimulated the Akt pathway in PC-3 cells, as demonstrated by elevations of ph-GSK3β, ph-p70S6K, and ph-4EBP1 (Fig. 3E). However, while the Akt inhibitor IV (1 μM) was effective at inhibiting ph-Akt, it failed to block TNF-α-induced NF-κB activation, suggesting that PL may affect NF-κB pathway independently, regardless of Akt activity in the cells.
PL Decreases Expression of IL-6, IL-8, and MMP-9 in Prostate Cancer Cells
Multiple genes regulated by NF-κB, including IL-6, IL-8, and MMP-9, are involved in tumorigenesis and have been implicated in progression of prostate cancer [46]. IL-6 is an important multifunctional cytokine involved in tumor progression toward androgen independent growth, whereas IL-8 and MMP-9 levels have been associated with increased metastatic potential [47,48]. Expression of IL-6, IL-8, and MMP-9 was examined in cell culture supernatants of PC-3 cells, pre-incubated with various concentrations of PL, followed by stimulation by TNF-α. As shown in Figure 4, pre-incubation with PL decreased TNF-α mediated expression of the cytokines, most pronounced in IL-6 and MMP-9, with IL-6 levels reduced below that of non-stimulated control cells.
Fig. 4.
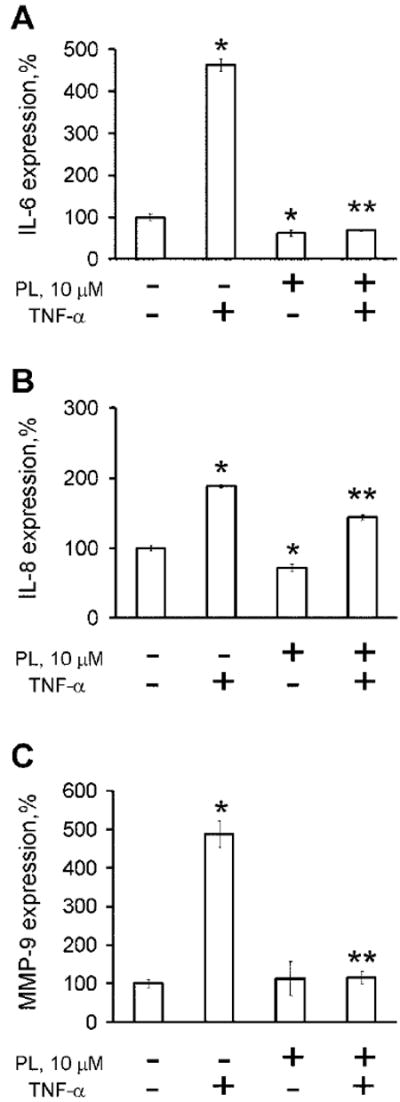
PL dramatically reduces expression of cytokines (A) IL-6, (B) IL-8, and (C) MMP-9 in PC-3 cells after TNF-α-mediated activation of NF-κB pathway. Statistical analysis was performed by one-way ANOVA. Statistically significant (P < 0.05) compared with cells cultured in medium alone (*) or TNF-α-induced cells (**).
PL Decreases Invasiveness and Adhesion of Prostate Cancer Cells
Invasion through the extracellular matrix represents a critical step in tumor metastasis. Studies indicate that metastatic activity of prostate cancer cells correlates with expression of pro-angiogenic factors, many of which are under NF-κB control [48]. Therefore, we anticipated that PL-mediated inhibition of NF-κB activity and expression of IL-6, IL-8, and MMP-9 proteins would have a functional impact on the metastatic potential of tumor cells. Findings presented in Figure 5 demonstrate that increasing levels of PL dramatically reduced invasiveness of the highly invasive PC-3 cells.
Fig. 5.
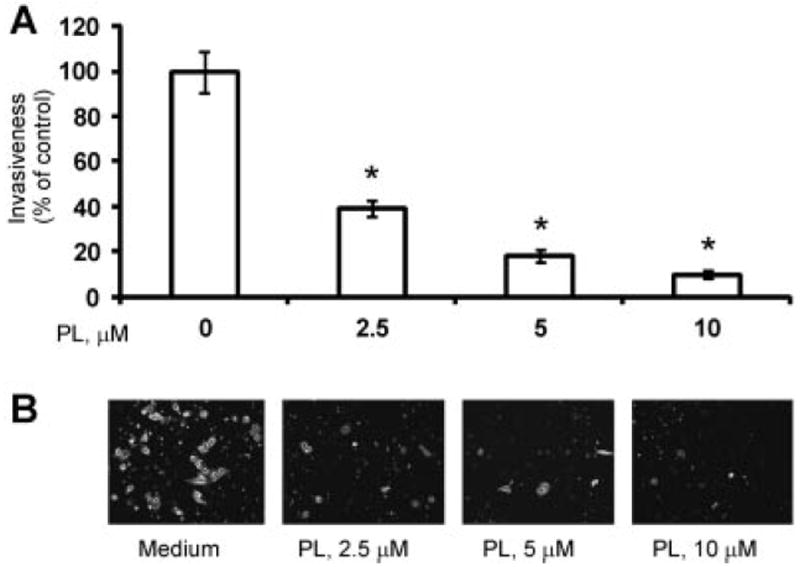
PL reduces invasiveness of PC-3 cells. A: Migration of PC-3 cells through the filters was quantified as described in the Materials and Methods section. Statistical analysis was performed by one-way ANOVA. Statistically significant (P < 0.05) compared with cells cultured in medium alone (*). Representative data from one of three experiments. B: Representative images of PC-3 cells treated under indicated conditions that invaded through the FluoroBlok membrane.
Cell-to-extracellular-matrix interactions have great importance in the ability of malignant cells to metastasize [49]. Figure 6 demonstrates a functional impact of PL on adhesion of PC-3 cells to fibronectin-coated plates, showing dose-dependent decrease in degree of adhesion.
Fig. 6.
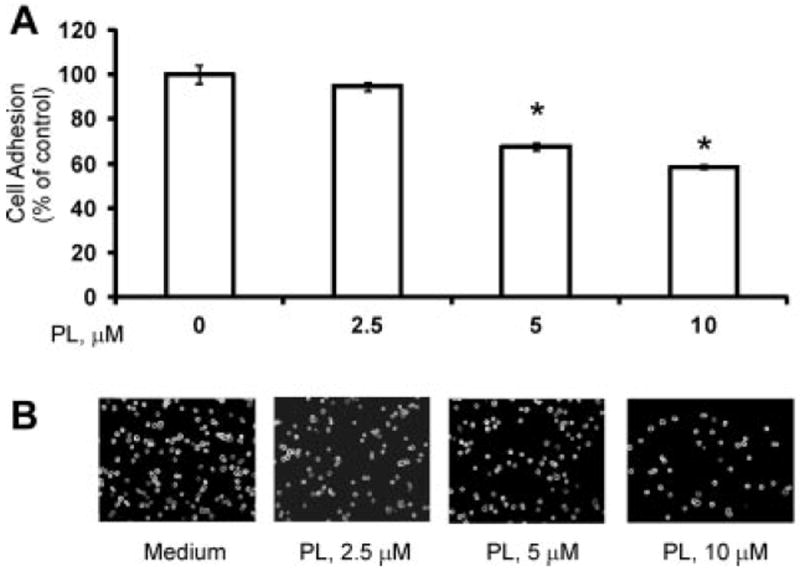
Effect of PL on the adhesion of PC-3 cells. Adhesion of PC-3 cells was determined as described in the Materials and Methods section. A: Adhesion capacity is depicted as tumor cell binding and related to100% binding of non-treated control. Statistical analysis was performed by one-way ANOVA. Statistically significant (P <0.05) compared with cells cultured in medium alone (*). Representative data from one of three experiments. B: Representative images of attached PC-3 cells.
PL Decreases Surface Expression of ICAM-1
Cell-to-cell interactions play a critical role in tumor’s metastatic potential and in prostate cancer have been correlated with increased gene expression and synthesis of the ICAM-1, which is regulated by NF-κB [48,50]. Figure 7 demonstrates that PL significantly inhibits TNF-α-mediated ICAM-1 up-regulation in the PC-3 cell line.
Fig. 7.
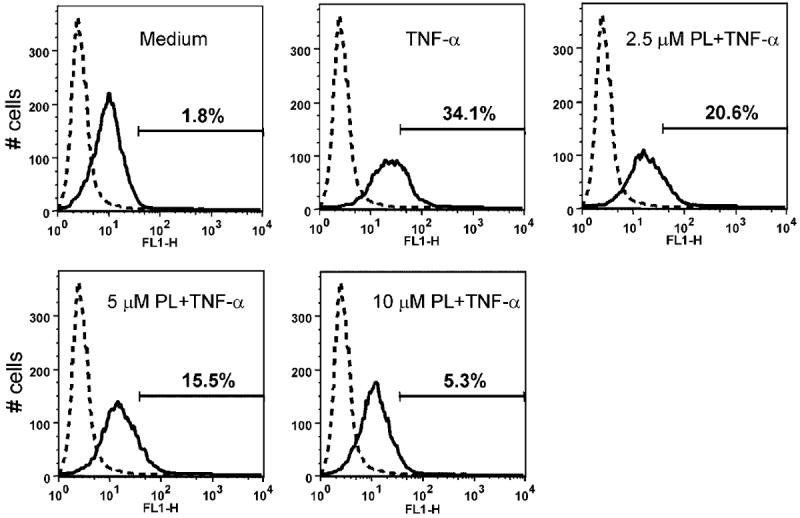
PL significantly inhibits TNF-α mediated ICAM-1 expression in PC-3 cell line. Normal mouse IgG-FITC was utilized as a negative control (dotted line). X-axis represents fluorescence intensity; Y-axis represents cell number. Representative data from one of three experiments.
DISCUSSION
NF-κB is a transcription factor that regulates multiple gene expression, affecting tumor growth, metastasis, and angiogenesis, and is therefore a potential target for cancer treatment and prevention [32,33]. A study by Son et al. [14] recently evaluated effects of PL on vascular smooth muscle cell proliferation and atherosclerotic lesion development, demonstrating a reduction in NF-κB activation. Our study undertook evaluation of PL effects on this ROS-dependent pathway, where we demonstrate in vitro that PL attenuates activation of NF-κB in both androgen-dependent and castrate-resistant prostate cancer cells. PL decreased cell proliferation, adhesion, and invasiveness over a range of concentrations (0–10 μM). PL demonstrated potent concentration-dependent attenuation of both, constitutive and TNF-α-inducible NF-κB activity. Specifically, PL blocked TNF-α mediated degradation of IkBα and thus nuclear translocation of RelA/p65 and p50 subunits. Previously reported, IL-6, IL-8, and MMP-9 are under NF-κB regulatory control, and our current study is consistent and supportive of the above findings [46,51].
It is well known that a number of constitutively activated signaling pathways, such as NF-κB and Akt, play critical roles in proliferation and survival of prostate cancer cells [15,52]. As was previously reported by McCall et al. [53], increased Akt signaling is observed in the progression to castrate-resistant disease. Studies have demonstrated that mTOR, downstream of Akt, stimulates NF-κB activity in prostate cancer cells via interaction with and stimulation of IKK [45]. Additionally, it has been shown that the NF-κB pathway has the ability to regulate Akt activity. Meng et al. [54] demonstrated that NF-κB inhibitors block TNF-α-induced Akt activation. However, TNF-mediated NF-κB activation was not reduced by the PI3K inhibitors such as Wortmannin and LY294002, although these inhibitors completely blocked the activation of Akt [54]. Our recent findings demonstrate that PL effectively inhibits Akt kinase activity and its downstream effectors in prostate (Fig. 3E), kidney, and breast cancer cells (unpublished data). Combined, these findings suggest that TNF-α-induced NF-κB translocation can proceed independent of Akt pathway, although cross-talk between the two pathways is present. PL affects both, TNF-α-induced NF-κB and Akt pathways. We observed that Akt inhibitor IV failed to block NF-κB translocation, while PL was successful in doing so (Fig. 3E). We therefore conclude that PL acts on NF-κB via an Akt-independent pathway. Other specific targets of PL are the subject of future investigations.
In vivo mouse models confirmed favorable toxicity profile of PL and its synergy in combination with 5-FU in sarcoma 180-transplanted mice [55,56]. Furthermore, anti-angiogenic and pro-apoptotic effects have been confirmed in xenograft tumors and transgenic models [22]. With effects detectable at micromolar concentrations and reports of favorable toxicity profile, PL may play an important role in primary or secondary chemoprevention of prostate cancer. Combined with recent reports of selectivity for neoplastic cells, these observations may guide future investigations in identifying PL utility in clinical practice. Additionally, demonstrating promising activity in androgen-independent prostate cancer cell lines, implication for management of advanced, castrate-resistant disease is underscored.
Aberrant activation of Akt/mTOR and NF-κB pathways contributes to both, initiation and progression of prostate cancer. Development of new strategies for prevention and treatment of prostate cancer represents a goal with enormous clinical and scientific merit. Our recent and current works demonstrate that PL acts as a multifocal inhibitor concurrently affecting several signaling pathways, that is, androgen receptor, Akt/mTOR and NF-κB in prostate cancer cells. These studies provide a scientific background for clinical evaluation of PL as a potential multitargeted anti-neoplastic agent.
CONCLUSIONS
PL is a naturally occurring compound with potent selective effects on neoplastic cells. These effects are demonstrated in an ROS-dependent pathway via NF-κB attenuation in vitro. PL affects cell proliferation, adhesion, and invasiveness, and consequently prevents development of aggressive growth characteristics of prostate cancer cells.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants (RO1 CA134463, RO3 CA167671, CCSG, P30 CA006927) and FCCC/Temple Nodal Grant to V.M.K. and the Department of Defense Physician Research Training Award (W81XWH-10-1-0187) and Bucks County Board of Associates Award to A.K.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.El-Amm J, Aragon-Ching JB. The changing landscape in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Med Oncol. 2013;5(1):25–40. doi: 10.1177/1758834012458137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 5.Andriole GL, Bostwick D, Brawley OW, Gomella L, Marberger M, Montorsi F, Pettaway C, Tammela TL, Teloken C, Tindall D, Freedland SJ, Somerville MC, Wilson TH, Fowler I, Castro R, Rittmaster RS, Group RS. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: Results from the REDUCE study. J Urol. 2011;185(1):126–131. doi: 10.1016/j.juro.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PC. Chemoprevention of prostate cancer. N Engl J Med. 2010;362(13):1237–1238. doi: 10.1056/NEJMe1001045. [DOI] [PubMed] [Google Scholar]
- 7.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365(2):97–99. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 8.Bernard CB, Krishnamurty HG, Chauret D, Durst T, Philogene BJR, Sanchezvindas P, Hasbun C, Poveda L, Sanroman L, Arnason JT. Insecticidal defenses of piperaceae from the neotropics. J Chem Ecol. 1995;21(6):801–814. doi: 10.1007/BF02033462. [DOI] [PubMed] [Google Scholar]
- 9.Reddy PS, Jamil K, Madhusudhan P, Anjani G, Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm Biol. 2001;39(3):236–238. [Google Scholar]
- 10.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475(7355):231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Bezerra DP, Pessoa C, de Moraes MO, de Alencar NMN, Mesquita RO, Lima MW, Alves APNN, Pessoa ODL, Chaves JH, Silveira ER, Costa-Lotufo LV. In vivo growth inhibition of sarcoma 180 by piperlonguminine, an alkaloid amide from the Piper species. J Appl Toxicol. 2008;28(5):599–607. doi: 10.1002/jat.1311. [DOI] [PubMed] [Google Scholar]
- 12.Golovine KV, Makhov PB, Teper E, Kutikov A, Canter D, Uzzo RG, Kolenko VM. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate. 2013;73(1):23–30. doi: 10.1002/pros.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong EH, Kim YJ, Kim YJ, Cho HJ, Yu SN, Kim KY, Chang JH, Ahn SC. Piplartine induces caspase-mediated apoptosis in PC-3 human prostate cancer cells. Oncol Rep. 2008;20(4):785–792. [PubMed] [Google Scholar]
- 14.Son DJ, Kim SY, Han SS, Kim CW, Kumar S, Park BS, Lee SE, Yun YP, Jo H, Park YH. Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem Biophys Res Commun. 2012;427(2):349–354. doi: 10.1016/j.bbrc.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: Important mediator or epiphenomenon? J Cell Biochem. 2004;91(1):100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 16.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52(3):183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10(16):5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 18.Eferl R, Wagner EF. AP-1: A double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64(3):224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore TD. The Rel/NF-kappaB signal transduction pathway: Introduction. Oncogene. 1999;18(49):6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 21.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 22.Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63(9):2206–2215. [PubMed] [Google Scholar]
- 23.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 24.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 25.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF CD95 and IL-1. Nature. 1997;385(6616):540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 26.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90(2):373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 27.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 28.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401(6748):82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57(1):141–146. [PubMed] [Google Scholar]
- 30.Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, Sonoda T, Hirano T, Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250(2):607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 31.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7(10):3298–3304. [PubMed] [Google Scholar]
- 32.Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N, Van Waes C. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26(2):119–129. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 34.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 2004;68(2):361–371. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S, Jackson C, Van Waes C. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002;99(4):538–548. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]
- 36.Hao W, Zhu Y, Zhou H. Prognostic value of interleukin-6 and interleukin-8 in laryngeal squamous cell cancer. Med Oncol. 2013;30(1):333. doi: 10.1007/s12032-012-0333-6. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima J, Tachibana M, Ueno M, Miyajima A, Baba S, Murai M. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin Cancer Res. 1998;4(7):1743–1748. [PubMed] [Google Scholar]
- 38.Kolenko V, Bloom T, Rayman P, Bukowski R, Hsi E, Finke J. Inhibition of NF-kappa B activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J Immunol. 1999;163(2):590–598. [PubMed] [Google Scholar]
- 39.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58(13):2720–2723. [PubMed] [Google Scholar]
- 40.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95(26):15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59(17):4291–4296. [PubMed] [Google Scholar]
- 42.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Jr, Whang YE. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277(13):11116–11125. doi: 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 43.Gustin JA, Maehama T, Dixon JE, Donner DB. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor kappa B activity. J Biol Chem. 2001;276(29):27740–27744. doi: 10.1074/jbc.M102559200. [DOI] [PubMed] [Google Scholar]
- 44.McCall P, Bennett L, Ahmad I, MacKenzie LM, Forbes IWG, Leung HY, Sansom OJ, Orange C, Seywright M, Underwood MA, Edwards J. NF kappa B signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br J Cancer. 2012;107(9):1554–1563. doi: 10.1038/bjc.2012.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22(11):1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis. 2006;27(10):1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 47.Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38(3):199–207. doi: 10.1002/(sici)1097-0045(19990215)38:3<199::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, Schwartz SA. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64(15):5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 49.Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 50.Farina AR, Cappabianca L, Mackay AR, Tiberio A, Tacconelli A, Tessitore A, Frati L, Martinotti S, Gulino A. Transcriptional regulation of intercellular adhesion molecule 1 by phorbol ester in human neuroblastoma cell line SK-N-SH involves jun- and fos-containing activator protein 1 site binding complex(es) Cell Growth Differ. 1997;8(7):789–800. [PubMed] [Google Scholar]
- 51.Crispen PL, Uzzo RG, Golovine K, Makhov P, Pollack A, Horwitz EM, Greenberg RE, Kolenko VM. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: Implications for chemoprevention. Prostate. 2007;67(6):582–590. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 52.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62(21):6141–6145. [PubMed] [Google Scholar]
- 53.McCall P, Gemmell LK, Mukherjee R, Bartlett JM, Edwards J. Phosphorylation of the androgen receptor is associated with reduced survival in hormone-refractory prostate cancer patients. Br J Cancer. 2008;98(6):1094–1101. doi: 10.1038/sj.bjc.6604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng F, Liu L, Chin PC, D’Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277(33):29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 55.Bezerra DP, Castro FO, Alves AP, Pessoa C, Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz J Med Biol Res. 2006;39(6):801–807. doi: 10.1590/s0100-879x2006000600014. [DOI] [PubMed] [Google Scholar]
- 56.Bezerra DP, de Castro FO, Alves AP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, de Alencar NM, Mesquita RO, Lima MW, Costa-Lotufo LV. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol. 2008;28(2):156–163. doi: 10.1002/jat.1261. [DOI] [PubMed] [Google Scholar]


