Abstract
Recent advancements at the Linac Coherent Light Source X-ray free-electron laser (XFEL) enabling successful serial femtosecond diffraction experiments using nanometre-sized crystals (NCs) have opened up the possibility of X-ray structure determination of proteins that produce only submicrometre crystals such as many membrane proteins. Careful crystal pre-characterization including compatibility testing of the sample delivery method is essential to ensure efficient use of the limited beamtime available at XFEL sources. This work demonstrates the utility of transmission electron microscopy for detecting and evaluating NCs within the carrier solutions of liquid injectors. The diffraction quality of these crystals may be assessed by examining the crystal lattice and by calculating the fast Fourier transform of the image. Injector reservoir solutions, as well as solutions collected post-injection, were evaluated for three types of protein NCs (i) the membrane protein PTHR1, (ii) the multi-protein complex Pol II-GFP and (iii) the soluble protein lysozyme. Our results indicate that the concentration and diffraction quality of NCs, particularly those with high solvent content and sensitivity to mechanical manipulation may be affected by the delivery process.
Keywords: femtosecond diffraction, crystallography, crystal injector, sample delivery, transmission electron microscopy (TEM), crystal characterization
1. Introduction
The intense, ultra-short pulses produced by the Linac Coherent Light Source (LCLS) at SLAC have expanded the boundaries of structural biology research, enabling the collection of atomic resolution X-ray diffraction patterns from protein crystals that are too small or too radiation sensitive for conventional data collection at synchrotrons. In less than 50 fs, a pulse delivered by the LCLS X-ray free-electron laser (XFEL) can expose a crystal to as many X-ray photons as a typical synchrotron beam produces in about a second. This has enabled a ‘diffraction before destruction’ [1] approach to overcoming radiation damage, where a single diffraction image is produced by a single LCLS pulse before a crystal succumbs to the damaging effects of X-ray exposure; and to obtain a complete dataset, single shot diffraction patterns from thousands of individual crystals are combined [2]. These experiments are carried out in vacuum (to reduce background scatter) at the Coherent X-ray Imaging endstation (CXI) at the LCLS [3]. Injectors are used to deliver crystals of submicrometre dimensions (or up to 5 µm) suspended in small diameter jets or drops of aqueous solutions [4] to a series of LCLS X-ray pulses, produced at a repetition rate of up to 120 Hz. These innovations have enabled serial femtosecond crystallography (SFX) experiments to solve crystal structures using diffraction data from nanometre-sized crystals (NCs) [5–10], opening the door to obtain atomic information from proteins whose intrinsic disorder prevented formation of micrometre-sized crystals such as many membrane proteins and large multi-protein complexes (MPCs).
The first SFX experiments used a gas dynamic virtual nozzle injector (GDVN) [4] for crystal delivery. The GDVN [2,10–15] is currently the primary injector supported at CXI for crystal screening experiments. Other sample delivery systems, such as the nanoflow electrospinning injector [16], have subsequently been developed and offer advantages such as reduced flow rate and thereby lower sample consumption by minimizing sample loss between X-ray pulses. Acoustic and micro-piezo activated drop-on-demand technologies are also in development.
The high demand for LCLS beamtime necessitates careful sample pre-characterization and injector compatibility testing to ensure efficient use of this limited resource. It is standard practice to test the crystal containing solutions with an offline sample injector prior to beam time to detect problems with jet formation due to solution bubbling, clogging from crystal clumping, or the solution drying out as it exits the injector nozzle and enters the vacuum chamber. These issues may often be eliminated by modifying the carrier solution, for example by minimizing the use of detergents or reducing salt concentration. For crystal and solution optimization, an offline setup of the GDVN injectors is available to users through the sample delivery group at the LCLS. These flow tests however do not detect the presence of diffraction quality crystals in the injector reservoir, or exiting the injector nozzle.
NCs of membrane proteins and large MPCs are often mechanically fragile. This fragility maybe further aggravated during the sample delivery process by exposure to high pressure and shear forces. Furthermore, injector efficiency may be reduced by NC clumping and aggregation in fittings such as those connecting the sample reservoir to the nozzle capillary. Therefore, methodologies to detect the presence and quality of NCs within the injector reservoir, and exiting the injector nozzle are essential to test the applicability of injector technologies to specific NC types, and to optimize the carrier solutions.
The work presented here demonstrates the potential of transmission electron microscopy (TEM) to serve as a tool for detecting and evaluating NCs both before and after the injection process. NC diffraction quality may be assessed by calculating the electron diffraction pattern of the NC lattice by fast Fourier transform (FFT).
2. Material and methods
Three types of NCs were tested. The parathyroid hormone receptor 1 (PTHR1) was selected as a representative membrane protein. The PTHR1 is a family B G-protein coupled receptor responsible for bone mineral ion homeostasis. PTHR1 is an example of a protein where only NCs, 200–500 nm in size, have been produced. The second type, a construct comprising a calmodulin-green fluorescent protein (GFP) chimaera bound to calmodulin-binding peptide (CBP) tagged Rpb4 subunit of RNA polymerase II (Pol II-GFP) serves as a representative MPC. This is a green-coloured protein with a large crystal lattice readily identifiable by TEM with crystal sizes ranging from 1 to 5 µm. The crystallization conditions for these systems were determined through direct examination of commercial crystallization screening drops using a novel selection protocol that combines (i) brightfield microscopy, UV fluorescence microscopy and dynamic light scattering as screening methods to detect crystallization drops containing NCs and (ii) TEM to accurately identify protein NCs and determine NC quality [17]. Lysozyme NCs were also tested as a soluble protein control.
(a). Protein expression and purification
PTHR1 and Pol II were expressed and purified as described previously [18]. GFP was expressed and purified by standard methods [19]. Pol II-GFP complex was assembled by adding 2.5 molar excess of GFP in the presence of 1 mM CaCl to promote calmodulin-CBP binding followed by isolation using a Superdex 200 10/300 GL (50 mM Hepes pH 7.5, 100 mM KCl, 4 mM dithiothreitol (DTT), 2 mM CaCl2 and 10 μM ZnCl2).
(b). Protein crystallization
Lysozyme NCs were grown with protein purchased from Sigma and was crystallized in the presence of 10% NaCl and 100 mM sodium acetate. Crystallography conditions for PTHR1 NCs were 200 mM NaCl, 100 mM Bis-Tris pH 5.5, 25% polyethylene glycol (PEG) 3350. Pol II-GFP nanocrystals were grown under 4–6% PEG 8000, 200 mM MgCl2, 1 mM CaCl2, 100 mM Tris pH 7.5 and 10 mM DTT.
(c). Gas dynamic virtual nozzle injector
An offline GDVN injector was set up on the laboratory bench, and an optical microscope was used to observe the nozzle and appearance of the ejected NC containing solution. The GDVN nozzle consists of two coaxial capillaries; a smaller diameter capillary (40 µm ID) that transports the crystal containing solution is centred inside a larger capillary that delivers a pressurized gas stream (typically helium) that surrounds and focuses the liquid stream as it exits the nozzle. The same nozzle was used to eject the PTHR1 and the Pol II-GFP solutions and a comparable nozzle was used with the lysozyme solution. For each tested solution, the gas and liquid pressure (of the order of 200–2000 psi) were varied to produce a continuous stream of liquid extending at least a few millimetres outside of the nozzle. (The X-ray interaction region is around 100 µm from the end of the nozzle). Table 1 lists the gas and liquid pressures used for each NC type. While the GDVN normally delivers solution into a vacuum chamber, for these experiments it was operated in air to facilitate collection of the post-injector solutions. The liquid stream from the GDVN is about 5 µm in diameter, travels at a velocity of about 10 m s−1 and may transport suspended crystals with edges of 10 µm or less. To prevent clogging, the crystal containing solutions may be first filtered, typically by a 10 µm porosity filter, before flowing through a 1/16’ outer diameter tubing reservoir connected to the central capillary of the GDVN nozzle. Solutions exiting the GDVN nozzle were collected onto an upper portion of an inside wall of a centrifuge tube which was immediately sealed. To help prevent dehydration, the bottom of the centrifuge tube contained a small amount of crystallization mother-liquor solution (protein-free).
Table 1.
NC crystal conditions used with the benchtop GDVN injector.
| crystal type | crystallization/carrier solution | crystal volume (%) | liquid pressure (psi) | shield gas pressure (psi) |
|---|---|---|---|---|
| lysozyme | 10% NaCl, 100 mM Na acetate | 80 | 500 | 550 |
| PTHR1 | 200 mM NaCl, 100 mM Bis-Tris pH 5.5, 25% PEG 3350 | 30 | 1200 | 932 |
| Pol II-GFP | 4–6% PEG 8K, 200 mM MgCl2, 1 mM CaCl2, 100 mM Tris pH 7.5 and 10 mM DTT | 60 | 750 | 300 |
(d). Transmission electron microscopy
NC samples were harvested both pre- and post-injector, then stored on ice until needed for grid preparation. Copper grids with carbon film (Electron Microscopy Sciences) were made hydrophlic in a glow discharge for 1 min at 25 mV (EmiTech KX100). The samples were incubated on a hydrophilic grid for 1 min, blotted and twice incubated in 2% uranyl acetate solution for 30 s. The resulting crystal confluence was approximately 70% pre-injector and 45% post-injector for the lysozyme samples, 45% pre-injector and 10% post-injector for the Pol II-GFP samples and 15% pre-injector and very low post-injector for the PTHR1 samples. A FEI Tecani T12 electron microscope operating at 120 kV, using a single-tilt specimen holder, equipped with a 2 k × 2 k Gatan UltraScan 1000 CCD camera for acquiring images, was used to visualize sample grids. Using the FEI T12 user interface program, images were acquired using auto-exposure and low dose mode. The focus of the images was adjusted by minimal contrast and by live FFT. The images were defocused by −500 to −1500 nm in order to obtain more distinct lattice fringes. A select region of the NC lattice underwent an FFT using DigitalMicrograph (Gatan, Inc).
(e). Diffraction screening at Coherent X-ray imaging
SFX experiments using solutions of Pol II-GFP and PTHR1 receptor were carried out at the LCLS CXI instrument [3] using 10 keV X-ray pulses of 40 fs duration with focus size of 200 nm at the X-ray interaction region. The GDVN injector was used for sample delivery using solutions with crystal concentrations of approximately 30% NCs w/v. Diffraction patterns were recorded at 120 Hz using a Cornell-SLAC Pixel Array detector [20] and analysed in real time using cctbx.xfel [21]. Only Pol II-GFP yielded diffraction patterns, and a 2.5% hit rate was obtained with 25% of hits producing indexable images. Hits were determined with a threshold of 200 ADU (determined from light averages of the data) and indexing was performed using the mod_hitfind module of cctbx.xfel with a target cell determined from previously acquired synchrotron datasets.
3. Results
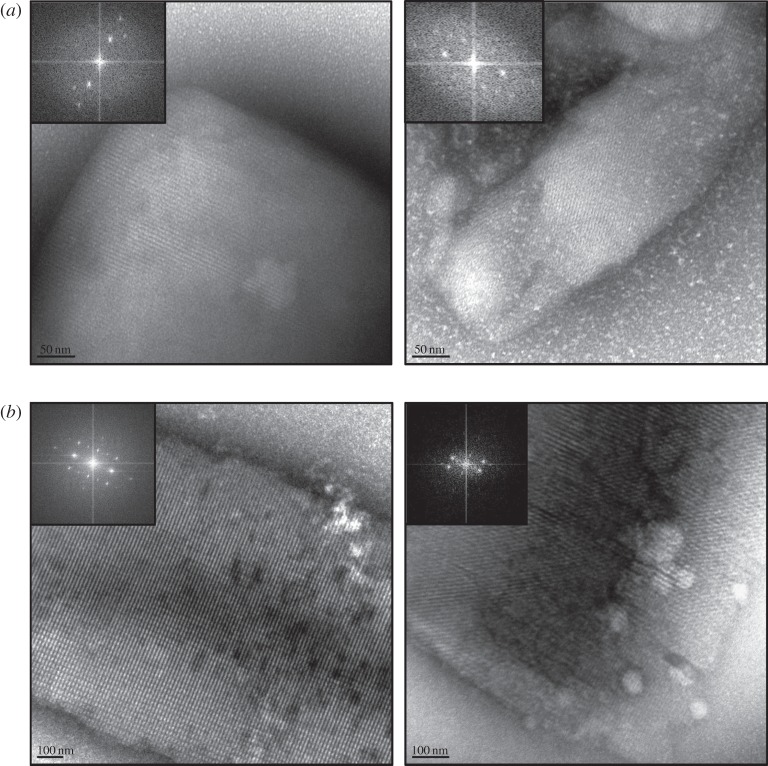
As shown in figure 1a,b both lyzozyme and Pol II-GFP survived the injector. Both pre- and post-injector NCs show clear identifiable lattices and at least third order evenly distributed Bragg spots obtained by FFT of the TEM images. The lattices for lysozyme were observed to be of similar quality both pre- and post-injector. As shown in figure 1b, the quality of the post-injector Pol II-GFP NC lattices was slightly decreased, as indicated by a reduction of Bragg spots from the FFT. As expected from our TEM pre-screening results, during X-ray screening at LCLS CXI the Pol II-GFP NCs produced a number of quality diffraction patterns to 4 Å resolutions, as shown in figure 2.
Figure 1.
(a) TEM images of Lysozyme pre- and post-injector. (b) TEM images of Pol II-GFP pre- and post-injector.
Figure 2.
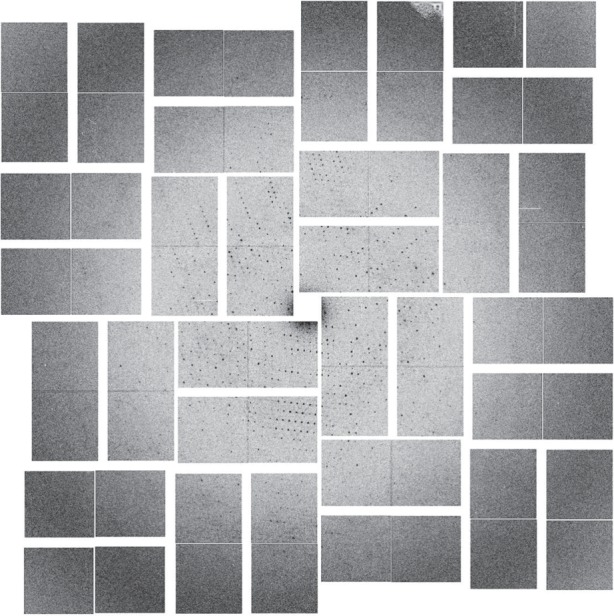
X-ray diffraction image from Pol II-GFP NCs collected at LCLS CXI in November 2013.
For PTHR1, the overall NC concentration was reduced post-injector. A few examples of crystals with a more distinct lattice (figure 3) were found in the post-injector grids that were more sparsely populated; however, the overall change in lattice quality was modest and did not differ pre- and post-injector. A significant contributor to the lower post-injector NC concentration observed in this case is the tendency of these NCs to clump together and gather in the fitting located just prior to the GDVN nozzle capillary. Modification of the internal form factor of the tubing-to-capillary connection may help resolve these problems. To reduce clumping during the experiment at CXI, crystallization conditions were optimized by adjusting PEG and salt concentrations. No diffraction was observed from PTHR1 during SFX experiments at CXI. However, a distinct low-resolution powder ring (40 Å) was observed when a loop of this NC containing reservoir solution was tested at the Stanford Synchrotron Radiation Lightsource BL12–2 at RT using a 20 × 20 µm X-ray beam at 12.5 keV. While the lack of diffraction observed during SFX experiments may be due to a low concentration of NC in the post-injector solution, resulting in zero diffraction ‘hits’, we attribute this negative outcome to crystal disorder. The FFT obtained from the TEM images of PTHR1, even for the more ordered lattice (figure 3), shows only low-order electron diffraction and predominantly in just one lattice direction. While initial results for this protein have been discouraging, we expect that successful SFX experiments using PTHR1 and the GDVN may be feasible through careful optimization of crystallization conditions with NC examination pre- and eventually post-injector using TEM.
Figure 3.
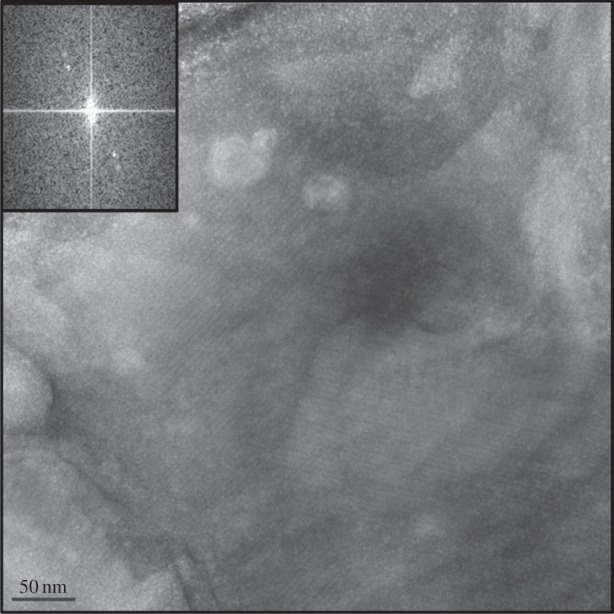
PTHR1 lattice and FFT (insert) observed in the post-injector sample.
4. Conclusion
X-ray free-electron lasers offer the possibility to solve three-dimensional structures of proteins that can only crystallize as NCs, and methods to distinguish NCs, optimize NC quality, and evaluate ideal sample delivery options prior to beamtime are essential components for efficient use (and in some cases successful experiments) of these high demand facilities. We have demonstrated the utility of negatively stained TEM as a straightforward tool for distinguishing NCs with edges shorter than 5 µm, and for evaluating the NC lattice both pre- and post-injector. X-ray diffraction quality of NCs may be assessed through examination of the NC lattice in TEM images both visually and by calculating the FFT of the image. While more work is necessary, these results indicate that evenly distributed third order or higher Bragg spots in the FFT of negative stained lattice images could be a prerequisite for obtaining measureable X-ray diffraction patterns at XFEL sources. Since cryo-electron microscopy does not rely on the use of harsh stains (potentially damaging NCs) and can provide higher resolution images, this method could constitute a more accurate method to evaluate NC quality. A future study to correlate electron diffraction with XFEL diffraction could assist the experimenter to select the best possible samples to be tested at an XFEL source.
The effects that the injection process in itself could have on diffraction quality have not been previously investigated using TEM. These effects could be of great consequence for fragile crystals with high solvent contents or for membrane proteins solubilized in detergents. Our initial results using the GDVN show that: (i) crystal concentration pre- and post-injector may vary significantly and (ii) analysis of post-injector crystals using TEM shows that diffraction quality of NCs could potentially be adversely affected by the delivery process. As indicated by the examination of Pol II GFP crystals that have 75–80% solvent content, these effects could be of particular relevance for samples with high solvent content. The success of SFX experiments may be appreciably improved through careful optimization of the carrier solutions and injector configuration for particular nanocrystal types through the use of TEM to assess diffraction quality both pre- and post-injector.
Acknowledgements
Portions of this research were carried out at the Linac Coherent Light Source, a National User Facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The CXI instrument was funded by the LCLS Ultrafast Science Instruments (LUSI) project funded by the US Department of Energy, Office of Basic Energy Sciences. We thank Marc Messerschmidt and Garth Williams of the LCLS and Robert L. Shoeman and Sabine Botha of the Max Plank Institute for Medical Research for support of data collection at CXI. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
References
- 1.Neutze R, Wouts R, van der Spoel D, Weckert E, Hajdu J. 2000. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 406, 752–757. ( 10.1038/35021099) [DOI] [PubMed] [Google Scholar]
- 2.Chapman HN, et al. 2011. Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77. ( 10.1038/nature09750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutet S, Williams GJ. 2010. The Coherent X-ray Imaging (CXI) instrument at the Linac Coherent Light Source (LCLS). New J. Phys. 12, 035024 ( 10.1088/1367-2630/12/3/035024) [DOI] [Google Scholar]
- 4.DePonte DP, Weierstall U, Schmidt K, Warner J, Starodub D, Spence JCH, Doak RB. 2008. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D: Appl. Phys. 41, 195505 ( 10.1088/0022-3727/41/19/195505) [DOI] [Google Scholar]
- 5.Aquila A, et al. 2012. Time-resolved protein nanocrystallography using an X-ray free-electron laser. Opt. Express 20, 2706–2716. ( 10.1364/OE.20.002706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutet S, et al. 2012. High-resolution protein structure determination by serial femtosecond crystallography. Science 337, 362–364. ( 10.1126/science.1217737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter MS, Fromme P. 2011. Toward structure determination using membrane-protein nanocrystals and microcrystals. Methods 55, 387–404. ( 10.1016/j.ymeth.2011.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern J, et al. 2013. Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 340, 491–495. ( 10.1126/science.1234273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koopmann R, et al. 2012. In vivo protein crystallization opens new routes in structural biology. Nat. Methods 9, 259–262. ( 10.1038/nmeth.1859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redecke L, et al. 2013. Natively inhibited Trypanosoma brucei cathepsin B structure determined by using an X-ray laser. Science 339, 227–230. ( 10.1126/science.1229663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson LC, et al. 2012. Lipidic phase membrane protein serial femtosecond crystallography. Nat. Methods 9, 263–265. ( 10.1038/nmeth.1867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomb L, et al. 2011. Radiation damage in protein serial femtosecond crystallography using an X-ray free-electron laser. Phys. Rev. B 84, 214111 ( 10.1103/PhysRevB.84.214111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barty A, et al. 2012. Self-terminating diffraction gates femtosecond X-ray nanocrystallography measurements. Nat. Photonics 6, 35–40. ( 10.1038/nphoton.2011.297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas RM. 2013. De novo protein crystal structure determination from X-ray free-electron laser data. Nature 505, 244–247. ( 10.1038/nature12773) [DOI] [PubMed] [Google Scholar]
- 15.DePonte DP, Doak RB, Hunter M, Liu Z, Weierstall U, Spence JCH. 2009. SEM imaging of liquid jets. Micron 40, 507–509. ( 10.1016/j.micron.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 16.Sierra RG, et al. 2012. Nanoflow electrospinning serial femtosecond crystallography. Acta Cryst. D68, 1584–1587. ( 10.1107/S0907444912038152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson HP, et al. In press. Use of Transmission Electron Microscopy to identify nanocrystals of challenging protein targets. Proc. Natl Acad. Sci. USA. [DOI] [PMC free article] [PubMed]
- 18.Pullara F, et al. 2013. A general path for large-scale solubilization of cellular proteins: from membrane receptors to multiprotein complexes. Protein Expr. Purif. 87, 111–119. ( 10.1016/j.pep.2012.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieryck W, Noubhani AM, Coulon D, Santarelli X. 2003. Cloning, expression and two-step purification of recombinant His-tag enhanced green fluorescent protein over-expressed in Escherichia coli. J. Chromatogr. B 786, 153–159. ( 10.1016/S1570-0232(02)00764-X) [DOI] [PubMed] [Google Scholar]
- 20.Hart P, et al. 2012. The CSPAD megapixel X-ray camera at LCLS. X-Ray free-electron lasers: beam diagnostics, beamline instrumentation, and applications. 8504, 8504°C.
- 21.Sauter NK, Hattne J, Grosse-Kunstleve RW, Echols N. 2013. New python-based methods for data processing. Acta Crystallogr. D69, 1274–1282. ( 10.1107/S0907444913000863) [DOI] [PMC free article] [PubMed] [Google Scholar]