Abstract
Bloom syndrome (BS) is an inherited genomic instability disorder caused by disruption of the BLM helicase and confers an extreme cancer predisposition. Here we report on a girl with BS who developed acute lymphoblastic leukaemia (ALL) at age nine, and treatment-related acute myeloid leukaemia (t-AML) aged 12. She was compound heterozygous for the novel BLM frameshift deletion c.1624delG and the previously described c.3415C>T nonsense mutation. Two haematological malignancies in a child with BS imply a fundamental role for BLM for normal haematopoiesis, in particular in the presence of genotoxic stress.
Keywords: Bloom syndrome, childhood leukaemia, treatment related leukaemia
Introduction
Bloom syndrome (BS, OMIM #210900) is an autosomal recessive disorder with proportionate pre- and postnatal growth retardation, inflammatory skin changes due to hypersensitivity to UV light, telangiectatic, hypo- and hyperpigmented skin flecks, and predisposition to malignancy. BS is caused by mutations in the BLM gene on chromosome 15q26.1, which encodes a RecQ helicase, and belongs to the group of genomic instability disorders with DNA repair defects (1-3). The mutation spectrum in BS patients is well defined and comprises missense and nonsense mutations, small and large deletions, and small duplications and insertions many of which represent founder mutations in distinct ethnic groups (3). More than half of the BS patients reported to the BS registry (http://weill.cornell.edu/bsr/genetics/) have developed cancer, 10-15% of which are haematological. In children several communications have described acute lymphoblastic leukemia (ALL) (4), myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) (5, 6), as has been in adults (7). Here we report the clinical findings and course of a girl with BS due to compound heterozygosity for a novel and a previously described BLM mutation, who developed ALL and secondary AML with a short latency, and discuss clinical and genetic implications.
Case report and cytogenetic analysis
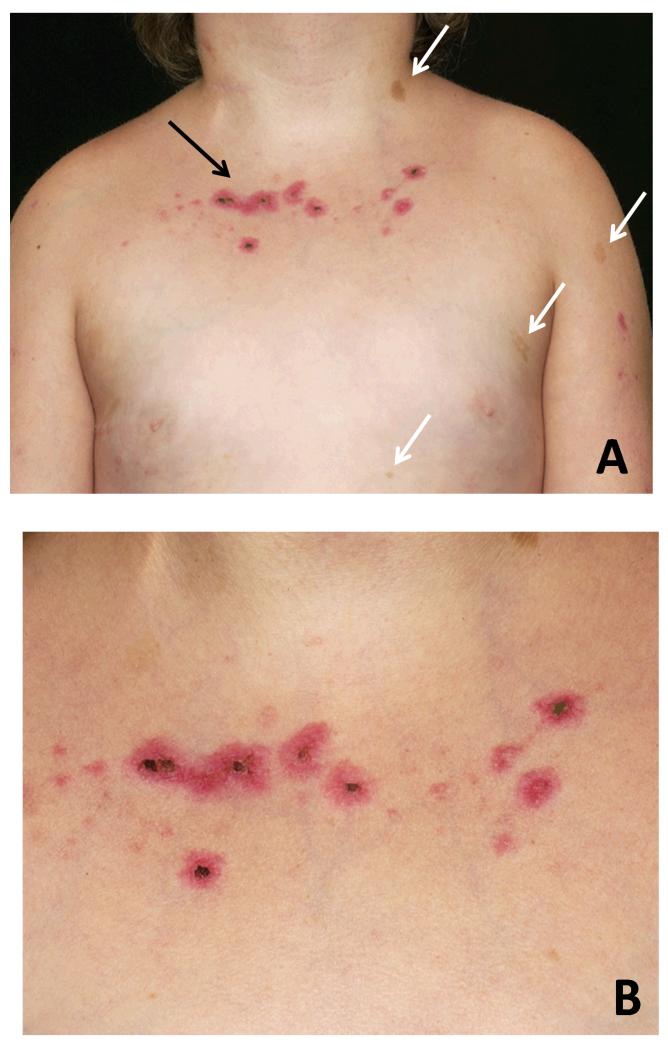
The girl was born to a non-consanguineous white British couple. Her birth weight was 1.88 kg (<0.4th centile). During early childhood she was noted to be short and to develop café-aulait patches (Figure 1A) and darker raised pigmented naevi on her arms and legs. Dysmorphic features with a long smooth philtrum, epicanthic folds, thin vermillion borders, prominent ears and triangular facies were also noted, but no diagnosis was made. At 9 years of age, the girl was referred for unexplained bruising. She had pancytopenia and circulating lymphoid blast cells consistent with acute lymphoblastic leukaemia. Bone marrow aspiration confirmed the diagnosis of pre-B cell ALL. Features of bone marrow blasts were consistent with the diagnosis of common ALL (Table 1). Induction chemotherapy was commenced according to the ALL 2003 trial (8), initially without significant side-effects. However, during a neutropenic episode after the first intensification she developed a painful photosensitive rash that healed slowly (Figure 1B).
Figure 1. Clinical features.
A: Four café-au-lait spots (white arrows) on the skin of the Bloom syndrome patient with a photosensitive rash (black arrow). B: Detailed view of the photosensitive rash that the girl developed while on chemotherapy.
Table 1. Morphology, immunophenotype and cytogenetic changes of ALL and subsequent MDS transforming into AML in the girl with Bloom syndrome.
| BM Morphology |
Immunophenotype | Cytogenetics | |
|---|---|---|---|
| ALL | 95% primitive mononuclear cells with lymphoblast morphology | DR+/TdT+/CD10++/CD38+ and cmu+ CD34/CD45 neg/weak | 46,XX. Non-clonal structural abnormalities noted. FISH for BCR/ABL, MLL, TEL/AML1 and IGH neg. |
| MDS/AML | Trilineage dysplasia. About 20% blasts. | CD34+ CD7 weak/CD13+/CD33+/CD117+/ CD36+ TdT−/ MPO−, CD41 − | 41~43,X,add(X)(q2),add(1)(p21),add(1)(q1)x2,,add(1)(p1)x2,add (3)(p21),-4,-5,-6,-6,add(7)(p13),add(9)(p13), add(9)(p13),+add (11)(p1),add(12)(p13),-13,-14,-15,-17,add(19)(p13) -21,+4~8. mar[cp7]/46,XX[1] |
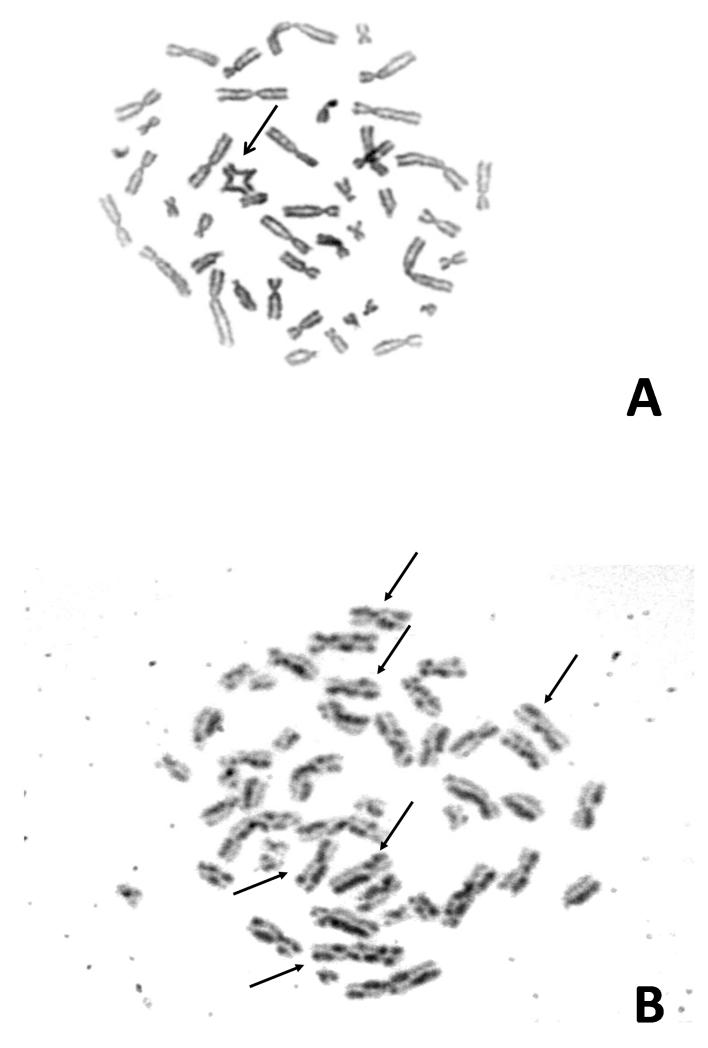
In view of the new diagnosis of ALL with a significant photosensitive skin reaction on chemotherapy in the presence of mild dysmorphic features and a history of IUGR, the possibility of a DNA repair disorder, in particular BS, was considered. Sister chromatid exchange (SCE) and karyotype analysis was carried out using standard protocols. In brief, four phytohaemagglutinin (PHA) stimulated whole blood cultures were established from the proband and an age matched control, respectively. Two unsychronised cultures were exposed to Bromodeoxyuridine (BrdU) at a concentration of 10 mg/ml, one unsychronised culture had no BrdU and the final culture was synchronised with thymidine and deoxycytidine. All of the cultures were harvested after 72 h. Metaphases obtained from the BrdU treated cultures were exposed to UV and 2xSSC for 12 min each and then stained for 2 min in Giemsa. 50 cells from each of these cultures were analysed to obtain the level of SCE. Metaphases obtained from the unsynchronised culture were solid stained with Giemsa. 50 cells were examined from this culture to obtain the level of spontaneous chromosome damage. Metaphases obtained from the synchronised culture were G banded using Trypsin and Giemsa. This culture was analysed to obtain the constitutional karyotype. The solid Giemsa stained slides revealed a greater than 10-fold increased rate of spontaneous chromosome breaks in this patient (0.24 aberrations/cell) compared to control cases (0.02 aberrations/cell), including distinct quadriradial reunion figures of homologous chromosomes (Figure 2A). After BrdU exposure of PHA-stimulated cultured lymphocytes there was evidence of a greater than 10-fold increase in SCE levels in this patient (92 SCEs/cell) compared to two control cases (6 and 5 SCEs/cell) (Figure 2B). This was consistent with a diagnosis of BS. Biochemistry profiling showed raised levels of cholesterol (10.9 mmol/L) and triglycerides (4.7 mmol/l), further supporting this diagnosis (9). A lymphoblastoid cell line of peripheral blood lymphocytes from this girl was established by EBV transformation. The diagnosis of BS was made three weeks into treatment. Morphological remission from ALL was documented at day 28 into treatment. However, minimal residual disease (MRD) analysis after induction was indicative of high risk according to the protocol. She continued chemotherapy following Regimen A (8), complicated by mild pancreatitis at week six of treatment. Vincristine and dexamethasone doses were reduced to 50% due to severe gastrointestinal side effects and hypertension. Two intensification blocks were scheduled with 25% cyclophosphamide and 66% cytarabine of the protocol recommended dose. Anthracyclines were considered too toxic from experience with other patients affected by related chromosomal instability syndromes (10), and omitted. Maintenance treatment was tolerated with frequent dose adaptations including dexamethasone pulses, due to bone marrow suppression and hypertension. She completed two years treatment and was at that time in molecular remission. Sixteen months into follow up for her ALL, she developed again thrombocytopenia. However, bone marrow aspiration did not show relapse of ALL, but hypercellular myelodysplastic syndrome (MDS RAEB 2) with tri-lineage myeloid dysplastic features, 10-15% blasts and complex cytogenetic aberrations (Table 1, Figure 3). Despite treatment with low dose cytarabine in order to delay the progression to AML, two months later she had 49% blasts with M6 morphology on bone marrow aspiration, consistent with AML. She was commenced on fludarabine (30 mg/m2/d) for 5 days and attenuated doses of cytarabine (750 mg/m2/d). This resulted in grade IV myelotoxicity and severe mucositis requiring total parenteral nutrition and intensive blood product and antibiotic support. Three weeks after chemotherapy bone marrow aspirate showed persistence of disease. A second course of chemotherapy was commenced, resulting again in severe mucositis and extreme myelosuppression, but blasts persisted on BM examination. In view of the refractory disease and extreme toxicity, active cytotoxic treatment was discontinued. She died three weeks later aged 12 years and 10 months.
Figure 2. Cytogenetic manifestations.
Giemsa-stained metaphases from 72-h-cultures of cells from the girl with Bloom syndrome. A: Double-symmetrical quadriradial figure (arrow) representing a rearrangement between homologous chromosomes from a PHA-stimulated whole blood culture of peripheral blood lymphocytes. B: Characteristic Harlequin chromosomes (arrows) in a metaphase from a PHA-stimulated peripheral blood lymphocyte culture exposed to BrdU, indicating a greatly increased rate of sister chromatid exchanges (SCEs). C: A metaphase from an EBV-transformed lymphoblastoid cell line showing only occasional SCEs (arrow) suggesting a normal SCE rate and somatic reversion of the line.
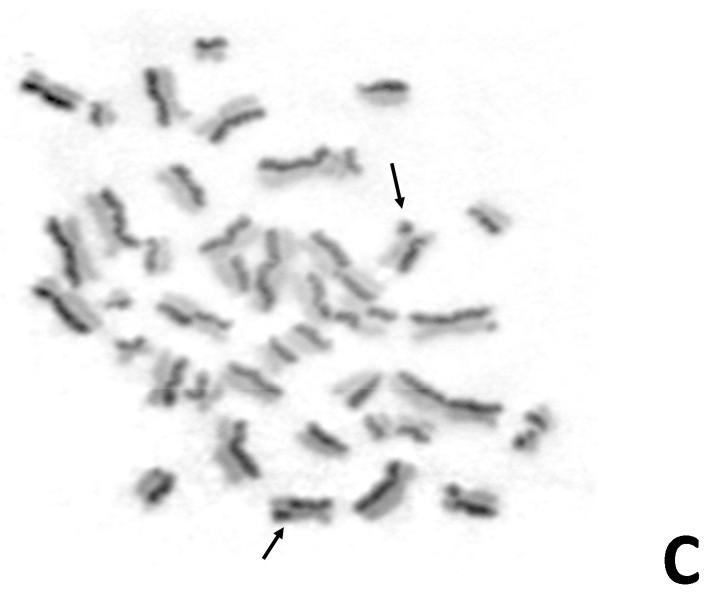
Figure 3. Karyotypic changes in an AML cell of the Bloom syndrome patient.
Karyotype from an unstimulated 40-h-culture bone marrow AML blast from the girl’s secondary AML: 41,X,add(X)(q26),add(1)(p2),+add(1)(q1)x2,add(3)(p21),-4,-5,-6,-6,add(7)(p13),add(9)(p13)x2,+add(11)(p11),add(12)(p13)-13,-14,-14,-15,-16,-17,add(19)(p13),-20,-21,-22+5mar.
Mutational analysis
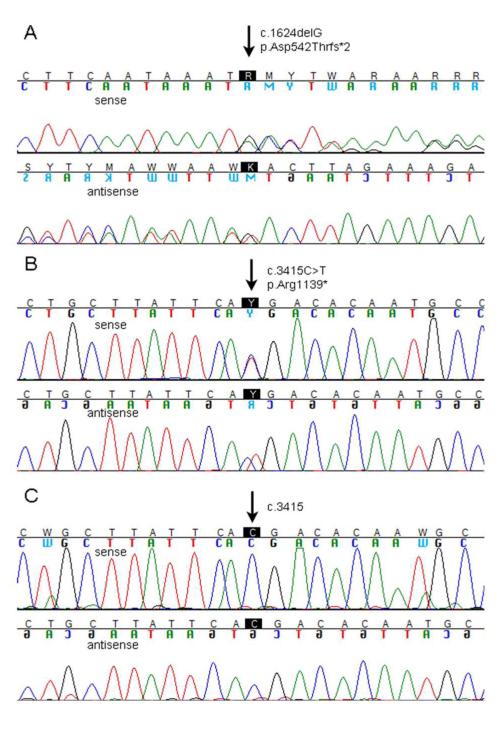
Mutation analysis was carried out on genomic DNA from peripheral blood, bone marrow aspirates at diagnosis of ALL and AML and during treatment, and a patient-derived lymphoblastoid cell line using exon scanning sequencing of the entire BLM gene (primer sequences available on request). In DNA extracted from peripheral blood lymphocytes, we detected the heterozygous BLM mutation c.1624delG in exon 7, which results in a shift of the reading frame and STOP after 3 bp, p.(Asp542Thrfs*2) (Figure 4A). As a second heterozygous mutation we detected c.3415C>T in exon 18, which has been described once previously (3), and results in an immediate STOP, (p.Arg1139*) (Figure 4B). Of note, both mutations appear to be very rare. The former is novel, the latter was observed in a patient of North American ancestry and classified by German et al. (2007) among 45 unique BLM mutations [3]. Both mutations truncate the protein, inconsistent with normal BLM function. Mutational analysis of a lymphoblastoid cell line from the patient confirmed the c.1624delG mutation. However, the c.3415C>T mutation was no longer detectable, implying total reversion in that cell type (Figure 4C). This fact was consistent with the observation of phenotypic reversion of the lymphoblastoid cell line displaying a normal rate of 4.1 ± 2.6 SCEs (mean ± SD) per metaphase (Figure 2C). To explore to what extent the genetic reversion that we detected in cultured lymphoblasts might have contributed to this girl’s clinical course, we repeated the mutation analysis in sequential samples obtained from bone marrow at diagnosis of the ALL and after induction chemotherapy, and at diagnosis and refractory disease of the AML. At all stages of disease progression both mutations were detected.
Figure 4. Mutation analysis in the girl with Bloom syndrome.
Electropherogams from Sanger sequencing. A: DNA from peripheral blood reveals the heterozygous mutation c.1624delG in BLM exon 7 on the sense (upper panel) and antisense (lower panel) strand. B: The other heterozygous mutation in peripheral blood DNA is c.3415C>T in BLM exon 18 shown on the sense (upper panel) and antisense (lower panel) strand. C: DNA from a lymphoblastoid cell line retains the heterozygous mutation c.1624delG on both strands, but has lost the mutation c.3415C>T (sense and antisense strand, respectively), confirming somatic reversion.
Discussion
BS is a cancer predisposition disorder with an inherited defect in DNA repair caused by disruption of the RecQ helicase encoded by the BLM gene. Mutational analysis of genomic DNA of the girl reported here revealed a novel mutation, c.1624delG that adds to the spectrum previously described in BLM patients from Welsh or English ethnic and geographical background (3). It also suggests that the c.3415C>T mutation, previously described in an individual with North American ancestry (3), might have its origin in the British Isles. Both mutations of the present BS patient are essentially null mutations, not permitting the expression of residual functional protein in compound heterozygous condition, suggesting that all disease manifestations of the patient are most probably ramifications of their presence. In contrast to the spectrum of malignancies in other chromosomal instability syndromes and in particular other disorders with RecQ helicase disruption, the distribution of cancers in BS is overall reflecting that of the normal population with a much younger age patterns and higher frequency, and includes lymphoid and myeloid leukaemia with median age of onset of leukaemia and lymphomas of 20 years (11). Although haematological malignancies are developing frequently in BS, MDS transforming to AML has been reported only in three paediatric cases (5, 6, 12), and one younger adult (7). These developed de novo and were not associated with prior treatment for earlier malignant disease. This is to our knowledge the first case with a myeloid malignancy secondary to treatment for preceding ALL in BS. There is no other data available of cytogenetic changes in acute lymphoblastic leukaemia associated with BS, but it is of note that the ALL in the present case was not hyperdiploid and lacked chromosomal rearrangements common for childhood ALL. Cytogenetic analysis of the MDS/AML subsequently revealed a complex karyotype in keeping with it representing a treatment (t)-related MDS/AML, and harboured chromosomal aberrations invariably associated with very poor prognosis. These included del5 and del7, which are frequently found in adults with t-AML after ALL (13). This occurred in the BS patient reported here with a latency similar to that of the other few reported t-AMLs that developed after ALL, but in much older individuals, and which represent only a small fraction of t-AMLs (14). This is in keeping with the accelerated aging phenotype of BS, and implies a fundamental role of the BLM protein for normal haematopoiesis and leukemia prevention, in particular in response to DNA-damaging agents. Loss of the mutation c.3415C>T associated with reversion of the cellular phenotype was detected in a lymphoblastoid cell line from the girl. This finding corresponds to common experience with other BS lymphoblastoid lines: German et al. (2007) reported that cytogenetic analysis determined low (i.e., normal) SCE levels in 15 out of 23 of their BS cell lines [3], though the mutation c.3415C>T in particular has not yet been recounted as revertible. The mechanisms by which such reversions emerge as well as the fact that approximately 20% of persons with typical BS have a proportion of lymphocytes (usually a small one) in blood exhibiting a low-SCE rate have been described previously (15). But even if there were a few undetectable pre-existing reverted cells in blood of the girl presented here --of the type that became prevalent in the lymphoblastoid line--they did not confer a benefit to the patient, as has never been described for any other BS patient. It may be concluded that the development of malignancies is faster in BS patients than the perseverance of mosaicism in the haematopoietic system. At any rate, the reversion was detectable in ALL or AML blood and bone marrow samples of the patient presented here suggesting that both haematological malignancies did not arise from revertant cells. Apparently it is the BS cellular phenotype that facilitates the development of leukaemia. We can also infer that the mechanism of the terminal chemo-unresponsiveness and refractory AML in this case must involve secondary genetic changes other than BS gene reversion.
Acknowledgements
SM is supported by CRUK, Leukemia Lymphoma Research, UK, and the Kay Kendall Leukaemia Fund.
Abbreviations
- BS
Bloom syndrome
- BLM
Bloom syndrome helicase gene or protein
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- t-(AML)
treatment related myeloid leukemia
- MRD
Minimal residual disease
- SCE
sister chromatid exchange
References
- 1.German J. Bloom’s syndrome. Dermatol Clin. 1995 Jan;13(1):7–18. [PubMed] [Google Scholar]
- 2.German J. Bloom’s syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet. 1997 Jan;93(1):100–106. doi: 10.1016/s0165-4608(96)00336-6. [DOI] [PubMed] [Google Scholar]
- 3.German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum Mutat. 2007 Aug;28(8):743–753. doi: 10.1002/humu.20501. [DOI] [PubMed] [Google Scholar]
- 4.Werner-Favre C, Wyss M, Cabrol C, Felix F, Guenin R, Laufer D, et al. Cytogenetic study in a mentally retarded child with Bloom syndrome and acute lymphoblastic leukemia. Am J Med Genet. 1984 Jun;18(2):215–221. doi: 10.1002/ajmg.1320180205. [DOI] [PubMed] [Google Scholar]
- 5.Aktas D, Koc A, Boduroglu K, Hicsonmez G, Tuncbilek E. Myelodysplastic syndrome associated with monosomy 7 in a child with Bloom syndrome. Cancer Genet Cytogenet. 2000 Jan 1;116(1):44–46. doi: 10.1016/s0165-4608(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 6.Poppe B, Van Limbergen H, Van Roy N, Vandecruys E, De Paepe A, Benoit Y, et al. Chromosomal aberrations in Bloom syndrome patients with myeloid malignancies. Cancer Genet Cytogenet. 2001 Jul 1;128(1):39–42. doi: 10.1016/s0165-4608(01)00392-2. [DOI] [PubMed] [Google Scholar]
- 7.Iwahara Y, Ishii K, Watanabe S, Taguchi H, Hara H, Miyoshi I. Bloom’s syndrome complicated by myelodysplastic syndrome and multiple neoplasia. Intern Med. 1993 May;32(5):399–402. doi: 10.2169/internalmedicine.32.399. [DOI] [PubMed] [Google Scholar]
- 8.Moorman AV, Richards SM, Robinson HM, Strefford JC, Gibson BE, Kinsey SE, et al. Prognosis of children with acute lymphoblastic leukemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21) Blood. 2007 Mar 15;109(6):2327–2330. doi: 10.1182/blood-2006-08-040436. [DOI] [PubMed] [Google Scholar]
- 9.Diaz A, Vogiatzi MG, Sanz MM, German J. Evaluation of short stature, carbohydrate metabolism and other endocrinopathies in Bloom’s syndrome. Horm Res. 2006;66(3):111–117. doi: 10.1159/000093826. [DOI] [PubMed] [Google Scholar]
- 10.Meyer S, Kingston H, Taylor AM, Byrd PJ, Last JI, Brennan BM, et al. Rhabdomyosarcoma in Nijmegen breakage syndrome: strong association with perianal primary site. Cancer Genet Cytogenet. 2004 Oct 15;154(2):169–174. doi: 10.1016/j.cancergencyto.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Monnat RJ., Jr Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin Cancer Biol. 2010 Oct;20(5):329–339. doi: 10.1016/j.semcancer.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brassesco MS, Valera ET, Scrideli CA, Tone LG. Bloom’s and myelodysplastic syndromes: Report of a rare pediatric case with gain of an isochromosome 5p. Leuk Res. 2012 Jan;36(1):e18–19. doi: 10.1016/j.leukres.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Verma D, O’Brien S, Thomas D, Faderl S, Koller C, Pierce S, et al. Therapy-related acute myelogenous leukemia and myelodysplastic syndrome in patients with acute lymphoblastic leukemia treated with the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimens. Cancer. 2009 Jan 1;115(1):101–106. doi: 10.1002/cncr.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilera DG, Vaklavas C, Tsimberidou AM, Wen S, Medeiros LJ, Corey SJ. Pediatric therapy-related myelodysplastic syndrome/acute myeloid leukemia: the MD Anderson Cancer Center experience. J Pediatr Hematol Oncol. 2009 Nov;31(11):803–811. doi: 10.1097/MPH.0b013e3181ba43dc. [DOI] [PubMed] [Google Scholar]
- 15.Ellis NA, Ciocci S, German J. Back mutation can produce phenotype reversion in Bloom syndrome somatic cells. Hum Genet. 2001 Feb;108(2):167–173. doi: 10.1007/s004390000447. [DOI] [PubMed] [Google Scholar]