Abstract
Malignant pleural mesothelioma (MPM) is a rare and aggressive cancer of the mesothelium with only a limited range of treatment options that are largely ineffective in improving survival. Recent efforts have turned toward the analysis of specific, dysregulated biologic pathways for insight into new treatment targets. Epigenetic regulation of tumor suppressor genes through chromatin condensation and decondensation has emerged as an important mechanism that leads to tumorogenesis. A family of histone acetyltransferases and deacetylases regulates this balance, with the latter facilitating chromatin condensation, thus preventing gene transcription, resulting in the loss of heterozygosity of tumor suppressors. Inhibition of this process, coupled with a similar inhibition of nonhistone protein deacetylation, ultimately leads to the promotion of apoptosis, cell cycle arrest, and inhibition of angiogenesis. An increasing amount of preclinical data highlighting the effectiveness of histone deacetylase inhibition in MPM cell lines and mouse xenograft models has led to a number of early phase clinical trials in patients with MPM. The results of these efforts have led to a multicenter, randomized, placebo-controlled phase III study of the histone deacetylase inhibitor vorinostat in patients with advanced MPM, offering hope for a new and effective therapy in patients with this disease.
Keywords: Pleural mesothelioma, Histone deacetylase inhibitor, Vorinostat
Malignant pleural mesothelioma (MPM) is a rare and aggressive cancer that arises from the mesothelial cells that line the pleural cavity. The incidence in the United States has increased over time, with 2000 to 3000 cases now diagnosed annually. The mortality rate is high: more than 80% of patients present with late stage disease, and the median survival does not exceed 12 months.1 Its association with asbestos exposure was first recognized in 1960.2 Studies using Surveillance, Epidemiology, and End Results data show current and predicted incidences of disease mirroring trends in asbestos use during the last century, with a latency of 20 to 40 years between peak consumption in the 1950s and 1960s to peak incidence of disease during this decade.3,4
The current treatment options for patients with MPM are limited and largely ineffective. Surgical options include thoracoscopy for palliation, pleurectomy/decortication, and extrapleural pneumonectomy, the last two of which can be offered in only a minority of patients, both for reasons of advanced disease and comorbid conditions.5–8 More recently, the use of induction chemotherapy before extrapleural pneumonectomy and postoperative radiation therapy has shown promise for improved outcomes.9,10 With regards to systemic therapy, a great number of early phase trials of chemotherapy and novel therapeutics have been conducted, but with disappointing results.11 Phase III trials have demonstrated improved survival with an antifolate plus cisplatin over treatment with cisplatin alone.12,13 Although these data provided the basis for a new standard in the first-line treatment of patients with unresectable disease, survival was improved by only a few months, and overall survival remains poor. The search for new, effective therapies that capitalize on the biology of MPM continues.
Epigenetic Regulation
Epigenetic modification has emerged as an important mechanism leading to tumorogenesis. These changes, distinct from the processes of mutagenesis, maintain a degree of heritability that provide the cancer cell an additional means by which to avoid the regulatory mechanisms that limit cell growth and proliferation. Both hypermethylation and histone regulation have been linked to the development of MPM. Data supporting the former, in which CpG islands within gene promoters are methylated and silenced, is sparse and based on the identification of simian virus 40 viral sequences in mesothelioma cell lines and tumor samples, with increased tumor suppressor gene methylation found in simian virus 40 large T-antigen containing specimens.14,15 Data for the latter is more robust and helps to provide a basis for ongoing trials in MPM using inhibitors of histone deacetylation.
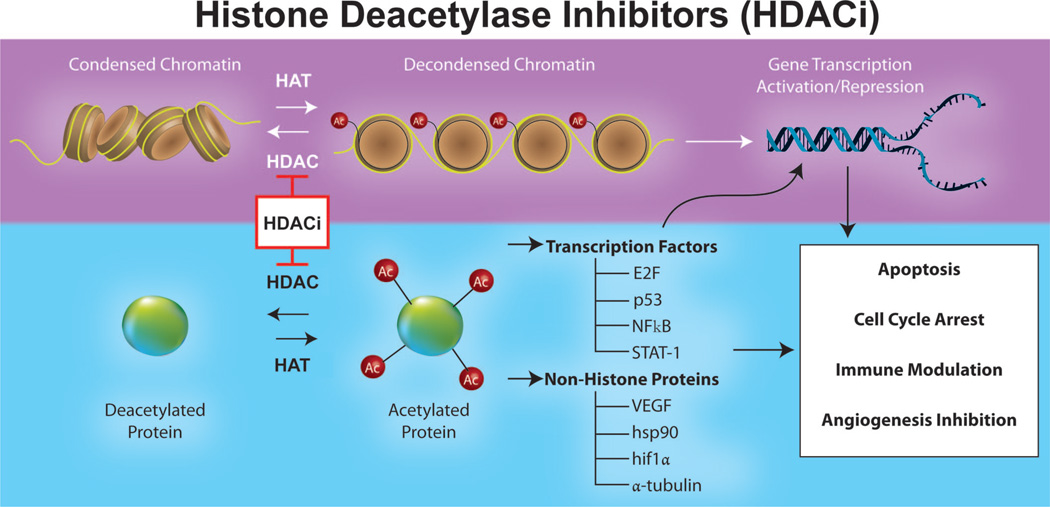
Histones are a family of basic proteins that serve as structural and regulatory components of chromatin. Chromatin is comprised of DNA, RNA, and both histone and nonhistone elements and serves to pack linear DNA efficiently within the cell. The fundamental subunit of chromatin is the nucleosome, which winds 147 bp of DNA around an octamer of histone subunits.16 Transcriptional activity can only occur when nucleosomes are decondensed as euchromatin, in a process that couples methylation of DNA with, among other reactions, methylation, acetylation, and phosphorylation of histones (Figure 1, top).16
FIGURE 1.
Schematic representation of the targets and effects of HDAC inhibitors (HDACi). In addition to directly regulating transcription through changes in chromatin structure, HDACi modulate the acetylation of transcription factors and other nonhistone proteins, leading to a range of biologic effects, including the promotion of apoptosis, cell cycle inhibition, immune modulation, and inhibition of angiogenesis.
Among these biochemical modifications, histone acetylation, controlled by a family of acetyltransferases and deacetylases (HDACs), has emerged as clinically important.17 Much of this interest has focused on HDACs, which serve to remove lysine residues from histone tails and nonhistone proteins, thereby preventing chromatin relaxation and gene transcription (Figure 1, left). Four classes of HDACs have been characterized, grouped in part by homology and in part by cellular localization, with aberrant recruitment and overexpression found in a wide range of cancers.18–25 A host of pharmacologic inhibitors have also been identified, divided by both structure and specificity for the different classes of HDACs.26
Histone Acetylation and MPM
In vitro data for the role of HDAC inhibition in MPM has centered on apoptosis, although the biologic effects of HDAC inhibitors (HDACi) are diverse and include cell cycle arrest, angiogenic inhibition, immunomodulation, and direct acetylation of signaling intermediates and transcription factors (Figure 1, bottom).26 The relevant studies include work by Cao et al.,27 who found a decrease in the expression of the antiapoptotic protein bcl-XL and the induction of apoptosis in MPM cell lines treated with sodium butyrate. Suberoylanilide hydroxamic acid was also found to sensitize MPM cells to TNF-related apoptosis- inducing ligand-mediated apoptosis, with strong downregulation of bcl-XL.28 Depsipeptide was similarly cytotoxic to MPM cells, an effect synergistically increased with flavopiridol, a cyclin dependent kinase inhibitor.29
More recently, caspase-dependent apoptosis has emerged as an important mechanism. Treatment of TNF-related apoptosis-inducing ligand-induced MPM cell lines with the HDACi LBH589 was found to increase caspase 3 and 7 expression in addition to apoptosis. This expression was linked to the degradation of the antiapoptotic protein X-linked inhibitor of apoptosis protein.30 Similarly, treatment of MPM cell lines with valproic acid increased caspase-dependent apoptosis when coupled with cisplatin and pemetrexed.31 This work by Vandermeers et al. was important in its study of HDACi alone and in combination with chemotherapy. In particular, while chemotherapy reliably led to increased annexin V staining and an increase in the sub-G1 population, HDACi monotherapy did so in only one of three cell lines tested. In addition, treatment with valproic acid plus chemotherapy led to a synergistic increase in reactive oxygen species formation and annexin V staining. Remarkably, a mouse xenograft model in this study showed complete suppression of human epithelioid MPM growth following treatment with valproic acid and chemotherapy, a response not seen with either valproic acid or chemotherapy alone.31
Indirect evidence for the importance of histone acetylation in MPM came from research by Shao et al.32 on the regulation of the tumor suppressor Wilms tumor-1 (WT-1). While its expression in lung tumors is restricted to MPM, little mechanistic data regarding this link had been published.33,34 In their study, Shao et al.32 found a decrease in WT-1 reporter activity and mRNA expression in 293T cells following overexpression of HDAC4 and HDAC5. This activity was reversed by cotransfection of the histone acetyltransferase p300, which was also found to increase histone H3 acetylation at the WT-1 intronic enhancer. Synergistic reporter activity was seen with the cotransfection of p300 and the transcription factors Sp1 (Specificity protein 1), c-Myb (Myeloblastosis oncogene homolog), and Ets-1 (Erythroblastosis E26 oncogene homolog 1), suggesting a role for histone acetylation in facilitating the interaction between transcription factors and the WT-1 gene, highlighting a possible target for HDAC inhibition in MPM.
Clinical Studies
Data for the effectiveness of HDACi in patients with MPM emerged in 2005, with the publication of a phase I study of vorinostat in patients with advanced solid tumors.35,36 Of the 73 patients treated, 13 held diagnoses of MPM. Six of the patients remained in the study for greater than 4 months. Four of these patients (30%) were found to have stable disease. Two patients met criteria for a radiographic partial response, though these were ultimately unconfirmed.36 Nevertheless, at the time of analysis, these patients were alive 27 and 21 months after initiating treatment, exceeding their predicted survival. Hematologic toxicity was mild. Fatigue and nausea/vomiting were the most common grade III toxicities (23%). A separate phase I study of vorinostat in combination with carboplatin and paclitaxel in patients with advanced solid tumors also led to disease stabilization in the single patient with MPM.37 These results were sufficiently compelling to lead to the initiation of a multicenter, randomized, placebo-controlled phase III study of vorinostat in patients with advanced MPM.38
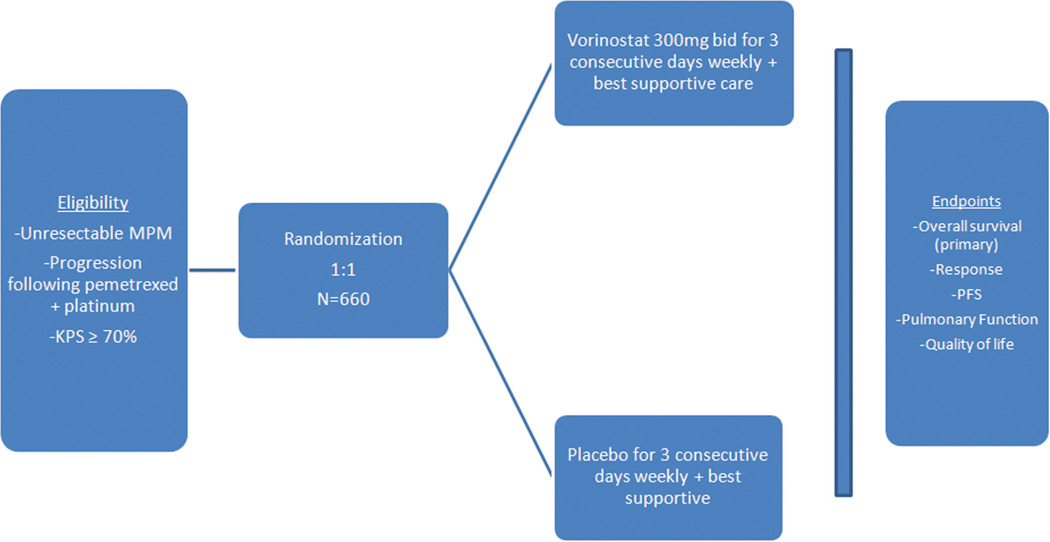
In this trial, patients who have progressed or relapsed following treatment with pemetrexed and cisplatin or carboplatin are randomized 1:1 to receive vorinostat 300 mg twice daily or a placebo (Figure 2). Medication is provided over 3 consecutive days per week in a 3-week cycle. The primary end point is overall survival, with secondary endpoints including objective response by RECIST, progression-free survival, pulmonary function, and quality of life. The planned target accrual is 660 patients. The study continues after two interim analyses.
FIGURE 2.
Study design for phase III trial comparing vorinostat versus placebo in patients with previously treated malignant pleural mesothelioma (MPM).
Data from a phase II trial of a related agent, belinostat (PXD101), in patients with advanced pleural mesothelioma has also been published.39 Thirteen patients were enrolled, the majority of whom had received prior chemotherapy and seven of whom had an epithelioid subtype. Three patients were able to receive only one cycle of treatment due to symptomatic or radiographic progression. Two patients (15%) attained stable disease as a best response, with no partial or complete responders. These results stand in contrast to those seen with vorinostat and run contrary to what might have been predicted based on preclinical data, where belinostat was found to be five-times more potent than vorinostat in in vitro growth inhibition studies.40 This may have been due to the relative aggressiveness of the mesothelioma in the belinostat trial, or differences in pharmacokinetics between the two drugs as posited by the authors.
While HDACi therapy alone seems to be effective in advanced MPM, preclinical data, including work by Vandermeers et al.,31 suggest that the coupling of HDACi therapy to chemotherapy should be even more so.41 A number of early phase trials in advanced solid tumors bear this out. A phase I study of vorinostat plus cisplatin and gemcitabine in chemonaive advanced stage non-small cell lung cancer patients demonstrated partial response rates of 32% (increasing to 45% if nonevaluable patients are excluded).42 Although a comparison with standard chemotherapy alone was not possible, these rates compare favorably to historical overall response rates of between 18 and 28% in similar patients treated with cisplatin and gemcitabine alone.43– 45 The added benefit of vorinostat to chemotherapy was seen more clearly in a recent randomized, placebo-controlled phase II trial of carboplatin and paclitaxel with or without vorinostat for first-line therapy for advanced non-small cell lung cancer.46 Ninety-four patients were randomized to receive carboplatin and paclitaxel with vorinostat 400 mg daily or a placebo. The response rate for vorinostat was 34 versus 13% for the placebo (p = 0.02). Median progression-free survival for vorinostat and the placebo was 5.8 and 4.1 months, respectively.
Although no such study of combined therapy in MPM has yet been conducted, a phase I trial of vorinostat in combination with cisplatin and pemetrexed in advanced solid tumors found an overall response rate of 10%, with 58% of patients exhibiting stable disease, including three of five patients (60%) with MPM.47 This combination was toxic, with grade 3/4 dehydration in 18% of patients, fatigue in 27%, electrolyte abnormalities in 27%, and myelosuppression in 22%. Further studies are necessary to determine the optimal dosing schedule for combining vorinostat with this chemotherapy regimen.
CONCLUSION
The need for more effective treatments for patients with advanced MPM is clear. Several preclinical studies provide a rationale for pursuing HDAC inhibition for the treatment of MPM, and clinical data are emerging. Hopefully, the results of the phase III study with vorinostat and other clinical trials with this class of drugs will demonstrate benefit and provide us with greater therapeutic options for our patients with MPM.
ACKNOWLEDGMENTS
We thank Tony Riley from the Medical Graphics Department at Memorial Sloan-Kettering for his invaluable assistance in the creation of the figure artwork.
Footnotes
Disclosure: Dr. Krug receives research funding from Merck & Co., Inc.
REFERENCES
- 1.Robinson BWS, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the north western cape province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price B, Ware A. Mesothelioma trends in the United States: an update based on surveillance, epidemiology, and end results program data for 1973 through 2003. Am J Epidemiol. 2004;159:107–112. doi: 10.1093/aje/kwh025. [DOI] [PubMed] [Google Scholar]
- 4.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973–2005. Cancer Causes Control. 2009;20:935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 5.Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1998;115:310–317. doi: 10.1016/S0022-5223(98)70274-0. discussion 317–318. [DOI] [PubMed] [Google Scholar]
- 6.Rusch V. Indications for pneumonectomy: extrapleural pneumonectomy. Chest Surg Clin N Am. 1999;9:327–338. [PubMed] [Google Scholar]
- 7.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative longterm survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. doi: 10.1016/s0022-5223(99)70469-1. discussion 63–65. [DOI] [PubMed] [Google Scholar]
- 8.Rusch VW, Piantadosi S, Holmes EC. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A lung cancer study group trial. J Thorac Cardiovasc Surg. 1991;102:1–9. [PubMed] [Google Scholar]
- 9.Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007–3013. doi: 10.1200/JCO.2008.20.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weder W, Stahel RA, Bernhard J, et al. Swiss Group for Clinical Cancer Research. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196–1202. doi: 10.1093/annonc/mdm093. [DOI] [PubMed] [Google Scholar]
- 11.Krug LM. An overview of chemotherapy for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1117–1136. doi: 10.1016/j.hoc.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 13.van Meerbeeck J, Manegold C, Gaafar R, et al. A randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the EORTC Lung cancer Group and NCIC (abstract 7021) J Clin Oncol. 2004;22:622S. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 14.Carbone M, Pass H, Rizzo P, et al. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- 15.Suzuki M, Toyooka S, Shivapurkar N, et al. Aberrant methylation profile of human malignant mesotheliomas and its relationship to SV40 infection. Oncogene. 2004;24:1302–1308. doi: 10.1038/sj.onc.1208263. [DOI] [PubMed] [Google Scholar]
- 16.Jenuwein T, Allis CD. Translating the Histone Code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 17.Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 18.Kalipso H, Luke G, Cook S, et al. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 19.Cote S, Rosenauer A, Bianchini A, et al. Response to histone deacetylase inhibition of novel PML/RARalpha mutants detected in retinoic acidresistant APL cells. Blood. 2002;100:2586–2596. doi: 10.1182/blood-2002-02-0614. [DOI] [PubMed] [Google Scholar]
- 20.Bereshchenko O, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Kwon H, Yoon B, et al. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Noh J, Lee J, et al. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS. 2005;113:264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Yamashita H, Toyama T, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004;10:6962–6968. doi: 10.1158/1078-0432.CCR-04-0455. [DOI] [PubMed] [Google Scholar]
- 24.Wilson AJ, Byun D, Popova N, et al. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006;281:13548–13558. doi: 10.1074/jbc.M510023200. [DOI] [PubMed] [Google Scholar]
- 25.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–1420. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 26.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 27.Cao XX, Mohuiddin I, Ece F, et al. Histone deacetylase inhibitor downregulation of Bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am J Respir Cell Mol Biol. 2001;25:562–568. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 28.Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of bcl-XL down-regulation. Biochem Biophys Res Commun. 2004;314:186–191. doi: 10.1016/j.bbrc.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen DM, Schrump WD, Chen GA, et al. Abrogation of p21 expression by flavopiridol enhances depsipeptide-mediated apoptosis in malignant pleural mesothelioma cells. Clin Cancer Res. 2004;10:1813–1825. doi: 10.1158/1078-0432.ccr-0901-3. [DOI] [PubMed] [Google Scholar]
- 30.Symanowski J, Vogelzang N, Zawel L, et al. A histone deacetylase inhibitor LBH589 downregulates XIAP in mesothelioma cell lines which is likely responsible for increased apoptosis with TRAIL. J Thorac Oncol. 2009;4:149–160. doi: 10.1097/JTO.0b013e318194f991. [DOI] [PubMed] [Google Scholar]
- 31.Vandermeers F, Hubert P, Delvenne P, et al. Valproate, in combination with pemetrexed and cisplatin, provides additional efficacy to the treatment of malignant mesothelioma. Clin Cancer Res. 2009;15:2818–2828. doi: 10.1158/1078-0432.CCR-08-1579. [DOI] [PubMed] [Google Scholar]
- 32.Shao YLJ, Cheng C, Cui L, et al. Reversible histone acetylation involved in transcriptional regulation of WT1 gene. Acta Biochim Biophys Sin. 2007;39:931–938. doi: 10.1111/j.1745-7270.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong JF, Pritchard-Jones K, Bickmore W, et al. The expression of the Wilms tumor gene, WT1, in the developing mammalian embryo. Mech Dev. 1992;40:85–97. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 34.Kumar-Singh S, Segers K, Rodeck U, et al. WT1 mutation in malignant mesothelioma and WT1 immunoreactivity in relation to p53 and growth factor receptor expression, cell-type transition, and prognosis. J Pathol. 1997;181:67–74. doi: 10.1002/(SICI)1096-9896(199701)181:1<67::AID-PATH723>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krug LM, Kelly WK, Curley T, et al. Clinical experience of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) in patients with malignant pleural mesothelioma. Presented at VII Meeting of the International Mesothelioma Interest Group (IMIG); Brescia, Italy. 2004. p. 155. [Google Scholar]
- 37.Ramalingam SS, Parise RA, Ramananthan RK, et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res. 2007;13:3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 38.Krug LM, Marangolo M, Kindler H, et al. A phase III, randomized, double-blind, placebo-controlled trial of oral vorinostat in patients with advanced malignant pleural mesothelioma (MPM) previously treated with systemic chemotherapy. Presented at 1st International Thoracic Oncology Congress; Dresden, Germany. 2008. [Google Scholar]
- 39.Ramalingam SS, Belani CP, Ruel C, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gimsing P. Belinostat: a new broad acting antineoplastic histone deacetylase inhibitor. Expert Opin Investig Drugs. 2009;18:501. doi: 10.1517/13543780902852560. [DOI] [PubMed] [Google Scholar]
- 41.Marchion D, Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007;7:583. doi: 10.1586/14737140.7.4.583. [DOI] [PubMed] [Google Scholar]
- 42.Trédaniel J, Descourt R, Moro-Sibilot D, et al. Vorinostat in combination with gemcitabine and cisplatinum in patients with advanced nonsmall cell lung cancer (NSCLC): a phase I dose-escalation study. J Clin Oncol. 2009;27:14601. [Google Scholar]
- 43.Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin, with or without cetuximab, as first-line therapy for patients with advanced or metastatic non-smallcell lung cancer. J Clin Oncol. 2007;25:5777–5784. doi: 10.1200/JCO.2007.13.0856. [DOI] [PubMed] [Google Scholar]
- 44.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy- naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 45.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 46.Ramalingam SS, Maitland M, Frankel P, et al. Randomized, double-blind, placebo-controlled phase II study of carboplatin and paclitaxel with or without vorinostat, a histone deacetylase inhibitor (HDAC), for first-line therapy of advanced non-small cell lung cancer (NCI 7863) J Clin Oncol. 2009;15s:8004. [Google Scholar]
- 47.Chen L, Vogelzang NJ, Blumenschein G, et al. Phase I trial of vorinostat (V) in combination with pemetrexed (Pem) and cisplatin (CDDP) in patients with advanced cancer. J Clin Oncol. 2007;25:18088. [Google Scholar]