Abstract
Cigarette-dependent smokers automatically and involuntarily orient attention towards smoking cues (SCs). This attentional bias is clinically significant, as it may contribute to relapse. Thus, identifying neural and genetic correlates of attentional bias is critical for improving interventions. Our previous studies show that the dopamine transporter (DAT) SLC6A3 genotype exerts profound effects on limbic responses to SCs. One potential mechanism underlying these effects is greater attentional bias for SCs. Here, we explored associations between attentional bias for SCs and neural responses to SCs among ‘sated’ smokers genotyped for the SLC6A3 polymorphism. Pseudo-Continuous arterial spin-labeled (pCASL) perfusion fMR images were acquired during SC exposure in 35 smokers genotyped for the SLC6A3 variable number of tandem repeats (VNTR) polymorphism (n=16, 9-repeats; n=19,10/10-repeats). Participants completed a visual dot-probe attentional bias task, which contained pictures of smoking and non-smoking pictures, to examine whether genetic variation in DAT influences attentional bias and to investigate relationships between attentional bias and neural responses to SCs. Although attentional bias to smoking pictures was not significantly different between 9-repeats and 10/10-repeats, 9-repeats showed a positive correlation between attentional bias and increased SC-induced brain activity in the amygdala; whereas, 10/10-repeats showed an inverse correlation in the medial orbitofrontal cortex (mOFC). In group comparisons, 9-repeats exhibited positive correlations between attentional bias and SCs in the mOFC and amygdala, relative to 10/10-repeats. Findings suggest that genetic variation in the DAT gene influences brain responses associated with attentional bias; thus, providing additional support for a SC-vulnerable endophenotype.
Keywords: Addiction, attentional bias, dopamine transporter, neuroimaging, smoking cues
INTRODUCTION
Many drug abuse and addiction theories suggest that drug-related cues maintain and contribute to drug use and relapse (O'Brien et al., 1998; Sinha and Li, 2007; Wikler and Pescor, 1967). For example, exposure to drug-related cues, such as seeing one’s drug of choice or associated paraphernalia, elicits increased physiological arousal and subjective craving (Carter and Tiffany, 1999; Droungas et al., 1995; Waters et al., 2009). Drug-related cues also influence cognitive processes, including attention; whereby, substance-dependent individuals automatically and involuntarily orient their attention toward drug-related stimuli (Field and Cox, 2008). This selective attention, or attentional bias, for drug-related cues is thought to develop as a result of dopamine-mediated incentive sensitization learning processes (Franken, 2003; Robinson and Berridge, 1993; Volkow et al., 2006).
According to recent addiction theories (Lang, Bradley & Cuthbert, 1997; Robinson and Berridge, 1993), drug-related stimuli acquire incentive-motivational properties as a result of repeated drug use. Specifically, repeated drug use causes dopamine (DA) release in mesocorticolimbic reward circuitry, comprising ventral tegmental area (VTA) neurons and their projections to the nucleus accumbens (NAc), prefrontal cortex, and other forebrain regions (Di Chiara, 1999; Di Chiara et al., 1999). Over time, the repeated drug-related DA response becomes sensitized, and consequently, drug-related cues acquire “incentive salience”. Thus, evidence suggests that DA-driven incentive salience influences attention toward drug-related cues, elicits approach behaviors, and contributes to craving and drug use (Robinson and Berridge, 2000).
Although a wealth of research on attentional bias exists, very few studies have examined the underlying neurobiological mechanisms of attentional bias for smoking cues (SCs). In one of the first fMRI studies of attentional bias, Janes and colleagues (Janes et al., 2010b) used an offline smoking emotional Stroop task and fMRI measures of brain reactivity to smoking versus neutral cues to examine the neural correlates of attentional bias for SCs among women smokers. Findings revealed that attentional bias was correlated with brain activity to SCs in limbic regions, namely the amygdala, hippocampus, and insula. A potential interpretation of Janes et al.’s (2010b) work is that emotive circuitry, which includes the amygdala, hippocampus, and insula, are acting to enhance identification of emotionally salient stimuli (i.e., reminders to smoke) by boosting sensory processing and shifting attention toward SCs. Thus, brain regions involved in incentive salience appear to underlie attentional bias to SCs; however, given recent evidence for a role of the dopamine transporter SLC6A3 (DAT) gene in modulating SC responses (Franklin et al., 2009; Franklin et al., 2011b), additional research is needed in order to examine whether genetically-mediated inter-individual differences in the SLC6A3 gene contribute to attentional bias.
In a previous study, our laboratory found that SCs activate limbic regions, including the ventral striatum, amygdala, insula, hippocampus and medial orbitofrontal cortex, independent of withdrawal (Franklin et al., 2007). Responses were robust; however, we noted considerable inter-individual variability. Due to the DAT’s role in removing synaptic DA after its release in response to addictive drugs and conditioned cues associated with repeated drug use (Jaber et al., 1997), we hypothesized that variation in the DAT may underlie the observed variable responses to SCs. Thus, we evaluated the impact of genetic variation in the DAT gene on brain perfusion to SCs. Briefly, smokers with the 9- variable number tandem repeat (VNTR) allele (9-repeats), which is associated with lower DAT expression (Fuke et al., 2001; Mill et al., 2002) and may potentiate DA responses, exhibited heightened neural responses to SCs compared with homozygous 10-repeat VNTR allele (10/10-repeats) smokers (Franklin et al., 2009; Franklin et al., 2011b). Given that both groups are equally dependent on cigarettes and smoking reminders and withdrawal are two major relapse-provoking factors, we suggest that 9-repeats might represent a subgroup of smokers who are more vulnerable to relapse in the presence of SCs, whereas smoking behavior in 10/10-repeats may be influenced more by withdrawal. Further, because greater attentional bias to smoking-related stimuli may be associated with increased likelihood of experiencing a lapse after establishing abstinence (Janes et al., 2010a), identifying neural correlates and putative genetic vulnerabilities of attentional bias for SCs is crucial in the development of effective therapeutics.
The aim of the present study was to examine the associations between attentional bias for SCs and brain responses to SCs among 9-repeat and 10/10-repeat smokers. Nicotine-dependent sated smokers completed a visual dot-probe attentional bias task and took part in a pseudo-continuous arterial spin-labeled (pCASL) perfusion fMRI SC-reactivity experiment. The visual dot-probe attentional bias task used in this study is an objective measure of attention between two co-present visual stimuli, and as such, avoids potential cognitive or mood interference from word-related Stroop or counting tasks (Ehrman et al., 2002; Field, Munafo & Franken, 2009). pCASL perfusion fMRI is quantitative and stable over time, and as such, is well-suited for examining sustained brain changes (Detre et al., 1992), such as those evoked by drug-related stimuli (Franklin et al., 2009; Franklin et al., 2007). Based on the work by Janes and colleagues (2010b) and our previous SC studies, we hypothesized that 9-repeats would have positive correlations between attentional bias and increased brain reactivity to SCs in limbic regions such as, the insula, amygdala, and hippocampus compared with 10/10-repeats; thus, providing a potential neural mechanism for greater cue-responsivity among 9-repeats.
METHODS AND MATERIALS
Participants
Thirty-five physically healthy smokers (16 males) ranging in age from 18 to 58 (36.2 ± 12.5) were recruited via radio advertisements and local list-serves stating that the study was intended for smokers contemplating quitting, but were not ready to quit in the near future. Of this sample, perfusion fMRI data on 15 were reported on previously (Franklin et al., 2011b), and 20 new participants were added to the sample as part of an ongoing smoking study. The final sample was 60% Caucasian American; 26% African American, and 14% multiple ethnicities. Table 1 provides additional demographics and smoking history characteristics.
Table 1.
Participant characteristics
| All N = 35 |
10/10-repeats n = 19 |
9-repeats n = 16 |
p | |
|---|---|---|---|---|
| Sex | 16 M (46%) | 8 M (42%) | 8 M (50%) | 0.64 |
| Race | 21 CA (60%), | 11 CA (58%), | 10 CA (63%), | |
| 9 AA (26%), | 5 AA (26%), | 4 AA (25%), | 0.950 | |
| 5 ME (14%) | 3 ME (16%) | 2 ME (12%) | ||
| Means ± (SEMs) | ||||
| Age | 36.6 (2.1) | 37.0 (2.8) | 36.2 (3.3) | 0.86 |
| Education | 14.5 (0.4) | 14.6 (0.6) | 14.4 (0.6) | 0.82 |
| Cigarettes per day | 15.4 (1.1) | 14.5 (1.3) | 16.4 (1.7) | 0.36 |
| Pack yearsa | 13.1 (2.0) | 11.6 (2.0) | 15.0 (3.8) | 0.42 |
| FTND scores | 4.4 (0.3) | 4.3 (0.4) | 4.6 (0.5) | 0.66 |
Pack years calculation: Cigarettes per day (÷) cigarettes in a pack (X) years smoking.
FTND = Fagerstrom Test for Nicotine Dependence; CA = Caucasian American; AA = African American; ME = Multiple Ethnicity; 10/10-repeats = homozygotes for the 10-repeat variable number tandem repeat (VNTR) allele of the DAT; 9-repeats = carriers of 1 or 2 copies of the 9-VNTR allele of the DAT. FTND scores ranged from 1 to 9.
Participants were screened, tested on study knowledge (by means of a study-specific quiz that consists of 10 true or false items), and consented prior to psychological and physical evaluations. The MINI International Neuropsychiatric Interview (Sheehan, Lecrubier & Sheehan, 1998) assessed current DSM-IV diagnosis of substance dependence other than nicotine and current severe psychiatric symptoms. The Fagerstrom Test for Nicotine Dependence (FTND) (Fagerstrom and Schneider, 1989) assessed severity of nicotine dependence. Exclusion criteria included: other current substance dependence, current Axis I DSM IV psychiatric diagnoses, significant medical conditions, an intellectual ability estimate score of ≤ 80 on the Weschler Abbreviated Scale of Intelligence (WASI) (Weschler, 1999), an abnormal structural MRI, a history of head trauma or injury causing loss of consciousness lasting greater than three minutes or associated with skull fracture or inter-cranial bleeding, or who had irremovable magnetically active objects on or within their body. Smokers received $100.00 for an initial consenting appointment and completion of MRI scanning and cognitive bias sessions. The study adhered to the Declaration of Helsinki and was approved by the University of Pennsylvania Institutional Review Board.
Genotyping
Blood samples were acquired during the initial physical examination, and genomic DNA was extracted from anti-coagulated venous blood samples using a standard salting out method (Lahiri and Nurnberger, 1991). Genotyping of the SLC6A3 40bp repeat polymorphism (rs28363170) was performed as previously described (Vandenbergh et al., 1992). Briefly, PCR was performed using a mix of 100ng genomic DNA, 1× Amplitaq buffer containing MgCl2, 200 nM dNTP mix, 150 nmol for each primer (Fwd: 5'-TGT GGT GTA GGG AAC GGC CTG AG-3' and Rev: 5'-CTT CCT GGA GGT CAC GGC TCAAGG-3') and 2.5 Units AmpliTaq per reaction. The PCR conditions included an initial melting step (94 °C; 4 min) followed by 40 cycles of melting (94 °C; 1 min), annealing (65 °C; 1 min) and extending (72 °C; 1 min). A final extension step was used (72 °C; 5 min). Agarose gel electrophoresis separated reaction products, and product sizes were determined by comparison to molecular weight standards and known sequenced samples. In addition, four samples were confirmed by sequencing. All samples were run in duplicate with 100% concordance rate and were read independently by two blinded investigators.
Visual Dot-Probe Attentional Bias Task
Participants completed an off-magnet visual dot-probe behavioral task designed to objectively measure attention shifts toward smoking pictures (Ehrman et al., 2002). Participants smoked ad lib until approximately 5 minutes before the task. The dot-probe task consisted of 20 color photographs of smoking-related content (e.g., pack of cigarettes) and 20 photographs not specifically related to smoking (e.g., pack of playing cards). Stimuli were matched for overall composition and degree of visual complexity. Participants were told that picture pairs would briefly flash on the screen followed by an asterisk (the dot probe) in the position previously occupied by one of the pictures. Participants were asked to indicate the position of the target as quickly and accurately as possible by using the left and right index fingers to strike the left or right response key, respectively. After a 20-trial practice block containing picture pairs without smoking-related stimuli, participants completed 80 stimulus pair trials. Each stimulus pair was presented four times, counterbalancing for picture/dot-probe location.
Each trial began with the presentation of a fixation point (X) in the center of the computer screen for 1 s immediately followed by a pair of photographs presented for 500 ms (one to the left and one to the right of fixation point). After the 500 ms presentation, an asterisk appeared in place of one of the images and remained on the screen until the participant responded or for 2 s, if there was no response. E-Prime software (Psychology Software Tools, Pittsburgh, PA) was used for task presentation and behavioral response recording in the form of reaction times (RTs) and accuracy. Incorrect trials were not used in mean reaction time calculations, and trials with 200 ms > RT > 1000 ms were eliminated (Ehrman et al., 2002; Townshend and Duka, 2001). For each subject, a relative attentional bias score was computed as RTnon-smoking – RTsmoking (Lubman et al., 2000) with positive bias scores reflecting attentional bias for SCs and negative scores reflecting bias towards nonsmoking cues.
Imaging Approach and Parameters
Pseudo-Continuous arterial spin-labeled (pCASL) perfusion fMRI, a quantitative estimate of cerebral blood flow (CBF) and indirect measurement of neural activity (Floyd et al., 2003), assessed brain activation in response to SC exposure. Prior to scanning, participants smoked ad lib to minimize the potential for nicotine withdrawal-induced craving that might accrue over the scanning session, and scanning occurred approximately 20–25 minutes after smoking to allow the acute cardiovascular effects of smoking to dissipate. The 35 minute scanning session included, in sequence, a five minute resting baseline scan where participants were instructed to lie still in the scanner with their eyes open; a 10 minute non-SC pCASL scan; a high resolution structural scan; and a 10 minute SC pCASL scan.
Ten-minute audio-visual clips were presented during pCASL scanning. The SC video included individuals differing in race, age, and sex who were smoking and using explicit language designed to induce appetitive desire for a cigarette. The non-SC video was similar in content; however, the video did not portray cigarette smoking or smoking reminders.
Imaging data were acquired on a 3.0 Tesla Trio whole-body scanner (Siemens AG, Erlangen, Germany) using a Bruker volume coil (volume coils are designed to provide a homogenous receiving sensitivity and are 1 channel; Bruker Biospin, Billerica, MA) for 15 subjects and a standard 8-channel receive-only array head coil for the remaining 20 subjects. For co-registration of the functional data, a T1-weighted 3D high resolution MPRAGE scan was acquired with FOV = 160 mm, TR/TE=1510/3 ms, 192 × 256 matrix, slice thickness 1 mm for 15 subjects and FOV = 250 mm, TR/TE = 1620/3 ms, 192 × 256 matrix, slice thickness 1 mm for the remaining 20 subjects. pCASL perfusion fMRI sequence was used for resting baseline, SC and non-SC data acquisition. Interleaved images with and without labeling were obtained using a gradient echo echo-planar imaging sequence with a delay of 1000 ms for 15 subjects or 700 ms for 20 subjects inserted between the end of the labeling pulse and image acquisition (FOV = 130 mm, matrix = 64 × 64 × 14, TR/TE = 3000/17 ms, flip angle = 90°, slice thickness = 6 mm with a 2 mm inter-slice gap for 15 subjects and a 1.2 mm inter-slice gap for 20 subjects.
Imaging Data Processing and Statistical Analyses
An SPM-based ASL data processing toolbox (Wang et al., 2008) was used for pCASL perfusion data analyses as described previously (Franklin et al., 2009; Franklin et al., 2011b). Briefly, ASL image pairs were realigned to the mean of all control images and spatially smoothed with a 3D isotropic Gaussian kernel at 10 mm FWHM. 100 CBF image series were generated from the 100 label/control ASL image pairs using a simplified two-compartment model with the sinc interpolation method for CBF calculations (Aguirre, Detre & Wang, 2005). The mean control image of each subject’s data was co-registered to the structural image using the mutual information based co-registration algorithm provided by SPM8. The same transformation parameters were applied to co-register the CBF maps to each subject's anatomical image. Subsequently, the structural image was spatially normalized to the Montreal Neurological Institute (MNI) standard brain. The resulting transformation matrix was used to align the CBF images to MNI space. A binary brain mask was used to exclude the non-brain areas in the CBF maps.
Contrasts between cue sets were defined in the general linear model (GLM) model to assess the voxel by voxel CBF difference for each subject. Using the corresponding parametric maps of the contrasts, region of interest (ROI) analyses of the CBF data were conducted using GLM. Based on our previous studies on SC-reactivity among cigarette-dependent smokers (Franklin et al., 2009; Franklin et al., 2011b), the ROI mask included the medial orbitofrontal cortex (mOFC), ventral striatum (VS), hippocampus, amygdala, anterior cingulate cortex (ACC), and insula. The ROI mask was created using the Harvard–Oxford probabilistic anatomical atlas provided with FMRIB Software Library (FSL) (Smith et al., 2004) and is available for viewing at http://franklinbrainimaging.com/. Significant voxels passed a voxelwise statistical threshold (p < 0.005) and, to control for multiple comparisons, were required to be part of a larger 400 µl cluster, as determined by a Monte-Carlo simulation and resulted in 5% probability (corrected) of a cluster surviving due to chance.
Correlations between brain responses to SCs and attentional bias scores were computed for all subjects and then for each DAT genotype group by performing a regression analysis on ROI contrast images of interest (SCs versus non-SCs), using attentional bias scores as the dependent variable. Significant functional clusters from the ROI analysis were used to extract mean CBF values from each subject. Mean CBF values were then correlated with attentional bias scores using IBM SPSS Statistics 19.0 (SPSS Inc., Chicago, IL). Group correlations (9-repeats, 10/10-repeats) were computed, and correlation coefficients representing associations between SC-induced brain activity and attentional bias scores were transformed in z using Fisher transformation before comparing correlations between 9-repeats and 10/10-repeats.
Demographic and Behavioral Statistical Analyses
Continuous demographic variables were summarized, by calculating means and standard error measurements (X ± SEMs). Nominal demographic variables were summarized by calculating proportions and compared across groups using chi-square analyses.
RESULTS
Group Assignment
Thirty-five smokers were genotyped for variance in the SLC6A3 gene (homozygous 10-repeats: n = 19; 9-repeats: n = 16). Smokers who carried at least one 9-repeat allele were classified and grouped together for analyses, as homozygotes for the 9-repeat allele are rare, and 10/10-repeat probands were classified as 10/10-repeats. Allele frequencies were 0.26 for 9-repeat carriers and 0.74 for 10/10-repeats, which is similar to what has been observed in other studies (Franklin et al., 2011b; Vandenbergh et al., 2002). Genotype frequencies did not deviate from that expected under Hardy-Weinberg equilibrium (χ2 = 0.08).
Participant Characteristics
There were no significant differences between genotypes in race, sex, cigarettes smoked per day, pack years (a measure to quantify intensity of chronic cigarette exposure since smoking initiation) or FTND scores (Table 1). Participants smoked between 10 and 30 cigarettes per day (15.4 ± 6.2), and FTND scores were 4.4 ± 0.3, indicating moderate nicotine dependence. On average, participants had an attentional bias score of 8.0 ± 3.5 ms, ranging from 44.3 to −35.8 ms. There were no significant differences between groups in attentional bias scores (p = 0.92; 9-repeats, mean = 8.4 ms ± 4.5; 10/10-repeats, mean = 7.7 ms ± 5.4).
Smoking Cue Reactivity
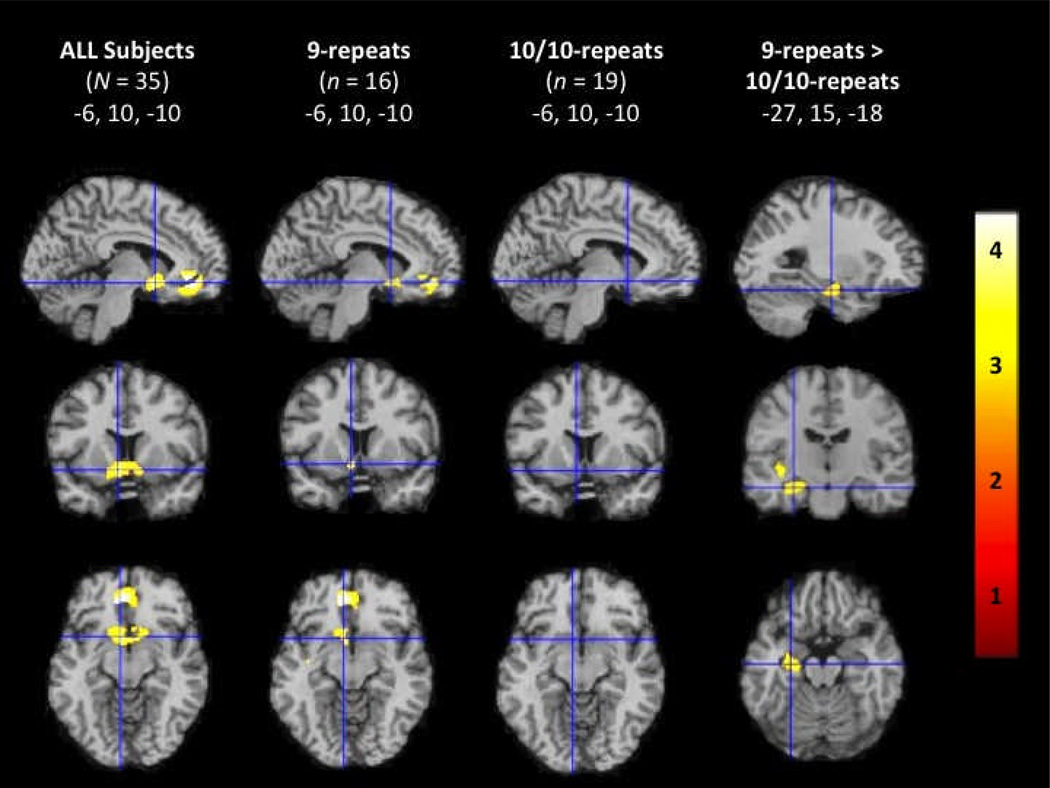
For the full sample of sated smokers, ROI analyses revealed significant activation to SCs relative to non-SCs in the mOFC and VS. 9-repeats exhibited significant activity in response to SCs in the mOFC and VS. There were no areas of statistically significant activations among 10/10-repeats. In comparison to 10/10-repeats, 9-repeats exhibited significantly greater activity in response to SCs vs non-SCs in the left parahippocampal gyrus/hippocampus and left insula (Figure 1, Table 2). When thresholds were reduced to p < 0.05, SC reactivity activation patterns were similar to our previous findings with increased activity in the VS/mOFC only in 9-repeats (Franklin et al., 2009; Franklin et al., 2011b). Representative sagittal, axial, and coronal sections co-registered to the MNI brain are shown in Figure 1. Coordinates listed in Table 2 were chosen from the suprathreshold voxel of each significant cluster using the Duvernoy Brain Atlas (Duvernoy, 1999) and the Atlas of the Human Brain (Mai, Voss & Paxinos, 2008).
Figure 1.
Region of interest analysis showing brain activity during smoking cues greater than non-smoking cues in all smokers, 9-repeats, 10/10-repeats, and 9-repeats vs 10/10-repeats. Representative fMR saggital, axial, and coronal brain slices, analyzed in SPM8, and overlain on the MNI brain. T-values range from 3.17 to 5.44, corrected at p < 0.005.
Table 2.
Brain perfusion during smoking cue exposure relative to non-smoking cue exposure
| All (n= 35) | 9-repeats (n= 16) | 10/10-repeats (n= 19) | 9-repeats > 10/10-repeats | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | x | y | z | T | x | y | z | T | x | y | z | T | x | y | z | T |
| L mOFC | −10 | 38 | −12 | 5.44 | −10 | 40 | −10 | 4.70 | ||||||||
| L VS | −6 | 10 | −10 | 4.07 | −8 | 18 | −8 | 3.69 | ||||||||
| L insula | −40 | −16 | −2 | 3.77 | ||||||||||||
| L para/hip | −30 | −12 | −16 | 3.50 | ||||||||||||
L = left; mOFC = medial orbitofrontal cortex; VS = ventral striatum; parahip/hip = parahippocampal/hippocampal gyrus. Coordinates are in MNI.
T-values are from the suprathreshold voxel in the cluster.
Attentional Bias and Neural Activity
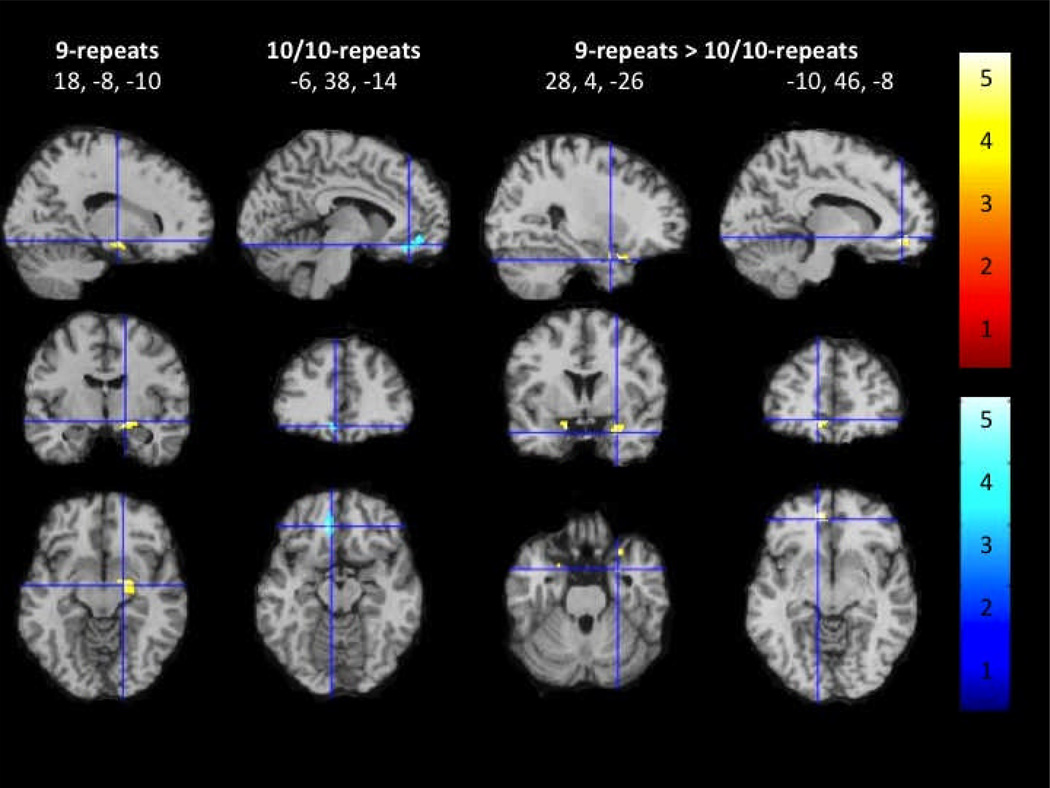
There were no significant correlations between attentional bias for SCs and brain reactivity to SCs vs non-SCs in a priori ROIs (i.e., insula, amygdala, hippocampus) when examining all smokers as a whole or among 10/10-repeats. For 9-repeats, however, a positive correlation was found between attentional bias for SCs and increased brain activity to SCs vs non-SCs in the right amygdala-hippocampal complex. Direct comparisons between groups revealed that attentional bias to SCs correlated with increased SC-reactivity in the bilateral amygdala for 9-repeats compared with 10/10-repeats.
An inverse correlation between attentional bias to SCs and brain reactivity to SCs vs non-SCs was observed in the mOFC. This correlation appeared to be carried by 10/10-repeats who also showed an inverse correlation between attentional bias for SCs and brain activity to SCs vs non-SCs in the mOFC. Further, DAT group comparisons showed that attentional bias to SCs correlated with increased SC-reactivity in the mOFC and bilateral amygdala for 9-repeats compared with 10/10-repeats (Figure 2). An interactive visual display of all brain data in all three planes can be found at http://franklinbrainimaging.com.
Figure 2.
Region of interest analysis showing correlation of attentional bias to smoking cues with brain responses to smoking cues vs non-smoking cues in 9-repeats, 10/10-repeats, and 9-repeats vs 10/10-repeats. Representative fMR saggital, axial, and coronal brain slices, analyzed in SPM8, and overlain on the MNI brain. T-values range from 3.24 to 5.19, corrected at p < 0.005.
DISCUSSION
The present study examined associations between attentional bias for SCs and SC-induced brain responses. Based on previous research (Franklin et al., 2009; Franklin et al., 2011b; Janes et al., 2010b), we hypothesized that 9-repeats would show positive correlations between attentional bias and increased brain reactivity to SCs in the insula, amygdala, and hippocampus compared with 10/10-repeats. Although 9-repeats and 10/10-repeats did not differ significantly on behavioral performance of attentional bias to SCs, analyses revealed differential associations between attentional bias to SCs and SC brain responses based on DAT genotype. Specifically, a positive correlation between attentional bias to SCs and brain activity during SC exposure was observed in the amygdala of 9-repeats, and direct group comparisons revealed that 9-repeats exhibited positive correlations between attentional bias and SCs in the mOFC and bilateral amygdala compared with 10/10-repeats. In earlier work, we hypothesized that 9-repeats might represent a group of smokers whose relapse is affected more by exposure to SCs compared with 10/10-repeats whose relapse may be influenced more by withdrawal from the pharmacological effects of nicotine in the brain. We found and confirmed that 9-repeats show greater brain responses than 10/10-repeats in reward-relevant brain regions, namely, the mOFC and VS, possibly identifying a subgroup of cue-vulnerable smokers (Franklin et al., 2009; Franklin et al., 2011b); however, the mechanism underlying the greater responses to SCs in the 9-repeats was unknown. Here, our results provide further evidence that heterogeneity in SC responsivity is likely related to genetic variance in the DAT, and the mechanism may be related to a difference in attentional bias to SCs in 9-repeats compared with 10/10-repeat homozygotes.
To our knowledge there is only one other published study examining the relationship between attentional bias and SC-reactivity using neuroimaging techniques. Specifically, Janes and colleagues (2010b) found a positive correlation between attentional bias to SCs and brain reactivity to SCs in the amygdala, insula, and parahippocampal gyrus among female smokers using an off-magnet smoking emotional stroop task. Here, using a visual dot-probe attentional bias task and perfusion fMRI, our findings among male and female smokers are modulated by DAT genotype with 9-repeats showing greater positive associations between attentional bias to SCs and SC-induced mOFC and amygdalar activation.
Theories of amygdalar and mOFC function suggest that the amygdala and mOFC are extensively interconnected and are involved in detecting and responding to emotionally salient stimuli (Dolan, 2007; Salzman and Fusi, 2010). Recent research suggests that attentional focus and reward evaluations elicit both mOFC and amygdala brain activity (Siep et al., 2009); whereby, the amygdala may detect emotionally salient information (Kanske and Kotz, 2011; Phillips et al., 2003) and the mOFC may be involved in the conscious reward experience (Gottfried, O'Doherty & Dolan, 2003; Small et al., 2001). The reciprocal connections between the amygdala and mOFC and their involvement in incentive motivational behavior is further supported by neuroimaging studies on patients with focal amygdala lesions showing that reward-related mOFC brain response is dependent on a functioning amygdala (Hampton et al., 2007). Future studies using a smoking attentional bias task during blood oxygen-level dependent (BOLD) fMRI scanning could examine functional connectivity between these two structures.
Of special interest are the inverse correlations between attentional bias to SCs and brain reactivity to SCs vs non-SCs in the mOFC among 10/10-repeats. This finding may provide a potential explanation for our previous findings that 10/10-repeats showed decreased SC-reactivity in the mOFC. Specifically, the 10/10-repeats may not be attending to SCs and/or may be evaluating the SCs differently than 9-repeats. Given that the smokers in the current study were satiated prior to the off-magnet attentional bias task and the SC-reactivity imaging task, we speculate that this satiated state eliminated brain responses in 10/10-repeats whose smoking behavior may be influenced to a greater extent by nicotine withdrawal than by SC exposure. Thus, the inverse correlation observed among 10/10-repeats may signify reduced cue valuation during satiety. We are currently collecting data to examine this possibility and plan to examine potential affective biases under withdrawal and satiated states among DAT genotypes.
The current (and our previous) findings suggesting that genetic variation in DAT influences individual responses to SCs show several intriguing similarities with a preclinical literature that focuses on the study of individual differences in the propensity to attribute incentive salience to reward cues (Flagel, Akil & Robinson, 2009; Robinson and Flagel, 2009; Saunders and Robinson, 2010). Briefly, when food or drug is paired with an identifiable cue, the cue itself becomes attractive and elicits approach behaviors in some rats (“sign-trackers,” STs); other rats direct their attention and behavior away from the cue towards reward delivery (“goal-trackers,” GTs) (Flagel et al., 2009; Flagel et al., 2007). Historically, STs are displaying Pavlovian conditioned responses to the cues that are paired with reward delivery (Hearst and Jenkins, 1974); whereas, GTs are potentially conditioned to the receipt of the reward itself (Boakes, 1977). It has also been postulated that STs are more vulnerable to addiction based on their responsiveness to cues (Saunders and Robinson, 2010, 2011); however, these studies indicate that STs and GTs self-administer cocaine at the same rate (Saunders and Robinson, 2010, 2011). This suggests to us that both STs and GTs have vulnerabilities that can lead to addiction, but the brain mechanisms underlying the addictive behaviors may differ. The authors posit that the individual differences in behavior between STs and GTs are likely related to variation in systems that respond to reward and attribute incentive salience to cues, specifically the dopaminergic system.
Although few studies have examined these putative differences in the brain between STs and GTs, existing research suggests that differences do exist (Tomie, Grimes & Pohorecky, 2008). For example, a recent study found higher levels of DAT mRNA in the VTA of GTs compared to STs (Flagel et al., 2007). One interpretation of this may be that lower levels of mRNA DAT in the VTA neurons of STs would lead to less DAT availability at the terminals of VTA afferents to the ventral striatum, and consequently, there would be slower reuptake of DA among STs in response to rewards and reward predictors. Thus, when a cue is present, DA remains longer in the synapse, leading to increased incentive salience and enhanced responses, such that STs exhibit maladaptive behavior in the presence of cues. This biomarker of reduced DAT mRNA in STs, who are cue-responsive, is in direct alignment with our fMRI data in seemingly cue-responsive 9-repeats. Although we believe the parallels between the preclinical and our findings support a cue-vulnerable endophenotype among 9-repeats, we plan to pursue studies exploring how DAT genotype variation influences other aspects of incentive salience and addictive behaviors, including treatment outcome.
A limitation of the current study is that behavioral measures are not fully supportive for differential attentional bias to SCs between DAT variants; however, attentional bias reaction times for smokers in the current study are similar to those presented in a previous study using the same visual dot-probe attentional bias task (Ehrman et al., 2002). We believe that the absence of a difference between groups is related to sample size, and that with a larger sample size, differences might be observed. Neurophysiological markers are presumed to be more sensitive, and thus, the results presented here and in Janes et al. 2010b substantiate the use of a correlational approach. A potential confound in our findings could be due to differences in data acquisition. For example, the first 15 subjects were scanned using a Bruker coil; whereas, the remaining subjects were scanned using an 8-channel coil. To explore whether data acquisition differences affected findings, we compared variances between both groups using a homogeneity of variance test and found that the variances were not significantly different. This study is also limited in that only one factor underlying cue vulnerability was examined: DAT genotype. We recognize that many factors are at play including race/ethnicity, sex, menstrual cycle, stress and variance in other genes (Franklin et al., 2008; McClernon et al., 2007; Okuyemi et al., 2006; Sinha, 2009). In fact, Okuyemi and colleagues (2006) found that African Americans had greater SC-reactivity relative to Caucasians, which could potentially indicate greater attentional bias based on our results. Thus, we will continue to acquire data in order to assess the interactions of these and other factors on relapse vulnerabilities.
The current results extend our prior finding that genetic variation in the DAT gene influences neural responsivity to SCs by identifying the potential mechanism underlying these genetic differences. The positive correlation between attentional bias to SCs and brain responses in the amygdala and mOFC during SC exposure in sated 9-repeats may provide a potential neural mechanism for the hyperresponsivity in reward-related regions, suggesting that 9-repeats may represent a cue-vulnerable endophenotype who may be at greater risk for relapse when exposed to SCs. The deleterious effects of this chronic relapsing disorder underscore the importance of identifying the neurophysiological vulnerabilities and protections that could improve treatment response. The available smoking cessation medications nicotine replacement therapy (NRT), bupropion and varenicline are efficacious in some smokers, but overall treatment success is modest (20–40%), reflecting heterogeneity in treatment response (Wu et al., 2006). All three medications combat withdrawal (Benowitz, 2009), varenicline blocks smoking reinforcement (Coe et al., 2005), and there is emerging evidence that bupropion and varenicline can reduce SC reactivity and SC-induced craving (Brandon et al., 2011; Culbertson et al., 2011; Franklin et al., 2011a). Thus, matching the appropriate medication to an individual’s specific vulnerabilities could substantially improve smoking outcomes for all of the existing treatments.
Acknowledgements
This work was supported by NIH grants 5-P60-DA-005186-18, 1R21DA025882 – 01A1, MH080729 and EB015893. The authors wish to thank Drs. John Detre and Ze Wang for optimization of the perfusion fMRI technique, and Yin Li for aiding in imaging data preprocessing. We also thank the nursing staff at the Center for the Studies of Addiction for conducting physical evaluations; clinicians Anita Hole PhD, Jesse Suh, PsyD and Kathleen Marquez, MA for conducting the psychological evaluations; Joshua Shin, BS, the lead technician on the study; and the MRI technicians at the Hospital of the University of Pennsylvania for conducting the scanning sessions.
Footnotes
Supplemental material: http://franklinbrainimaging.com/
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Authors Contribution
RRW, TRF, RE and ARC were responsible for the study concept and design. TRF acquired the clinical data. FWL performed the genetic analysis. RRW and KJ conducted the data analysis, and TRF, ARC, CO assisted in the interpretation of findings. RRW drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Aguirre GK, Detre JA, Wang J. Perfusion fMRI for functional neuroimaging. Int Rev Neurobiol. 2005;66:213–236. doi: 10.1016/S0074-7742(05)66007-2. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Boakes., editor. Performance on learning to associate a stimulus with positive reinforcement. Hillsdale, NJ: Erlbaum; 1977. [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Archives of general psychiatry. 2011;68:505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with Magnetic Resonance Imaging and Blood Supply. 2nd ed. New York City, NY: Springer-Verlag Wien; 1999. [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Archives of general psychiatry. 2011a;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt) 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O'Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. 2011b;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins H. Sign-tracking: the stimulus-reinforcer relation and directed action. Austin: Monograph of the Psychonomic Society; 1974. [Google Scholar]
- Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–633. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010a;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ. Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology. 2010b;35:2339–2345. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Emotion triggers executive attention: anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum Brain Mapp. 2011;32:198–208. doi: 10.1002/hbm.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. Attention and Orienting: Sensory and Motivational Processes. 1997;477:97–135. [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychol Med. 2000;30:169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Mai JK, Voss T, Paxinos G. Atlas of the Human Brain. New York City, NY: Elsevier Academic Press; 2008. [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3’ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Powell JN, Savage CR, Hall SB, Nollen N, Holsen LM, McClernon FJ, Ahluwalia JS. Enhanced cue-elicited brain activation in African American compared with Caucasian smokers: an fMRI study. Addict Biol. 2006;11:97–106. doi: 10.1111/j.1369-1600.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95. 2000;(Suppl 2):S191–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan B, Lecrubier Y, Sheehan K. The Mini International Neuropsychiatric Interview (MINI): The development and validation of structured diagnostic interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol Psychiatry. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O’Connor R, Stauffer RL, Vogler GP, Kozlowski LT. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different. Nicotine Tob Res. 2002;4:333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Carter BL, Robinson JD, Wetter DW, Lam CY, Kerst W, Cinciripini PM. Attentional bias is associated with incentive-related physiological and subjective measures. Exp Clin Psychopharmacol. 2009;17:247–257. doi: 10.1037/a0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence manual. San Antonio: Harcourt Brace & Company; 1999. [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and "relapse" in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]