Abstract
Background: Pain following extraction of an impacted third molar is widely used to assess analgesic efficacy, especially that of a single dose of a drug. The analgesic activity of conventional nimesulide (CN) has been documented in a variety of types of acute and chronic pain. Beta-cyclodextrin nimesulide (BN) is a new formulation in which nimesulide is included in a cyclodextrin molecule, which increases its solubility in water and its dilution rate, allowing extended, rapid absorption of the drug.
Objective: The aim of this study was to assess the efficacy and tolerability of a single dose of BN compared with CN in patients with pain following extraction of an impacted third molar.
Methods: This was a prospective, randomized, double-blind, double-dummy study conducted at 3 dentistry centers in Venezuela. The patients were randomized to 1 of 2 groups. One group received a single dose of BN (400-mg tablet, equivalent to 100 mg of nimesulide); the other group received a single dose of CN (100-mg tablet). Both groups also received a placebo. The efficacy variables were (1) pain intensity (PI), assessed on a visual analog scale (VAS) at the following times: 0, 5, 10, 15, 30, and 45 minutes and 1, 2, 4, 6, 8, 10, and 12 hours after drug administration; (2) time to first measurable difference in PI from baseline (PID) (PID ≥1 cm on the VAS; ie, the beginning of analgesic action); (3) maximum PID (max PID); (4) sum of PIDs in the 12-hour observation period; (5) pain relief (PR), as rated on a 5-point scale; (6) maximum PR; and (7) sum of the PR scores in the 12-hour observation period (ie, total PR). For the tolerability analysis, all adverse events (AEs) were to be recorded, and the investigators were to assess whether each AE was drug related.
Results: Seventy-two patients were enrolled in the study. Of these, 62 patients (40 women, 22 men; mean [SD] age, 20.1 [5.9] years) were assessed; 35 were treated with BN and 27 with CN. PI reduction was more rapid and greater in the BN group. The first measurable change in PI (PID ≥1 on the VAS) was reached within 5 minutes by 39% and 15% of the patients in the BN and CN groups, respectively, and within 10 minutes by 52% and 30% of the patients in the BN and CN groups, respectively. The max PID was reached <1 hour in 32% and 15% of patients in the BN and CN groups, respectively. No AEs were reported.
Conclusions: In this study population, both BN and CN were similarly effective in relieving pain after extraction of an impacted third molar, and both drugs were well tolerated. PI changes were statistically significantly more rapid and greater with BN than CN.
Keywords: beta-cyclodextrin nimesulide, dental pain
INTRODUCTION
Following extraction of an impacted third molar, pain is usually acute, with a short duration that reaches the highest intensity within 3 hours of the procedure. This type of pain is used widely to assess analgesic efficacy, especially that of a single dose of a drug.
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are sufficiently effective for the relief of mild and moderate pain; these drugs are particularly suited to use in an outpatient setting. The analgesic activity of conventional nimesulide (CN), an NSAID, has been documented in a variety of types of acute and chronic pain. Beta-cyclodextrin nimesulide (BN) is a new formulation in which nimesulide is included in a cyclodextrin molecule, which increases its solubility in water and its dilution rate, allowing extended, rapid absorption of the drug. The high nimesulide pKa (negative logarithm of the acid ionization constant)1 and the cyclodextrin inclusion allow the drug to pass rapidly through the mucus membranes into the plasma, giving BN 2 significant advantages—rapid therapeutic action (an ideal characteristic for an analgesic agent) and short permanence in the mucus membranes, further decreasing the low ulcerogenic activity of CN.2
In BN, coformulation with cyclodextrin increases the drug dissolution rate. Furthermore, cyclodextrins are not absorbed across the gastrointestinal (GI) mucosa.2
The efficacy and tolerability of BN have been assessed in several clinical trials in patients with pain after arthroscopic surgery3 or dental surgery.4 In these trials, both products were effective and well tolerated in the treatment of acute pain.
The purpose of this study was to determine the efficacy and tolerability of a single oral dose of BN compared with CN in patients with pain following extraction of an impacted third molar.
PATIENTS AND METHODS
Patients
Patients attending 1 of 3 dentistry centers in Venezuela for extraction of an impacted third molar and who provided written informed consent were screened for entry into the study. Patients of both sexes, aged 12 to 60 years, with pain after surgery rated between 4 and 8 cm (ie, moderate to severe) on a 10-cm visual analog scale (VAS) were eligible for the study. VAS scores ranging from 0 cm (no pain) to 10 cm (worst pain imaginable) are used widely for pain measurement and are recommended by guideline committees for gauging therapy for individual patients.1
Exclusion criteria were suspected or known hypersensitivity to NSAIDs; a history of cardiac, hepatic, or renal disease; and a history or the presence of GI disease. Pregnant, possibly pregnant, or lactating women were not eligible for the study. Patients with alcohol dependence or abuse, or diagnosed hypertension, diabetes, or hematologic or coagulation disorders also were excluded from the study.
Study Design
For this multicenter, prospective, randomized, double-blind, double-dummy study, enrolled patients were randomized and distributed in blocks of 10, to 1 of 2 groups. One group received a single dose of BN (400-mg tablet equivalent to 100 mg of nimesulide); the other group received a single dose of CN (100-mg tablet), according to a double-blind design. Both groups also received a placebo. The protocol was approved by an independent ethics committee.
The third molar was removed using a standard technique and local anesthesia. The duration of the surgery (from the first incision until the end of suturing) was recorded for each patient.
Consumption of short-acting analgesics in the last 6 hours prior to surgery or long-acting analgesics within 24 hours prior to surgery was prohibited.
During the study, consumption of any NSAID except acetaminophen, which was considered to be the rescue medication, was prohibited. Acetaminophen 500-mg tablets could be given 30 minutes after study drug administration if pain relief was nonsignificant. Steroids could be given after study drug administration. In both groups, the dose and the route of administration were recorded.
One to 5 hours after surgery, patients who developed pain that measured 4 to 8 cm on the VAS were entered into the study. After administration of a single dose of 1 of the study drugs, patients rated pain intensity (PI) and pain relief (PR) at 0, 5, 10, 15, 30, and 45 minutes and 1, 2, 4, 6, 8, 10, and 12 hours. The times were determined using the patient's wristwatch. The timing measurements were recorded in the same way for all patients, under double-blind conditions.
Efficacy Variables
The efficacy variables were (1) PI, as rated on the 10-cm VAS (0 cm = no pain to 10 cm = the worst imaginable pain); (2) time to first measurable difference in PI from baseline (PID) (PID ≥1 cm on the VAS; ie, the beginning of analgesic action); (3) maximum PID (max PID); (4) sum of PIDs in the 12-hour observation period (SPID) (measured at 15, 30, and 45 minutes and 12 hours); (5) PR, as rated on a 5-point Pain Relief scale2–4 (0 = no relief, 1 = a little relief, 2 = some relief, 3 = a lot of relief, 4 = complete relief); (6) maximum PR; and (7) sum of the PR scores in the 12-hour observation period (ie, total PR [TOPAR]). The equations used to calculate PID, SPID, and TOPAR are as follows:PID=PI−PI1→12SPID=Σ(PI0−PID1→12)TOPAR=Σ(PR·h)1→12
Tolerability Analysis
During the 12-hour observation period, all adverse events (AEs), as volunteered by the patients or determined by questioning by study investigators, were recorded. The investigators assessed whether each AE was drug related.
Statistical Analysis
PI, PID, and SPID were compared using the paired t test (within groups) and the unpaired t test (between groups).
PR and TOPAR were assessed using the Wilcoxon rank sum test (within groups) and the Mann-Whitney test (between groups).
The study had a power of 90% to detect a 2-cm change in VAS between treatments and a power of 90% to detect a ≥20% difference between treatments. A probability level of P≤0.05 was considered significant.
RESULTS
A total of 72 patients were enrolled (BN group, n = 39; CN group, n = 33). Of these, 4 patients (10%) in the BN group and 6 patients (18%) in the CN group had no pain, so the data from these patients were not analyzed. Only patients who received the study medication were considered in the analysis (N = 62; 40 women, 22 men; mean [SD] age, 20.1 [5.9] years; BN group, n = 35; CN group, n = 27) (Table I). The age, sex, body weight, height, and blood pressure were similar in the 2 groups.
Table I.
Demographic characteristics of study patients (N = 62).∗
| Characteristic | BN (n = 35) | CN (n = 27) |
|---|---|---|
| Age, mean, y | 19.5 | 20.9 |
| Sex, no. (%) | ||
| Women | 21 (60) | 19 (70) |
| Men | 14 (40) | 8 (30) |
| Body weight, kg | 62.0 (13.1) | 62.2 (11.6) |
| Height, m | 1.6 (0.1) | 1.7 (0.1) |
| Blood pressure, mm Hg | ||
| Systolic | 106.9 (26.5) | 114.5 (6.4) |
| Diastolic | 68.1 (9.1) | 69.1 (7.3) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
Values are presented as mean (SD) unless otherwise noted. No statistically significant between-group differences were found.
The indications for removal of a third impacted molar, duration of surgery, and time to the onset of pain were similar in the 2 groups (Table II).
Table II.
Indications for removal of an impacted third molar, duration of surgery, and time until onset of pain in study patients (N = 62).∗
| Parameter | BN (n = 35) | CN (n = 27) |
|---|---|---|
| Indication, no. (%) of patients† | ||
| Inadequate space | 18 (51) | 12 (44) |
| Orthodontia | 5 (14) | 8 (30) |
| Incorrect position | 7 (20) | 4 (15) |
| Duration of surgery, no. (%) of patients‡ | ||
| <15 min | 7 (20) | 6 (22) |
| ≥15 min–1 h | 28 (80) | 17 (63) |
| Mean time to onset of pain, h:min | 3:00 | 3:29 |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
No statistically significant between-group differences were found.
Percentages may not add to 100% due to rounding. Data not available for 5 patients in the BN group and 3 patients in the CN group.
Data not available for 4 patients in the CN group.
The majority of patients in the BN and CN groups (33 patients [94%] and 23 patients [85%], respectively) received a single dose of steroids parenterally as anti-inflammatory medication at the time of the extraction. A majority of patients (28 [80%] and 22 [81%] patients in the BN and CN groups, respectively) also received antibiotic prophylaxis. Both steroids and antibiotics are used routinely by maxillary surgeons in Venezuela. Both treatments were distributed similarly in the 2 groups (data not shown).
Efficacy Analyses
Pain Intensity
Fifty-eight patients were included in the efficacy analyses. Data were unavailable in 4 patients. PI was similar in the BN and CN groups at the onset of pain (5.53 cm vs 5.38 cm, respectively). The first measurable change in PI (ie, PID ≥1 cm on the VAS) occurred in 12 patients (39%) in the BN group and 4 patients (15%) in the CN group within 5 minutes of drug administration. Sixteen (52%) and 8 (30%) patients in the BN and CN groups, respectively, reached a measurable change in PI within 10 minutes (Table III).
Table III.
Time to first measurable difference in pain intensity (PID) (ie, PID ≥1 cm on the visual analog scale) in study patients (N = 58).
| No. (%) of Patients with First Measurable PID |
||
|---|---|---|
| Time After StudyDrug Administration | BN (n = 31)∗ | CN (n = 27) |
| ≤5 min† | 12 (39) | 4 (15) |
| 10 min | 4 (13) | 4 (15) |
| 15 min | 2 (7) | 5 (19) |
| 30 min | 3 (10) | 7 (26) |
| 45 min | 2 (7) | 2 (7) |
| ≥1 h | 6 (19) | 5 (19) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
Data not available in 4 patients, and pain was not relieved in 2 patients in time for the evaluation.
P<0.05 between groups.
The first significant change in PI was observed in the BN group at 10 minutes after study drug administration (PI = 4.41 cm; P = 0.042 vs baseline); for the CN group, the first significant change in PI was observed at 15 minutes (PI = 4.54 cm; P = 0.008 vs baseline) (Table IV).
Table IV.
Mean (SD) pain intensity scores (cm) on the 10-cm visual analog scale∗ (VAS) in study patients (N = 58).
| VAS Score |
||
|---|---|---|
| Time After Study Drug Administration | BN (n = 31)† | CN (n = 27) |
| Baseline | 5.53 (1.84) | 5.38 (1.06) |
| 5 min | 5.04 (2.01) | 5.22 (1.20) |
| 10 min | 4.41 (2.46)‡ | 4.84 (1.68) |
| 15 min | 4.09 (2.44)§ | 4.54 (1.77)∥ |
| 30 min | 3.51 (2.53)¶ | 3.76 (2.06) |
| 45 min | 2.78 (2.50) | 3.02 (2.29) |
| 1 h | 2.34 (2.62) | 2.43 (2.24) |
| 2 h | 2.05 (2.65) | 1.52 (2.25) |
| 4 h | 1.33 (2.09) | 1.09 (1.92) |
| 6 h | 0.96 (1.72) | 0.40 (1.02) |
| 8 h | 0.85 (1.41) | 0.27 (0.87) |
| 10 h | 0.78 (1.34) | 0.08 (0.28)# |
| 12 h | 0.62 (1.27) | 0.18 (0.74) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
Scale: 0 cm=no pain to 10 cm=worst imaginable pain.
Data not available in 4 patients.
P=0.042 versus baseline.
P=0.010 versus baseline.
P=0.008 versus baseline.
P=0.002 versus baseline.
P=0.013 versus BN group.
Throughout the 12-hour observation period, the only significant between-group difference in PI occurred at 10 hours (BN, 0.75 cm and CN, 0.08 cm; P = 0.013); however, the difference was not clinically significant because both of these VAS scores were close to 0 cm (ie, no pain) on the pain scale (Figure 1).
Figure 1.
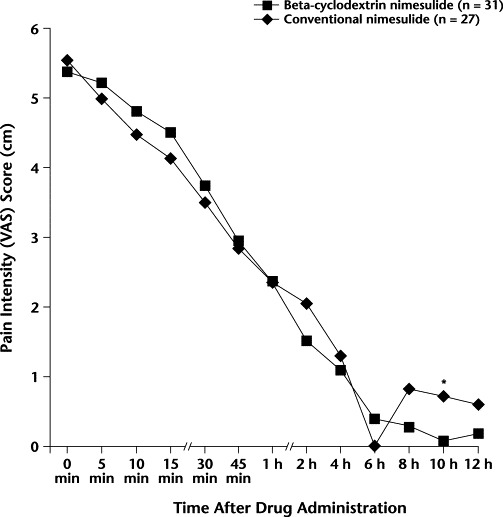
Pain intensity scores on the 10-cm visual analog scale (VAS) (0 cm = no pain to 10 cm = worst imaginable pain) in the 2 treatment groups throughout the 12-hour observation period. ∗P = 0.013 versus conventional nimesulide.
The max PID was reached by 10 (32%) and 4 (15%) patients in the BN and CN groups, respectively, before 1 hour postadministration (Table V).
Table V.
Time to maximum difference from baseline in pain intensity (max PID) in study patients (N = 58).
| No. (%) of Patients Reaching max PID |
||
|---|---|---|
| Time After Study Drug Administration | BN (n = 31)∗ | CN (n = 27) |
| <1 h | 10 (32) | 4 (15) |
| ≥1 h | 21 (68) | 23 (85) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
Data not available in 4 patients.
PID was similar in the BN and CN groups at all time points after study drug administration, except at 5 minutes (1.16 vs 0.17, respectively; P = 0.04) (Figure 2).
Figure 2.
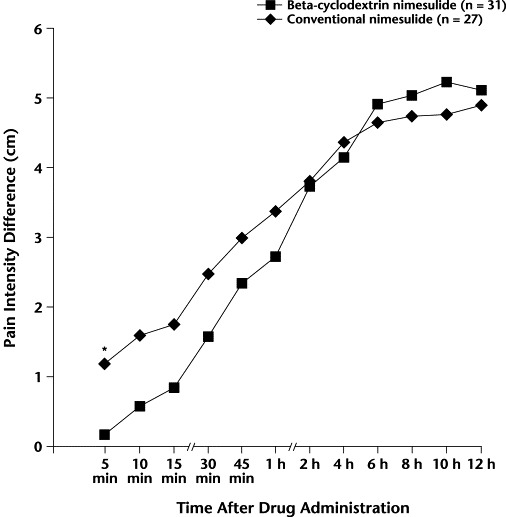
Difference from baseline in pain intensity score on the 10-cm visual analog scale (0 cm = no pain to 10 cm = worst imaginable pain). ∗P = 0.04 versus conventional nimesulide.
The SPID was similar in both groups at 15, 30, and 45 minutes; the finding at 12 hours showed a significant difference in favor of BN (BN, 62.49 cm vs CN, 36.34 cm; P<0.001) (Table VI).
Table VI.
Mean (SD) sum of the pain intensity differences (SPID) from baseline in study patients (N = 58).
| SPID |
||
|---|---|---|
| Time After Study Drug Administration | BN (n = 30)∗ | CN (n = 27) |
| 15 min | 4.52 (8.32) | 1.59 (3.48) |
| 30 min | 6.98 (11.20) | 3.15 (4.99) |
| 45 min | 9.99 (14.00) | 5.47 (6.63) |
| 12 h† | 62.49 (35.08) | 36.34 (14.88) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
Data not available in 4 patients.
P<0.001 between groups.
Total Pain Relief
The first significant change in PR was observed in both groups after 5 minutes. Table VII shows the TOPAR scores (sum of pain relief). The max PR was similar in both groups.
Table VII.
Mean (SD) total pain relief (TOPAR) scores in study patients (N = 58).
| TOPAR Score |
||
|---|---|---|
| Time After Study Drug Administration | BN (n = 35) | CN (n = 27) |
| 10 min | 1.69 (2.23) | 1.44 (1.52) |
| 15 min | 3.08 (3.50)∗ | 2.77 (2.14)∗ |
| 30 min | 5.05 (4.51)∗ | 4.92 (2.90)∗ |
| 45 min | 7.40 (5.53)∗ | 7.29 (3.82)∗ |
| 12 h | 26.91 (12.38)∗ | 31.26 (8.41)∗ |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
P<0.001, by Wilcoxon rank sum test, within-group difference versus previous TOPAR score.
Most patients in the BN and CN groups (27 patients [87%] and 19 patients (95%), respectively) considered the treatment to be effective (ie, very good or good) (Table VIII).
Table VIII.
Patient ratings of treatment efficacy.∗
| No. (%) of Patients |
||
|---|---|---|
| Efficacy Rating | BN (n = 31)† | CN (n = 20) |
| Very good | 19 (61) | 12 (60) |
| Good | 8 (26) | 7 (35) |
| Moderate | 0 (0) | 1 (5) |
| Poor | 2 (7) | 0 (0) |
| Very poor | 1 (3) | 0 (0) |
BN = beta-cyclodextrin nimesulide; CN = conventional nimesulide.
No statistically significant between-group differences were found.
Data not available in 5 patients.
Tolerability Analysis
No AEs were reported in either treatment group.
DISCUSSION
In BN formulations, nimesulide is united with cyclodextrin molecules to increase its solubility and, consequently, its absorption rate. This increase in the absorption rate is reflected in the drug plasma levels, which reach the analgesic level more rapidly than with CN formulations.
For the primary efficacy outcome of PI, the first statistically significant change from the baseline score occurred in the BN group 10 minutes after drug administration and in the CN group 15 minutes after administration; this difference was significant, considering the rapid rate at which both drugs reach therapeutic plasma levels.
This result, in addition to the fact that 55% of patients in the BN group versus 30% in the CN group reached a measurable change (PID ≥1 cm) in the pain scale within 10 minutes and that more patients reached max PID (32% vs 15%, respectively) before 1 hour in the BN group, supports the rapidity of the analgesic effect.
The SPID was somewhat higher in the BN group than in the CN group throughout the observation period, although the difference did not reach statistical significance until hour 12. The decreases from baseline in PI after 12 hours were greater in the BN than in the CN group. Rapid dissolution did not seem to affect the duration of analgesia.
The first measurable change in PR occurred in both groups at the same time. Both drugs had a rapid onset of action.
No significant between-group differences were found in TOPAR at 15, 30, and 45 minutes, and at 12 hours; however, in both groups a significant increase in TOPAR versus previous TOPAR score was found at all time points. It is important to remember that this evaluation is not redundant; it reflects a different aspect of the pain experience.
Most patients in both groups rated the products as effective for the relief of pain.
Results obtained in this study are similar to those obtained by Scolari et al4 in patients with pain following extraction of an impacted third molar and by Berruto et al5 in patients after arthroscopic knee surgery. In the latter study, VAS score decreased significantly (P<0.01) more rapidly in the BN than in the CN group.
In the current study, CN provided unusually rapid pain relief compared with that found in several other studies, in which the first measurable change in PI occurred after 30 minutes.4–6
Considering that pain control after dental surgery is seen as a good model to assess the analgesic activity of NSAIDs, the results obtained in the current study can be expected to be confirmed in other clinical studies in which a rapid analgesic effect is required.
Future studies similar to this one should examine the efficacy of BN and CN in other models of acute pain (eg, dysmenorrhea, sports injuries).
CONCLUSIONS
In this study population, both BN and CN were similarly effective in relieving pain after extraction of an impacted third molar, and both drugs were well tolerated. PI changes were statistically significantly more rapid and greater with BN than CN.
Footnotes
Reproduction in whole or part is not permitted.
References
- 1.Mantha S., Thisted R., Foss J. A proposal to use confidence intervals for visual analog scale data for pain measurement to determine clinical significance. Anesth Analg. 1993;77:1041–1047. doi: 10.1213/00000539-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Miro A., Quaglia F., Calignano A. Physicochemical and pharmacological properties of nimesulide/B-cyclodextrin formulations. STP Pharma Sciences. 2000;10:157–164. [Google Scholar]
- 3.Vizzardi M., Visconti S., Pedrotti L. Nimesulide beta cyclodextrin (Nimesulide-Betadex) versus nimesulide in the treatment of pain after arthroscopic surgery. Curr Ther Res Clin Exp. 1998;59:162–171. [Google Scholar]
- 4.Scolari G., Lazzarin F., Fornaseri C. A comparison of nimesulide cyclodextrin and nimesulide in postoperative dental pain. Int J Clin Pract. 1999;53:345–348. [PubMed] [Google Scholar]
- 5.Berruto M., Marzano N., Pedrotti L. Faster analgesic effect of nimesulide beta cyclodextrin (nimesulide betadex) in comparison with nimesulide in post surgery pain. Preliminary report on a double blind clinical trial. Minerva Ortopedica e Traumatologica. 1997;48:437–443. [in Italian] [Google Scholar]
- 6.Perilli E., Minola M., Colombo F. Nimesulide Betadex: An example of actuality: Pharmaceutical technology as part of a new design of a pharmaceutical product. Farmaci. 1998;22:1–8. [in Italian] [Google Scholar]


