Abstract
Background: Aminophylline, a theophylline compound that contains ethylenediamine, has untoward side effects on many organ systems.
Objective: The goal of this case report was to illustrate the occurrence of acute adverse events (ie, chest discomfort and myocardial enzyme elevation) that may be associated with aminophylline treatment.
Methods: To uncover previous studies/reports on this subject, a literature search (1950–2003) was conducted on MEDLINE, UpToDate, and Doctor's Guide, using the search terms aminophylline toxicity, theophylline toxokinetics, pharmacotoxic myocardial injury, hypersensitivity myocarditis, and diagnosis of myocardial infarction with biomarkers of cardiac injury. A 76-year-old, obese, female patient was admitted to University Hospital (Rion, Greece) for an acute exacerbation of chronic bronchitis. Beginning on day 0 of hospitalization, the patient was treated with aminophylline 750 mg IV, given in a 24hour constant infusion, for persistent wheezing. We monitored the patient's condition using electrocardiography, echocardiography, and blood chemistry analysis.
Results: While undergoing aminophylline treatment, the patient developed vague chest discomfort and myocardial enzyme elevation due to aminophylline-induced cardiotoxicity. Mild wheezing was still present on physical examination on day 2 of hospitalization. The serum creatine kinase (CK) level was slightly increased. On day 6 of hospitalization, the patient's symptoms worsened, with mild epigastric discomfort, tachycardia, fatigue, and tightness in the chest. Blood gas analysis revealed mild hypoxia and hypocapnia. Pulmonary perfusion scan showed a low risk for pulmonary thromboembolism, as indicated by the absence of segmental perfusion defects. Blood chemistry analysis showed increased serum CK (×2.5) and CK isoenzyme (CK-MB) fraction (×8.6) levels. Echocardiography on day 7 showed a slight hypertrophy of the septum, with normal dimensions of the ventricles and a 70% ejection fraction. Aminophylline treatment was permanently discontinued, and the patient's signs and symptoms promptly improved.
Conclusions: In the case presented here, the exclusion of usual causes of increased serum CK and CK-MB fraction levels, together with the increased serum aminophylline concentration and, most importantly, the rapid alleviation of symptoms and normalization of myocardial enzymes in absolute temporal relationship to the discontinuation of the drug, suggested that aminophylline treatment might be associated with elevated levels of myocardial enzymes. (Curr Ther Res Clin Exp. 2003;64:379–386)
Keywords: aminophylline, adverse events, cardiotoxicity
Introduction
Aminophylline, a theophylline compound that contains ethylenediamine, has untoward side effects on many organ systems. This drug has a low therapeutic index, and drug-induced toxicity may be manifested as serum drug concentrations within the therapeutic range.1 Many concomitantly administered drugs have been demonstrated to increase serum aminophylline concentrations (unpublished data, American Hospital Formulary Service Drug Information, 2003).
The goal of this case report was to illustrate the occurrence of acute adverse events (AEs) (ie, chest discomfort and myocardial enzyme elevation) that may be associated with aminophylline treatment.
Patient and methods
To uncover previous studies/reports on this subject, a literature search (1950–2003) was conducted on MEDLINE, UpToDate, and Doctor's Guide, using the search terms aminophylline toxicity, theophylline toxokinetics, pharmacotoxic myocardial injury, hypersensitivity myocarditis, and diagnosis of myocardial infarction with biomarkers of cardiac injury.
A 76-year-old, obese, female patient (height, 167 cm; body weight, 92 kg) was admitted to University Hospital (Rion, Greece) because of an acute exacerbation of chronic bronchitis. She also had mild hypertension, which had been treated with indapamide tablets 2.5 mg once daily for the past 10 years. She had never received beta-blockers. On physical examination, the patient was dyspneic, her heart rate was 88 bpm, respiratory rate 24 breaths/min, and body temperature 37.4°C. She had moderate wheezing in both lungs. Chest radiography was normal. Hypoxia was found on blood gas analysis (partial pressure of oxygen, 75 mm Hg; partial pressure of carbon dioxide, 35 mm Hg; and pH, 7.5, while breathing room air). The results of hematologic and blood chemistry analyses were normal.
Oxygen was provided to the patient via a mask, and ceftriaxone 2 g once daily was administered IV. Budesonide (400 μg every 12 hours given by nebulizer) and salbutamol plus ipratropium (0.5 = 2.5 mg every 6 hours given by nebulizer) were added to the treatment regimen. Because of persistence of wheezing, aminophylline 750 mg IV, given in a 24-hour constant infusion, was added on day 0 of hospitalization. The patient did not receive heparin.
We monitored the patient's condition using electrocardiography (ECG), echocardiography, and blood chemistry analysis.
Results
Laboratory data recorded during hospitalization are shown in Tables I and II. The patient's condition moderately improved during days 1 and 2 of hospitalization, but on day 3 the patient experienced vague chest pressure. ECG was normal, except for sinus tachycardia (100 bpm), and the blood gas levels were improved, but mild wheezing was still present on physical examination. The hematologic and biochemical profiles were normal, except for a slight increase in serum creatine kinase (CK) level. On day 6 of hospitalization, the patient's symptoms worsened, with mild epigastric discomfort, tachycardia, fatigue, and tightness in the chest. Chest radiography now showed no changes; the ECG showed a sinus rhythm of 90 bpm, with a right bundle branch block. Blood gas levels were unchanged, revealing mild hypoxia and hypocapnia. The plasma levels of D-dimers were normal.
Table I.
Fluctuation of serum creatine kinase (CK) and CK isoenzyme (CK-MB) fraction levels (U/L) during and after intravenous administration of aminophylline.
| Day of Hospitalization |
|||||||
|---|---|---|---|---|---|---|---|
| Component | Normal Value | 0 | 1 | 3 | 6 | 7∗ | 9 |
| CK | 50–200 | 170 | 166 | 195 | 477 | 392 | 174 |
| CK-MB fraction | <lt;10 | 15 | 14 | 19 | 155 | 156 | 12 |
Aminophylline treatment discontinued.
Table II.
Serum laboratory data measured during hospitalization.
| Day of Hospitalization |
||||||
|---|---|---|---|---|---|---|
| Component | Normal Value | 0 | 3 | 6 | 7∗ | 9 |
| ALT, U/L | 10–40 | 15 | 14 | 18 | 21 | 20 |
| AST, U/L | 20–48 | 15 | 12 | 15 | 16 | 12 |
| Bilirubin (total), mg/dL | 0.3–1.2 | 0.4 | 0.5 | 0.4 | 0.3 | 0.3 |
| CK, U/L | 50–200 | 170 | 195 | 477 | 392 | 174 |
| CK-MB fraction, U/L | <lt;10 | 16 | 24 | 155 | 156 | 14 |
| Creatinine, mg/dL | 0.6–1.2 | 0.8 | 1.0 | 1.0 | 1.1 | 1.0 |
| cTnl, mg/mL | <lt;0.06 | – | 0 | 0 | 0 | 0 |
| Glucose, mg/dL | 70–110 | 108 | 115 | 120 | 125 | 112 |
| LDH, U/L | 50–200 | 220 | 242 | 235 | 223 | 215 |
| Urea nitrogen, mg/dL | 8–23 | 55 | 44 | 48 | 44 | 54 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; CK = creatine kinase; CK-MB = creatine kinase isoenzyme; cTnl = cardiac troponin l; LDH = lactate dehydrogenase.
Aminophylline treatment discontinued.
Venous ultrasonography of the legs was normal, and a pulmonary perfusion scan showed a low risk for pulmonary thromboembolism, as indicated by the absence of segmental perfusion defects. Blood chemistry analysis showed increased serum CK (×2.5) and CK isoenzyme (CK-MB) fraction (×8.6) levels. The serum cardiac troponin I (cTnI) level was normal.
Thyroid hormone and thyroid-stimulating hormone levels were normal. The serology results for a viral infection were negative. During days 6 and 7, serial measurements of serum CK and CK-MB fraction levels increased at the same rate. Serum aspartate aminotransferase, cTnI, and lactate dehydrogenase levels were normal, and ECG showed no indication of myocardial infarction (MI). Echocardiography performed on day 7 of hospitalization showed slight hypertrophy of the septum, with normal dimensions of the ventricles and a 70% ejection fraction. The serum aminophylline concentration on day 7 was 19 μg/mL (therapeutic level, 10–20 μg/mL). Due to the signs, symptoms, and elevated enzyme levels found, aminophylline treatment was discontinued. The patient's signs and symptoms improved promptly on discontinuation (Figure), and the serum CK and CK-MB fraction levels returned to normal after 2 days.
Figure.
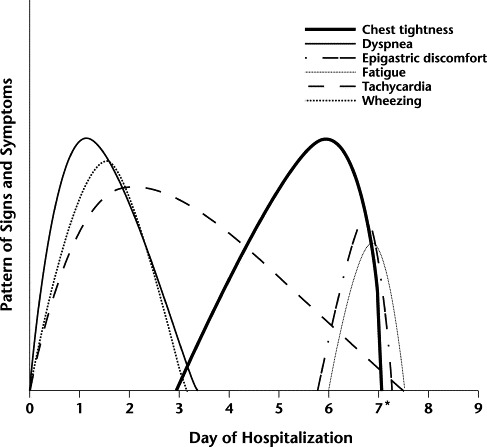
Patient's signs and symptoms during hospitalization. ∗Aminophylline treatment discontinued.
Discussion
Although many drugs administered concomitantly with aminophylline have been demonstrated to increase the serum aminophylline concentration, none of the drugs given to the presented patient (indapamide, cephalosporins, inhaled corticosteroids, beta-agonists, and anticholinergic drugs) have been reported to interact with aminophylline or to affect its serum concentration. Moreover, these drugs have not been associated with cardiotoxic AEs or the elevation of myocardial enzyme levels.
We had difficulty establishing an etiologic diagnosis; adverse drug reactions commonly are overlooked in the differential diagnosis. At first, we attributed the patient's signs and symptoms to her underlying condition, trying to rule out causes of deterioration (eg, infection, pulmonary embolism). The increased serum CK and CK-MB fraction levels, together with vague chest pressure and tightness, suggested an MI.
The clinical profiles, presentations, and outcomes of women with acute coronary syndromes are quite different from those of their male counterparts. Women often have atypical complaints, which was a major consideration in ruling out ischemic heart disease as the cause of symptoms in the presented case. These differences between the sexes cannot be entirely accounted for by differences in baseline characteristics and may reflect pathophysiologic and anatomic differences between women and men.2 Even major risk factors of coronary heart disease, excluding hypercholesterolemia, such as smoking, diabetes mellitus, elevated triglyceride levels, and left ventricular hypertrophy, were found to have a greater effect on women than on men.3 ECG revealed only nonspecific changes in the presented patient, but a substantial number of patients with acute MI may have an ECG with only nonspecific alterations or even a normal ECG.4 Furthermore, many patients have no recognizable symptoms. For example, <lt;50% of elderly people with an acute MI present with chest discomfort.5 For these reasons, more reliance has been placed on the assessment of biochemical markers of myocardial injury.6
The patient presented here did not fulfill the criteria for an acute, evolving, or established MI. The typical increase and gradual decrease in serum cTnI level did not occur, nor did the more rapid increase and decrease in CK-MB fraction level; also, there were no pathologic Q waves or other ECG changes indicative of ischemia (ST-segment or T-wave changes) on serial ECG.7 Troponins are the markers of choice and should be used instead of the CK-MB fraction.8 Troponins are highly specific for myocardial injury, increase early (at 4–6 hours after MI), and remain elevated for up to 10 days. The CK-MB fraction level may be helpful in determining the timing of events and should be used predominantly for that purpose because it increases early but remains elevated for only 36 to 48 hours. cTnI is a sensitive, highly specific, and long-lived marker of myocardial injury.8–10 Serial measurements of serum cTnI helped us to rule out MI in this patient.
Elevated CK-MB fraction also can be found in skeletal muscle.11 Our patient had no clinical signs of myopathy or skeletal muscle damage. She also had normal renal and thyroid function. Because elevations of biochemical markers are diagnostic of cardiac injury but not necessarily MI, we considered other mechanisms of cardiac injury, such as myocarditis and drug cardiotoxicity.
The increased serum CK and CK-MB fraction levels could be associated with myocardial necrosis due to myocarditis. The normal serum cTnI level did not rule out this diagnosis.12 Involvement of the myocardium has been reported in 1% to 5% of patients with acute viral infections. However, establishing a diagnosis of myocarditis is difficult because a noninvasive “gold standard” method has not been identified. Many cases are thought to be subclinical and without cardiac symptoms. Also, subtle cardiac signs and symptoms may be overshadowed by systemic manifestations of an underlying infection or disease process.
In the patient presented here, no clinical or laboratory findings showed evidence of a bacterial or viral infection affecting the cardiac muscle. The patient experienced vague chest pressure and tightness, and it is known that myocarditis can mimic myocardial ischemia due to localized inflammation or endothelial dysfunction. However, in myocarditis, chest pain usually is associated with concomitant pericarditis, which was ruled out on echocardiography in this patient. The patient had mild sinus tachycardia and a right bundle branch block, which are more common than serious atrial or ventricular arrhythmias observed in myocarditis, but she was receiving aminophylline and nebulized beta-agonists.
Echocardiography is the most effective means of detecting decreased ventricular function in suspected myocarditis, even when subclinical. Although fulminant and acute myocarditis involve left ventricular systolic dysfunction, patients with fulminant disease have near-normal left ventricular diastolic dimensions and increased septal thickness. In contrast, patients with acute myocarditis have increased left ventricular diastolic dimensions and normal septal thickness.13 Our patient did not fulfill these criteria, having only marginal septal hypertrophy. Hypersensitivity myocarditis related to a drug allergy could be a possibility in this patient, but her cardiac symptoms and ECG findings did not occur in a setting consistent with drug allergy, such as skin rash, fever, and eosinophilia. She also was not receiving any of the commonly implicated drugs (ie, methyldopa, hydrochlorothiazide, ampicillin, furosemide, digoxin, tetracycline, phenytoin, benzodiazepines, tricyclic antidepressants, and lithium).
Echocardiography, in conjunction with a lack of changes on serial ECG and normal serum cTnI levels, provided good evidence against myocardial disease in the presented patient.
Conclusions
In the case presented here, the exclusion of the usual causes of increased serum CK and CK-MB fraction levels, together with the increased serum aminophylline concentration and, most importantly, the rapid alleviation of symptoms and normalization of enzymes that are myocardial injury markers in absolute temporal relationship to the discontinuation of the drug, suggest that aminophylline treatment might be associated with elevated levels of myocardial enzymes.
Footnotes
Reproduction in whole or part is not permitted.
References
- 1.Katz G, Kewitz G, Vozeh S, Follath F. Relation of dose, serum concentration and side effects in intravenous aminophylline therapy. Schweiz Med Wochenschr. 1981;111:2054–2056. [in German] [PubMed] [Google Scholar]
- 2.Hochman J.S, Tamis J.E, Thompson T.D, The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 3.Jonsdottir L.S, Sigfusson N, Gudnason V. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 4.Brush J.E, Jr., Brand D.A, Acampora D. Use of the initial electrocardiogram to predict in-hospital complications of acute myocardial infarction. N Engl J Med. 1985;312:1137–1141. doi: 10.1056/NEJM198505023121801. [DOI] [PubMed] [Google Scholar]
- 5.Wroblewski M, Mikulowski P, Steen B. Symptoms of myocardial infarction in old age: Clinical case, retrospective and prospective studies. Age Aging. 1986;15:99–104. doi: 10.1093/ageing/15.2.99. [DOI] [PubMed] [Google Scholar]
- 6.Alpert J.S, Thygesen K, Antman E, Bassand J.P. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe A.S, Ravkilde J, Roberts R. It's time for a change to a troponin standard. Circulation. 2000;102:1216–1220. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E, Antman E, Beasley J.W. ACC/AHA guidelines for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) J Am Coll Cardiol. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 9.Guest T.M, Ramanathan A.V, Tuteur P.G. Myocardial injury in critically ill patients: A frequently unrecognized complication. JAMA. 1995;273:1945–1949. [PubMed] [Google Scholar]
- 10.Antman E.M. Decision making with cardiac troponin tests. N Engl J Med. 2002;346:2079–2082. doi: 10.1056/NEJMe020049. [DOI] [PubMed] [Google Scholar]
- 11.Tsung J.S, Tsung S.S. Creatine kinase isoenzymes in extracts of various human skeletal muscles. Clin Chem. 1986;32:1568–1570. [PubMed] [Google Scholar]
- 12.Lauer B, Niederau C, Kuhl U. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–1359. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 13.Felker G.M, Boehmer J.P, Hruban R.H. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]


