Abstract
Background: Dysmenorrhea produces painful abdominal cramps that can disrupt the personal lives and productivity of women.
Objective: The aim of this study was to compare the analgesic efficacy, including onset and duration of pain relief, peak effect, and total effect, and tolerability of ibuprofen arginate with those of conventional ibuprofen in patients with moderate to severe pain associated with primary dysmenorrhea.
Methods: Patients were administered a single dose of ibuprofen arginate (200 or 400 mg), conventional ibuprofen (200 or 400 mg), or placebo during each of 5 menstrual cycles in a single-center, double-blind, randomized, double-dummy, 5-cycle, crossover study. Patients recorded their pain intensity and pain relief at regularly scheduled intervals (10, 20, 30, 40, 50, 60, and 90 minutes and 2, 3, 4, 5, and 6 hours) after taking the study medication, and all study observations were recorded in a patient diary. Pain intensity was rated using the following 4-point categoric rating scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Pain relief was rated on a 5-point scale as 0 = none, 1 = a little, 2 = some, 3 = a lot, and 4 = complete relief. Tolerability of ibuprofen arginate was based on a comparison of the incidence of spontaneously reported adverse events in each of the treatment groups.
Results: One hundred four patients entered the study. Of these, 81.7% were white; the mean (SD) age was 27.5 (5.0) years. A total of 65.4% of patients reported moderate pain from dysmenorrhea, and the remaining 34.6% reported severe pain; 20.2% of patients did not complete the study. The median time to achieve meaningful pain relief was ∼30 minutes faster with ibuprofen arginate 400 mg than with either dose of conventional ibuprofen. Tolerability was similar across all treatments.
Conclusions: In this study population of patients experiencing acute pain as a result of primary dysmenorrhea, ibuprofen arginate was associated with effective, tolerable analgesia and a more rapid onset of action than conventional ibuprofen. The faster onset of analgesia may have a role in clinical practice in treating women with dysmenorrhea. A faster onset of action may be important to women whose personal relationships, productivity, or ability to sleep is being adversely affected by pain.
Keywords: dysmenorrhea; pain management; ibuprofen; randomized, doubleblind, placebo-controlled clinical trial
Introduction
Primary dysmenorrhea is defined as cramping pain in the lower abdomen occurring just before or during menstruation. The prevalence of dysmenorrhea in the United States is estimated to be as high as 90%.1 Primary dysmenorrhea often begins 2 to 3 years after menarche and lasts for several years. Symptoms may decrease after pregnancy.2 In addition to pain, women with primary dysmenorrhea may experience nausea, vomiting, diarrhea, headaches, weakness, and/or fainting.3 Primary dysmenorrhea generally affects the personal lives and productivity of women. In addition, sleep disturbance is common, which may exacerbate the effect of pain on daytime functioning.4
The menstrual cramps and associated symptoms of primary dysmenorrhea are known to be associated with excess prostaglandin production, resulting in increased uterine tone and strong, frequent uterine contractions. Because nonsteroidal anti-inflammatory drugs (NSAIDs) interfere with prostaglandin synthesis, they are a logical choice for the treatment of primary dysmenorrhea, and nearly all women treated in experimental settings with NSAIDs have responded positively.5
Ibuprofen is an NSAID proved to be a safe and effective analgesic for treating many different types of pain.6–8 Specifically, studies9–11 have shown the effectiveness of conventional ibuprofen in relieving the pain of primary dysmenorrhea. However, conventional ibuprofen is absorbed slowly and has a slow onset of action compared with ibuprofen arginine (time to maximum plasma drug concentration 90 minutes vs 30 minutes, respectively).12 In fact, ibuprofen is a relatively weak acid (pKa 4.4) that is sparingly soluble in water and acidic media. This weak acidity results in a relatively long residence time in the acidic gastric environment, slowing ibuprofen absorption.
The L-arginine salt of ibuprofen (ibuprofen arginate) is highly soluble and formed by the combination of racemic ibuprofen with the amino acid L-arginine. L-Arginine, a naturally occurring essential amino acid, renders conventional ibuprofen more soluble in water and facilitates its rapid absorption across the gastric and enteric mucosa. By combining ibuprofen with L-arginine, the potential exists for delivering the well-known analgesic properties of conventional ibuprofen faster and more effectively. Physicochemical characterization of ibuprofen arginate indicates that ibuprofen and L-arginine are released at the time of administration and that the dissolved ibuprofen behaves the same as conventional ibuprofen from the free acid (unpublished data, Zambon Group S.p.A. [Milan, Italy], RPT/KRM 93-02, 1993). Each 200-mg ibuprofen arginate tablet delivers 200 mg of ibuprofen and 185 mg of L-arginine.
Peak plasma levels of oral formulations of ibuprofen arginate are significantly higher than those of conventional ibuprofen and are reached 15 to 30 minutes after administration. In commercially available oral ibuprofen products, the maximum plasma ibuprofen levels are reached 1 to 2 hours after administration13 (also unpublished data, Zambon Group S.p.A., RPT/KRM 93-03, 1993).
Thus, adding L-arginine to ibuprofen improves the overall bioavailability of the ibuprofen component. This observation has been confirmed in several clinical studies (unpublished data, Zambon Group S.p.A., IA-US-02, IA-US-04, 1997).
The aim of this study was to compare the analgesic efficacy, including onset and duration of relief, peak effect, and total effect, and tolerability of ibuprofen arginate with those of conventional ibuprofen in patients with moderate to severe pain associated with primary dysmenorrhea.
Patients and methods
Women with dysmenorrhea were followed up in this single-center, double-blind, randomized, placebo-controlled, double-dummy, 5-cycle, crossover study. The study was conducted according to the principles of the Declaration of Helsinki and approved by the Clinical Investigation Review Board at the Biomedical Research Group, Inc. (Austin, Texas). Four gynecologists who were subinvestigators referred patients to the principal investigators for the purpose of participating in the trial.
Patients
All patients had a history of recurrent episodes of documented dysmenorrhea in at least 80% of their menstrual cycles during the year before entering the study. They also had moderate to severe pain associated with primary dysmenorrhea at the time study medication was ingested.
All patients were known to be in good health, with no other likely causes of abdominal pain, including endometriosis, gastric or peptic ulcer, inflammatory bowel disease, leiomyoma, and pelvic inflammatory disease. Patients with concurrent conditions that could cause secondary dysmenorrhea (eg, endometriosis, salpingitis, adhesions) were not eligible for the trial. Patients also were excluded if they were taking oral contraceptives or had taken oral contraceptives for either of the 2 menstrual cycles immediately preceding the study.
Caffeine-containing beverages or foods were not allowed from 1 hour before to 2 hours after taking study drug. Patients also were not permitted to use any concomitant medications, including psychotropics, antidepressants, sedative-hypnotics, muscle relaxants, or other NSAIDs that could confound assessments of pain relief.
All patients provided written informed consent to participate.
Methods
Patients received a single dose of each of the 5 study medications over 5 consecutive menstrual cycles in a randomized sequence: (1) ibuprofen arginate∗ 200 mg; (2) ibuprofen arginate 400 mg; (3) conventional ibuprofen† 200 mg; (4) conventional ibuprofen 400 mg; and (5) placebo. Patients were randomized to sequences using the method described by Ratkowsky et al.14 Patients self-administered study medication only after their menstrual blood flow began and their lower abdominal pain reached moderate to severe intensity. To comply with the double-blind, double-dummy design, all patients ingested 4 tablets (a combination of active drug[s] and/or placebo) at each cycle. Data were collected at regular intervals for 6 hours after each dose of study medication was administered.
The ibuprofen arginate tablets were white and capsule-shaped. They were supplied with a matching placebo by the manufacturer. The conventional ibuprofen tablets were white, compressed, and round. They were purchased over the counter (OTC) and repackaged in an unmarked bottle under the US Food and Drug Administration Good Manufacturing Practice regulation.
Efficacy and tolerability assessments
Patients were instructed to record the exact time they took study drug, time to onset of any pain relief, and time to onset of meaningful pain relief using a clock assigned to them when they enrolled. Patients recorded their pain intensity and pain relief at regularly scheduled intervals (10, 20, 30, 40, 50, 60, and 90 minutes and 2, 3, 4, 5, and 6 hours) after taking the study medication, and all observations were recorded in a patient diary. Pain intensity was rated using the following 4-point categoric rating scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Pain relief was rated on a 5-point scale as 0 = none, 1 = a little, 2 = some, 3 = a lot, and 4 = complete relief. Patients visited the study site within 10 days of taking each dose of study drug to return all completed forms and receive study drug for their next cycle.
Any patient who did not achieve adequate pain relief was permitted to take an alternate medication at any time for each of the cycles in which they participated. However, patients were encouraged to wait at least 1 hour, if possible, before remedicating with another analgesic.
Pain intensity difference (PID) and pain relief were analyzed at each time point. Summary measures were areas under the time–concentration curve from 0 to 6 hours for the sum of PIDs (SPID) and total pain relief (TOTPAR) and peak pain relief.
Primary criteria for efficacy assessment were PID and pain relief scores at intervals up to 6 hours after treatment.
Secondary criteria included the assessment of the weighted SPID (from baseline), pain relief scores, TOTPAR, and peak pain relief. Summary measures for onset of analgesia included time to any relief, time to a little relief, time to some relief, and time to meaningful relief. Duration of relief was assessed by analyzing the time to remedication.
Adverse events (AEs) were listed and summarized by body system. Treatments were compared for the incidence of AEs using the Fisher exact test. Tolerability of ibuprofen arginate was based on a comparison of the incidence of spontaneously reported AEs in each of the treatment groups. Relationships of AEs to study drugs were assessed only in severe AEs.
Statistical analysis
The last score before remedication was used for all time points after remedication. With the exception of times to onset of analgesia and remedication, comparisons of active groups to placebo were done using analysis of variance, with factors for patient, period, and treatment. Comparisons among active groups were performed separately using the previously mentioned model. The placebo group was not included in this analysis. Times to onset of analgesia and remedication were analyzed using the previously mentioned analysis after a rank transformation. Proportions of patients with onset of analgesia who remedicated were analyzed overall using a chi-square test. Paired comparisons were performed using a modification of the McNemar test to allow all patients to be included. This was done using the Mantel-Haenszel chi-square test stratified by patient, but including patients with only 1 of 2 treatments in a separate stratum. The significance level used was set at ≤0.05. Analyses were performed using SAS version 6.12 (SAS Institute Inc., Cary, North Carolina).
Results
A total of 104 patients participated in this study. Eighty-five patients (81.7%) were white; the mean (SD) age was 27.5 (5.0) years. Sixty-eight patients (65.4%) reported moderate pain from dysmenorrhea, and the remaining 36 patients (34.6%) reported severe pain.
Twenty-one patients (20.2%) did not complete the study. Nine of these patients (42.9%) were noncompliant (skipped ≥2 consecutive cycles), 9 (42.9%) violated the study protocol, 2 (9.5%) were lost to follow-up (did not return to the study site in compliance with the protocol after having completed 2 cycles and 1 cycle, respectively), and 1 (4.8%) discontinued for administrative reasons. Of the 9 protocol violations, 4 (44.4%) were due to previously participating in another study using ibuprofen arginate, 3 (33.3%) became pregnant between cycles, 1 (11.1%) was an employee of an investigator, and 1 (11.1%) took an excluded medication for premenstrual symptoms.
Eighty-three of the patients (79.8%) completed all 5 cycles. Ninety-nine patients (95.2%) had ≥1 cycle included in the efficacy analysis, and all 104 patients (100.0%) had ≥1 cycle included in the tolerability analysis (Table I). Overall, the patients who received the study drug completed a total of 431 cycles that were included in the efficacy analysis and 448 cycles that were included in the tolerability analysis. The numbers of cycles valid for efficacy and tolerability analyses are shown in Table II.
Table I.
Number (%) of patients valid for efficacy and tolerability analyses (N = 104).
| Cycle |
||||||
|---|---|---|---|---|---|---|
| Analysis | 1 | 2 | 3 | 4 | 5 | Overall |
| Efficacy | 95 (91.3) | 89 (85.6) | 82 (78.8) | 83 (79.8) | 82 (78.8) | 99 (95.2) |
| Tolerability | 104 (100.0) | 92 (88.5) | 85 (81.7) | 84 (80.8) | 83 (79.8) | 104 (100.0) |
Table II.
Number (%) of cycles valid for efficacy and tolerability analyses.
| Ibuprofen Arginate |
Conventional Ibuprofen |
|||||
|---|---|---|---|---|---|---|
| Analysis | 200 mg | 400 mg | 200 mg | 400 mg | Placebo | Total No.of Cycles |
| Efficacy∗ | 84 (19.5) | 87 (20.2) | 87 (20.2) | 87 (20.2) | 86 (20.0) | 431 |
| Tolerability | 88 (19.6) | 90 (20.1) | 90 (20.1) | 91 (20.3) | 89 (19.9) | 448 |
Percentages do not total 100% due to rounding.
Efficacy analysis
Time to onset of meaningful pain relief was significantly faster with ibuprofen arginate 400 mg than with either dose of conventional ibuprofen. With conventional ibuprofen 200 or 400 mg, meaningful relief was achieved at 90 minutes and 86 minutes, respectively, compared with a time to meaningful relief of 56 minutes with ibuprofen arginate 400 mg (P<lt;0.05). Time to onset of pain relief with ibuprofen arginate 400 mg was significantly faster than with conventional ibuprofen 200 mg for all measures of onset (time to any, a little, some, and meaningful relief) (all P<lt;0.05; Figure). The cumulative number of cycles in which patients remedicated is summarized in Table III. Cycles invalid for efficacy analysis are shown in Table IV.
Figure.
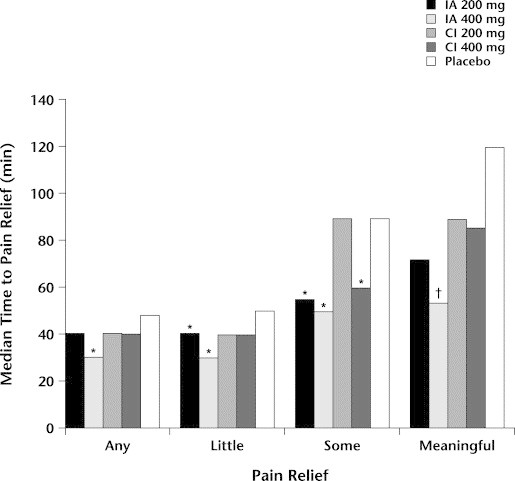
Median time to onset of pain relief. ∗P<lt;0.05 versus conventional ibuprofen 200 mg. †P<lt;0.05 versus conventional ibuprofen 200 and 400 mg. IA = ibuprofen arginate; CI = conventional ibuprofen.
Table III.
Cumulative number (%) of cycles in which patients remedicated.
| Ibuprofen Arginate |
Conventional Ibuprofen |
||||
|---|---|---|---|---|---|
| Time | 200 mg (84 Cycles) | 400 mg (87 Cycles) | 200 mg (87 Cycles) | 400 mg (87 Cycles) | Placebo (86 Cycles) |
| 90 min | 6 (7.1)∗ | 5 (5.7)∗ | 5 (5.7)∗ | 4 (4.6)∗ | 14 (16.3) |
| 2 h | 9 (10.7)∗ | 6 (6.9)∗ | 12 (13.8) | 8 (9.2)∗ | 20 (23.3) |
| 3 h | 13 (15.5)∗ | 9 (10.3)∗ | 16 (18.4) | 11 (12.6)∗ | 25 (29.1) |
| 4 h | 15 (17.9)∗ | 13 (14.9)∗ | 20 (23.0) | 15 (17.2)∗ | 30 (34.9) |
| 5 h | 17 (20.2)∗ | 16 (18.4)∗ | 21 (24.1)∗ | 15 (17.2)∗ | 32 (37.2) |
| 6 h | 24 (28.6)∗ | 19 (21.8)∗ | 24 (27.6)∗ | 15 (17.2)∗ | 33 (38.4) |
P<lt;0.05 versus placebo.
Table IV.
Cycles invalid for efficacy analysis.
| Treatment/Patient No. | Cycle | Reason |
|---|---|---|
| Ibuprofen arginate 200 mg | ||
| 7 | 2 | Patient ate within 1 hour of dosing |
| 36 | 1 | Patient was in Zambon dental pain study |
| 49 | 4 | Patient ate within 1 hour of dosing |
| 63 | 1 | Patient took only 2 tablets |
| Ibuprofen arginate 400 mg | ||
| 59 | 1 | Patient took only 2 tablets |
| 68 | 3 | Patient took a prohibited drug 31 minutes postdose |
| 100 | 1 | Patient took only 2 tablets |
| Conventional ibuprofen 200 mg | ||
| 24 | 2 | Patient was in Zambon dental pain study |
| 25 | 1 | Patient ate within 1 hour of dosing |
| 44 | 1 | Patient was an employee of an investigator |
| Conventional ibuprofen 400 mg | ||
| 14 | 2 | Patient ate within 1 hour of dosing |
| 15 | 1 | Patient was in Zambon dental pain study |
| 19 | 3 | Patient ate within 1 hour of dosing |
| 42 | 5 | Patient ate within 1 hour of dosing |
| Placebo | ||
| 13 | 1 | Patient was in Zambon dental pain study |
| 24 | 1 | Patient was in Zambon dental pain study |
| 83 | 3 | Patient ate within 1 hour of dosing |
Time to remedication was significantly different between ibuprofen arginate and placebo at all time points (all P<lt;0.05). Significant differences versus placebo were found only at 90-minute and at 5- and 6-hour assessments for conventional ibuprofen 200 mg (all P<lt;0.05). No significant differences were seen between the active drugs, confirming that faster onset of action does not necessarily shorten the overall duration of the analgesic effect. The majority of patients in all active treatment groups did not remedicate. This indicates that once onset of analgesia is achieved, the durations of the action of study medications were similar. However, there was a clinical advantage to reaching onset of analgesia as quickly as possible. Statistically significant differences were noted in TOTPAR between ibuprofen arginate 200 mg, ibuprofen arginate 400 mg, and conventional ibuprofen 400 mg compared with conventional ibuprofen 200 mg (all P<lt;0.05).
Ibuprofen arginate 400 mg TOTPAR values were significantly higher than ibuprofen arginate 200 mg and conventional ibuprofen 200 mg (P<lt;0.05). The values for ibuprofen arginate 200 mg were similar to those of conventional ibuprofen 400 mg.
Statistically significant differences were noted for ibuprofen arginate 200 mg, ibuprofen arginate 400 mg, and conventional ibuprofen 400 mg versus placebo for all efficacy measures (all P<lt;0.05). Statistically significant differences between conventional ibuprofen 200 mg and placebo were detected only for SPID and peak pain relief (both P<lt;0.05).
Tolerability analysis
No statistically significant differences were found between the active treatments and placebo in the incidence of overall or individual AEs. The most common AEs were headache, nausea, and dizziness (Table V). No patient discontinued the study due to an AE. None of the AEs were considered treatment related.
Table V.
No. (%) of patients experiencing adverse events in each cycle in ≥3% of patients in any treatment group.
| Ibuprofen Arginate |
Conventional Ibuprofen |
||||
|---|---|---|---|---|---|
| Adverse Event | 200 mg (88 Cycles) | 400 mg (90 Cycles) | 200 mg (90 Cycles) | 400 mg (91 Cycles) | Placebo (89 Cycles) |
| Headache | 5 (5.7) | 3 (3.3) | 2 (2.2) | 0 (0.0) | 4 (4.5) |
| Nausea | 1 (1.1) | 2 (2.2) | 3 (3.3) | 2 (2.2) | 3 (3.4) |
| Dizziness | 0 (0.0) | 2 (2.2) | 3 (3.3) | 0 (0.0) | 1 (1.1) |
Discussion
Ibuprofen arginate was developed to retain the well-known, validated efficacy and tolerability of ibuprofen as an effective analgesic, anti-inflammatory, and antipyretic drug with a shorter absorption time of ibuprofen allowing faster pain relief in patients with acute painful conditions.15–17 This clinical trial supports the hypothesis that ibuprofen arginate is as efficacious as conventional ibuprofen, but with a faster onset of action. In this study, ibuprofen arginate was shown to have significantly faster pain relief than conventional ibuprofen. Furthermore, patients experienced meaningful pain relief, which represents a greater level of pain relief, earlier with ibuprofen arginate than with conventional ibuprofen (30–34 minutes earlier).
AE profiles were comparable in the 5 treatment groups. The tolerability profile of racemic ibuprofen is well established and extensively documented in the literature.18–22 Maximum consumption of 6 tablets/d of 200 mg ibuprofen arginate (corresponding to the maximum recommended OTC daily dosage) would result in total daily dosages of L-arginine equivalent to 16 mg/kg. This corresponds to a daily intake of 1100 mg L-arginine, which is considered to be unlikely to present any significant risk for serious AEs, considering that the recommended dietary allowance of arginine is 4.7 to 5.4 g. L-Arginine is commercially available as a dietary supplement and has been administered in 400- to 500-mg doses IV with relatively little toxicity.23,24
The present study demonstrates that ibuprofen arginate (200 and 400 mg) provides analgesia that is tolerable and as effective as that of conventional ibuprofen, but with a faster onset of action for the treatment of primary dysmenorrhea.
The onset of analgesia, in terms of meaningful pain relief, was ∼30 minutes faster for ibuprofen arginate 400 mg than for conventional ibuprofen 400 mg. The duration of analgesia in terms of the percentage of ibuprofen arginate–treated patients remedicating by 6 hours was not different from ibuprofen and was significantly less than placebo.
Conclusions
In this study population of patients experiencing acute pain as a result of primary dysmenorrhea, median time to pain relief was significantly faster for ibuprofen arginate 400 mg than ibuprofen arginate 200 mg or either dose of conventional ibuprofen. This difference may have a role in clinical practice in treating women with dysmenorrhea. A faster onset of action may be important to women whose personal relationships, productivity, or ability to sleep is being adversely affected by pain.
Footnotes
Reproduction in whole or part is not permitted.
Trademark: Espidifenh, Faspich, Spedifenh (Zambon Group S.p.A., Milan, Italy).
Trademark: Motrin® (McNeil Consumer & Specialty Pharmaceuticals, a Division of McNeil-PPC, Inc., Fort Washington, Pennsylvania).
References
- 1.Coco A.S. Primary dysmenorrhea. Am Fam Physician. 1999;60:489–496. [PubMed] [Google Scholar]
- 2.Deligeoroglou E. Dysmenorrhea. Ann N Y Acad Sci. 2000;900:237–244. doi: 10.1111/j.1749-6632.2000.tb06235.x. [DOI] [PubMed] [Google Scholar]
- 3.Menstrual cramps (dysmenorrhea). Available at: http://www.mckinley.uiuc.edu/health-info/womenhlt/mencramp.html. Accessed June 4, 2001.
- 4.Baker F.C, Driver H.S, Rogers G.G. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol. 1999;277(6 Pt 1):E1013–E1021. doi: 10.1152/ajpendo.1999.277.6.E1013. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro S.S, Diem K. The effect of ibuprofen in the treatment of dysmenorrhea. Curr Ther Res Clin Exp. 1981;30:327–334. [Google Scholar]
- 6.Mehlisch D.R. Ketoprofen, ibuprofen, and placebo in the treatment of primary dysmenorrhea: A double-blind crossover comparison. J Clin Pharmacol. 1988;28(Suppl 12):S29–S33. doi: 10.1002/j.1552-4604.1988.tb05974.x. [DOI] [PubMed] [Google Scholar]
- 7.Mehlisch D.R. Double-blind crossover comparison of ketoprofen, naproxen, and placebo in patients with primary dysmenorrhea. Clin Ther. 1990;12:398–409. [PubMed] [Google Scholar]
- 8.Mehlisch D.R, Fulmer R.I. A crossover comparison of bromfenac sodium, naproxen sodium, and placebo for relief of pain from primary dysmenorrhea. J Womens Health. 1997;6:83–92. doi: 10.1089/jwh.1997.6.83. [DOI] [PubMed] [Google Scholar]
- 9.Cash H.C, Humpston D, Kasap H.S. Feldene in the symptomatic treatment of primary dysmenorrhea. Practitioner. 1982;226:1338–1341. [PubMed] [Google Scholar]
- 10.Gookin K.S, Forman E.S, Vecchio T.J. Comparative efficacy of ibuprofen, indomethacin, and placebo in the treatment of primary dysmenorrhea. South Med J. 1983;76:1361–1362. doi: 10.1097/00007611-198311000-00008. 1367. [DOI] [PubMed] [Google Scholar]
- 11.Weaver M, Mehlisch DR, Brown P, Bauman A. Onset and duration of analgesia comparing ketoprofen 12.5 mg and 25 mg, ibuprofen 200 mg and placebo in the treatment of primary dysmenorrhea. Paper presented at: Annual Meeting of the American Society of Clinical Pharmacology and Therapeutics; March 6–9, 1997; San Diego, Calif.
- 12.Fini A, Zecchi V, Tartarani A. Dissolution profiles of NSAIDs, carboxylic acids and their salts with different counter ions. Pharm Acta Helv. 1985;60:58–62. [Google Scholar]
- 13.Moote C. Ibuprofen in the management of pain. Clin Invest Drugs. 1996;11(Suppl 1):1–7. [Google Scholar]
- 14.Ratkowsky D.A, Evans M.A, Alldredge J.R, editors. Cross-Over Experiments: Design, Analysis, and Application. Marcel Dekker; New York: 1993. pp. 121–184. [Google Scholar]
- 15.Black P, Max M.B, Desjardins P. A randomized, double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin Ther. 2002;24:1072–1089. doi: 10.1016/s0149-2918(02)80020-0. [DOI] [PubMed] [Google Scholar]
- 16.Mehlisch D.R, Ardia A, Pallotta T. A controlled comparative study of ibuprofen arginate versus conventional ibuprofen in the treatment of postoperative dental pain. J Clin Pharmacol. 2002;42:904–911. doi: 10.1177/009127002401102821. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins P, Black P, Papageorge M. Ibuprofen arginate provides effective relief from postoperative dental pain with a more rapid onset of action than ibuprofen. Eur J Clin Pharmacol. 2002;58:387–394. doi: 10.1007/s00228-002-0491-0. [DOI] [PubMed] [Google Scholar]
- 18.Adams S.S, Bough R.G, Cliffe E.E. Some aspects of pharmacology, metabolism, and toxicology of ibuprofen: I. Pharmacology and metabolism. Rheumatol Phys Med. 1970;10(Suppl 10):9–26. doi: 10.1093/rheumatology/x.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 19.Adams S.S, Bough R.G, Cliffe E.E. Absorption, distribution and toxicity of ibuprofen. Toxicol Appl Pharmacol. 1969;15:310–330. doi: 10.1016/0041-008x(69)90032-5. [DOI] [PubMed] [Google Scholar]
- 20.Busson M. Update on ibuprofen: Review article. J Int Med Res. 1986;14:53–62. doi: 10.1177/030006058601400201. [DOI] [PubMed] [Google Scholar]
- 21.Brune K. The pharmacological profile of non-opioid (OTC) analgesics: Aspirin, paracetamol (acetaminophen), ibuprofen, and phenazones. Agents Actions Suppl. 1988;25:9–19. [PubMed] [Google Scholar]
- 22.O'Reilly D.S, Fraser W.D, Penney M.D. Arginine infusion blocks the action of parathyroid hormone but not arginine vasopressin on the renal tubule in man. J Endocrinol. 1986;111:501–506. doi: 10.1677/joe.0.1110501. [DOI] [PubMed] [Google Scholar]
- 23.Block R.J, Bolling D, editors. The Essential Amino Acid Requirements of Man. Charles C. Thomas; Springfield, Ill: 1947. The amino acid composition of proteins and foods, analytical methods and results; pp. 307–309. [Google Scholar]
- 24.Visek W.J. Arginine needs, physiological state and usual diets. A reevaluation. J Nutr. 1986;116:36–46. doi: 10.1093/jn/116.1.36. [DOI] [PubMed] [Google Scholar]


