Abstract
Background: In infants and children, treatment of Kawasaki disease (KD) with high-dose intravenous immunoglobulin (IVIG) and acetylsalicylic acid ([ASA] aspirin) diminishes inflammatory response and reduces the risk for coronary artery abnormalities. However, patients with high serum concentrations of tumor necrosis factor (TNF)-alpha, which is associated with vascular damage, may develop coronary artery lesions even with treatment. The hemorheologic agent pentoxifylline blocks the production of TNF-alpha and may be an appropriate adjunctive therapy to IVIG and ASA.
Objective: The objective of this study was to assess the pharmacokinetic characteristics and tolerability of a new oral syrup formulation of pentoxifylline as an adjunct to IVIG and ASA in the treatment of KD in children.
Methods: Hospitalized boys and girls aged 6 months to 5 years and who were diagnosed with KD within the first 10 days of illness were eligible. Patients were assigned to 1 of 4 pentoxifylline treatment groups, by dose level (dose levels 1, 2, 3, and 4: 10, 15, 20, and 25 mg/kg daily, respectively, divided into 3 doses). Six plasma samples collected at the time the first dose was administered, and 4 samples collected after administration of the last dose on study day 6, were assessed by high-performance liquid chromatography using noncompartmental and 1-compartment pharmacokinetic analyses for pentoxifylline and its active metabolite (M-1). TNF-alpha levels on days 1 and 6 were assessed using electroimmunoassay.
Results: Fourteen boys and 10 girls were enrolled. The mean age, body weight, and illness day at study entry were 34.5 months, 13.8 kg, and 6, respectively. Pentoxifylline exhibited nonlinear kinetic characteristics, with median area under the plasma concentration–time curve from time 0 to infinity(AUC0–∞) values of 622, 3428, 8416, and 10,347 ng/mL · h for dose levels 1 to 4, respectively, on study day 1. Pentoxifylline noncompartmental oral clearance and volume of distribution were significantly lower, and dose-normalized AUC0–∞ was significantly higher, for dose level 3 than dose level 1. M-1 parameters were not significantly different between dose levels. No accumulation of pentoxifylline or M-1 was noted. Fifteen of 24 patients (63%) reported mild to moderate adverse events that may or may not have been treatment related. Frequency and severity did not differ significantly between dose levels.
Conclusions: In the children in this study, pentoxifylline was well tolerated at the doses studied. No notable differences in clinical outcomes were observed between dose levels, and dose levels 3 and 4 (20 and 25 mg/kg daily, respectively) resulted in similar exposure to both pentoxifylline and M-1. Future efficacy and tolerability studies should use a daily dose of 20 mg/kg of pentoxifylline in acute KD.
Keywords: pentoxifylline, pharmacokinetics, pediatrics, Kawasaki disease, TNF-alpha
INTRODUCTION
Kawasaki disease (KD) is an acute, multisystem vasculitis of unknown cause that mainly affects infants and young children. It has replaced acute rheumatic fever as the most common cause of acquired heart disease in childhood in the United States.1 Clinical symptoms of KD include fever, rash, mucosal inflammation, lymphadenopathy, and extremity changes, including erythema, swelling, and desquamation. If untreated, the vasculitis causes damage to coronary arteries in 20% to 25% of patients, which can lead to coronary artery aneurysm, myocardial infarction, coronary artery stenosis and obstruction, valvular disease, and sudden death.2 Treatment with high-dose intravenous immunoglobulin (IVIG) and acetylsalicylic acid ([ASA] aspirin) diminishes the inflammatory response and reduces the prevalence of coronary artery abnormalities.3–5 Approximately 15% of patients do not respond to the first dose of IVIG and require additional therapy. A subset of these patients (10%) develop coronary artery lesions.6 Overall, 2% to 6% of patients who receive appropriate treatment still develop coronary artery abnormalities.7
Tumor necrosis factor (TNF)-alpha is an inducible cytokine that is markedly elevated during the acute phase of KD and may be involved in the pathogenesis of vascular damage.8–10 Patients with higher concentrations of TNF-alpha are more likely to develop coronary artery lesions, even when treated with IVIG. Therefore, modulation of TNF-alpha and its effects may benefit patients with acute KD.9,10 The hemorheologic agent pentoxifylline blocks the production of TNF-alpha in monocytes and macrophages, thereby lowering serum TNF-alpha concentrations.11
Pentoxifylline is a synthetic dimethylxanthine derivative prescribed for complications of peripheral vascular diseases. The pharmacokinetic characteristics of pentoxifylline in adults have been well characterized.12–14 Although pentoxifylline is almost completely absorbed, it undergoes significant first-pass metabolism resulting in a bioavailability of 20% to 33%. Pentoxifylline is extensively metabolized and forms an active 1-(5′-hydroxyhexyl)-3,7-dimethylxanthine metabolite (M-1) through aldo-keto-reductase. Pentoxifylline and M-1 have similar potency, have short half-lives of 0.4 to 2.0 hours, and exhibit nonlinearity with respect to serum area under the plasma concentration–time curve (AUC) and half-life. Currently, no evidence suggests significant protein binding of pentoxifylline or M-1. Pentoxifylline is not known to induce its own metabolism after multiple oral doses of the drug. Other recognized metabolites have lower plasma concentrations and less or no activity.
Pentoxifylline, given as the crushed adult tablet, has been assessed as adjunctive therapy to IVIG and ASA in Japanese children with acute KD. In 1 study,15 assessment of patients treated with adjunct pentoxifylline therapy showed a trend toward fewer coronary artery lesions, but no information on pharmacokinetic characteristics was reported.
The potential for altered pharmacokinetic characteristics in patients with KD complicates the choice of an appropriate pentoxifylline dose. Both altered protein binding and decreased bioavailability have been associated with the low ASA levels documented in patients during the acute phase of KD.16,17 In addition, preliminary studies15 of pentoxifylline in KD suggested a dose-related effect on the prevention of coronary artery abnormalities. To make a rational dose selection when assessing the potential benefits of pentoxifylline, an accurate description of pentoxifylline pharmacokinetics in KD is needed.
The objective of this study was to assess the pharmacokinetic characteristics and tolerability of a new oral syrup formulation of pentoxifylline as adjunct therapy to IVIG and ASA in the treatment of KD in children.
PATIENTS AND METHODS
Study Design
This was a prospective, unblinded, multicenter, Phase I to II, dose-escalating study. Participants were enrolled from January 1998 to July 2001 at 5 pediatric pharmacology research unit (PPRU) study sites, as follows: University of California at San Diego/Children's Hospital and Health Center ([UCSD/CHHC] San Diego, California), University of Tennessee Health Sciences Center ([UTHSC] Memphis, Tennessee), Rainbow Babies and Children's Hospital ([RBCH] Cleveland, Ohio), Children's Hospital Research Foundation of Columbus ([CHRFC] Columbus, Ohio), and Children's Hospital of Michigan–Wayne State University ([CHM-WSU] Detroit, Michigan). The institutional review boards at each of the study sites approved the protocol, and written informed consent was obtained from the parent(s), or legal guardians as appropriate, for every participant before study enrollment.
Participants were enrolled consecutively, 6 patients per dose level (levels 1, 2, 3, and 4: 10, 15, 20, and 25 mg/kg daily, respectively, divided into 3 doses). All participants received a single dose of IVIG (2 g/kg) and daily doses of ASA (80 to 100 mg/kg), per the current standard of care in the United States.18 Participants also received pentoxifylline oral solution 3 times daily with meals or feedings (infants) for 6±2 days. The first dose of pentoxifylline was administered by study personnel 6 to 24 hours after the start of the IVIG infusion. Clinical data collected included demographic data (age, sex, ethnicity, body weight), vital signs (measured daily until discharge), and laboratory tests performed before IVIG administration (complete blood cell count with differential, platelet count, erythrocyte sedimentation rate, chemistry panel including gamma-glutamyltransferase [GGT] and alanine aminotransferase [ALT], quantitative C-reactive protein, albumin, TNF-alpha, and urinalysis). Echocardiography was performed before IVIG administration and again at study day 6±2 (hereafter referred to as study day 6) or according to the standard of care at each study site. Coronary artery dilatation was defined as a Z score of >2 according to the criteria of de Zorzi et al.19
The pentoxifylline solution contained 20 mg/mL of pentoxifylline in alcohol-free syrup.
Patient Population
Hospitalized boys and girls aged 6 months to 5 years and who were diagnosed with KD within the first 10 days of illness were eligible. Patients were required to have fever plus 4 of the 5 standard diagnostic criteria18 and to have IV access for obtaining blood samples for the pharmacokinetic studies. Patients were excluded if they had a known allergy or hypersensitivity to methylxanthines.
Tolerability Assessment
As each dose level was completed, participants were assessed for significant dose-limiting toxicity before proceeding to the next-higher dose level. Study nurses collected data on adverse events (AEs) at least once daily during hospitalization using a standardized collection form. Parents were given a digital thermometer for measuring axillary temperature and instructed on its use before their child's discharge from the hospital. Study personnel contacted parents daily until the first follow-up visit at study day 6 and asked them to record on their home logs fever, nausea, vomiting, abdominal complaints, and any other AEs.
Sample Collection
Plasma samples of 1.5 mL were collected before dosing and at 0.25, 0.5, 1, 2, 3, and 5 hours after the first dose of pentoxifylline on study day 1. On study day 6, additional samples were collected at 0.5, 1, 2, and 3 hours after dosing. TNF-alpha levels were measured before pentoxifylline administration on study days 1 and 6. Patients who did not complete the pharmacokinetic assessment on study day 1 due to problems with IV access were replaced.
Analytical Procedures
Pentoxifylline and M-1 were measured in the Louisiana State University Medical Center's PPRU laboratory (Shreveport, Louisiana). Plasma samples containing pentoxifylline and M-1 were prepared for analysis by adding an acetonitrile-containing internal standard (phenacetin). Samples were mixed to precipitate proteins, centrifuged at 1200 rcf for 10 minutes, and the supernatant was dried under nitrogen. After reconstitution with mobile phase, a 20 μL aliquot was injected onto the high-performance liquid chromatography system for analysis. Separation was achieved using a 100- and 200-mm eta-octadecylsilane column in tandem and a mobile phase consisting of 24% acetonitrile in 1% acetic acid and 0.3% triethylamine. The detection wavelength was 272 nm using a diode array detector. Calibration curves using peak area ratios of drug to internal standard for pentoxifylline and M-1 were linear from 63 to 6000 ng/mL, with a correlation of 0.997 for pentoxifylline and 0.997 for M-1. The lower limit of quantization was 63 ng/mL, with mean back-calculated values for M-1 and pentoxifylline of 68.7 (2.8) ng/mL and 70.0 (3.9) ng/mL, respectively; with biases of −9.1% and −11.2%, respectively; and coefficient of variations (CV%s) of 4.1 and 5.5, respectively. The high point on the standard curve was 6000 ng/mL for M-1 and pentoxifylline, with a mean back-calculated value of 6015.4 (162.4) ng/mL and 5961.8 (208.7) ng/mL, respectively; with biases of −0.26% and 0.64%, respectively; and CV%s of 2.7 and 3.5, respectively.
TNF-alpha samples were frozen at −70°C, batched, and assessed using a commercially available enzyme-linked immunosorbent assay kit (Quantikine® HS TNF-alpha Immunoassay, R&D Systems, Inc., Minneapolis, Minnesota). The lower and upper limits of quantization were 0.5 and 32.0 pg/mL, respectively.
Pharmacokinetic and Pharmacodynamic Analyses
The maximum plasma concentration (Cmax) and the corresponding time (Tmax) were determined using observation of the plasma concentration–time profile for each patient. The AUC0–last for pentoxifylline and M-1 were estimated using the trapezoidal rule up to the last measurable concentration. The extrapolated area after the last concentration was estimated as Clast/λz, where Clast was the last measurable concentration and λz was the terminal slope of the curve. AUC from time 0 to infinity (AUC0–∞) was calculated as AUC0–last + Clast/λz. Noncompartmental oral clearance rate (Cl/FNC) was calculated as the ratio of dose to AUC0–∞. Apparent volume of distribution (VdNC/F) was calculated as Cl/FNC over λz. Half-life (t1/2) was calculated as 0.693/λz. Plasma pentoxifylline concentrations collected in the subacute phase (study day 6) were compared with those collected during the acute phase (study day 1). AUC0–∞, Cl/F1-C (oral clearance from 1-compartment model), and Vd1-C/F (volume of distribution from 1-compartment model) also were determined using a 1-compartment model with first-order absorption. This second modeling approach was used because the data were sparse, limiting our ability to estimate parameters for some patients. Obtaining similar parameters with each approach indicates that the limitations of each methodology did not affect the results. WinNonlin, version 2.0 (Pharsight, Mountain View, California) was used for the pharmacokinetic analyses.
Statistical Analysis
Dose proportionality of pentoxifylline and M-1 noncompartmental pharmacokinetic parameters was assessed by testing VdNC/F, Cl/FNC, Tmax, t1/2, and dose-normalized Cmax and AUC0–∞ using the Kruskal-Wallis 1-way analysis of variance on ranks. AUC0–∞ and Cmax were normalized to the daily dose level of 10 mg/kg by dividing the values in dose levels 2, 3, and 4 by 1.5, 2.0, and 2.5, respectively. When a statistically significant difference was detected, the Kruskal-Wallis multiple-comparison Z-value test with Bonferroni criteria was used for pairwise comparisons of medians. The differences in concentrations of pentoxifylline, M-1, and TNF-alpha between study day 1 and study day 6 were assessed with the Wilcoxon signed rank test for paired data. The Spearman rank correlation coefficient was used to examine the relationships between (1) age and oral clearance and (2) active drug exposure and percentage change in TNF-alpha from study days 1 to 6. The statistical tests were performed using the Number Cruncher Statistical System (NCSS) 2001/PASS 2000 software (NCSS Statistical Software, Kaysville, Utah). Statistical significance was set at P<0.05.
RESULTS
Patients' Demographic Characteristics
Twenty-four children were enrolled at 5 PPRU study sites, as follows: UCSD/CHHC, n = 12; UTHSC, n = 5; RBCH, n = 5; CHRFC, n = 1; and CHM-WSU, n = 1.
The participants in each dose-level group (n = 6 patients per group) had similar demographic characteristics. The mean (SD) age, body weight, and illness day at study entry were 34.5 (18.2) months, 13.8 (3.7) kg, and 6 (1), respectively. Fourteen of 24 patients were boys. Eight of the patients (33%) were white, 7 (29%) were black, 3 (13%) were Hispanic, 1 (4%) was Asian, and 5 (21%) were >1 race.
Pharmacokinetic Characteristics
All 24 patients (100%) completed the pharmacokinetic assessment on study day 1, and 10 participants (42%) underwent the additional pharmacokinetic assessment on study day 6. Of the 14 participants who did not complete the sampling on study day 6, 2 (14%) were lost to follow-up, and 12 (86%) either had no IV access or their parents declined the second assessment. One participant in the group receiving 15 mg/kg inadvertently was given 15 mg/kg per dose rather than 15 mg/kg per day, for a total dose of 45 mg/kg daily, and was excluded from the pharmacokinetic analyses.
Single-dose noncompartmentalized and 1-compartment pharmacokinetic parameters for pentoxifylline and M-1 from study day 1 are listed in Table I. For the pentoxifylline 1-compartment model, the terminal slope of the concentration–time curve could not be determined due to an insufficient number of measurable concentrations in 2 patients and the flatness of the curve in 2 additional patients. For M-1, the terminal slope could not be determined due to the flatness of the curve in 2 patients for the 1-compartment analysis and an insufficient number of measurable concentrations in 1 patient for both the noncompartmental and 1-compartment analyses. The median AUC0–∞ values were 622, 3428, 8416, and 10,347 ng/mL·h for dose levels 1 to 4, respectively, on study day 1. The median pentoxifylline and M-1 plasma concentration–time profiles are shown in Figure 1. The pentoxifylline exposure for dose levels 3 and 4 was greater than for dose levels 1 and 2, and the M-1 exposure was greatest for dose level 3.
Table I.
Single-dose noncompartmentalized and 1-compartment pharmacokinetic parameters for pentoxifylline (PTX) and 1-(5′-hydroxyhexyl)-3,7-dimethylxanthine (M-1) from study day 1.
| Noncompartmental Analysis |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dose Level | Cmax, ng/mL | Tmax, h | Cl/FNC, L/kg·h | VdNC/F, L/kg | t1/2, h | AUC0–∞, ng/mL·h | PTX + M-1 AUC, μM×h | M-1/PTX AUC Ratio |
| 1 PTX (n = 6) | ||||||||
| Median | 315 | 0.4 | 6.1 | 6.2 | 0.7 | 622 | 6.4 | 1.7 |
| Range | 83–2582 | 0.3–2.0 | 1.8–13.6 | 1.5–16.9 | 0.5–1.2 | 252–1819 | 1.9–10.5 | 0.6–2.1 |
| M-1 (n = 6) | ||||||||
| Median | 632 | 0.3 | – | – | 0.8 | 914 | – | – |
| Range | 192–1190 | 0.3–0.5 | – | – | 0.6–1.4 | 279–1629 | – | – |
| 2 PTX (n = 5) | ||||||||
| Median | 797 | 0.5 | 1.5 | 4.9 | 1.0 | 3428 | 21.2 | 1.7 |
| Range | 342–2924 | 0.3–0.5 | 0.9–23.5 | 1.5–7.3 | 0.2–2.3 | 213–5426 | 4.3–62.6 | 0.7–4.6 |
| M-1 (n = 5) | ||||||||
| Median | 833 | 1.0 | – | – | 1.3 | 2477 | – | – |
| Range | 435–2715 | 0.5–3.0 | – | – | 0.4–2.6 | 785–12,055 | – | – |
| 3 PTX (n = 6) | ||||||||
| Median | 3016 | 0.8 | 0.9 | 1.0 | 0.9 | 8416 | 58.8 | 1.0 |
| Range | 1025–5230 | 0.3–1.0 | 0.5–3.8 | 0.6–3.4 | 0.2–2.3 | ,1771–13,653 | 20.0–96.4 | 0.4–2.2 |
| M-1 (n = 6) | ||||||||
| Median | 2607 | 1.0 | – | – | 1.3 | 8000 | – | – |
| Range | 803–3391 | 0.5–2.0 | – | – | 0.5–3.1 | 1696–13,235 | – | – |
| 4 PTX (n = 6) | ||||||||
| Median | 3235 | 0.5 | 0.9 | 2.3 | 1.3 | 10,347 | 49.4 | 0.4 |
| Range | 2007–8270 | 0.3–1.0 | 0.5–4.0 | 1.0–6.7 | 0.6–3.5 | ,2080–15,922 | 10.1–83.5 | 0.04–0.8 |
| M-1 (n = 5) | ||||||||
| Median | 1424 | 1.0 | – | – | 1.3 | 6037 | – | – |
| Range | 341–2169 | 0.5–2.0 | – | – | 0.7–3.4 | 727–8193 | – | – |
| 1-Compartment Model Analysis |
|||
|---|---|---|---|
| Cl/F1-C, L/kg·h | Vd1-C/F, L/kg | AUC0–∞, ng/mL·h | |
| 1 PTX (n = 5) | |||
| Median | 4.3 | 4.9 | 749 |
| Range | 4.0–14.2 | 4.3–15.9 | 241–839 |
| M-1 (n = 6) | |||
| Median | – | – | 701 |
| Range | – | – | 209–1637 |
| 2 PTX (n = 4) | |||
| Median | 1.4 | 4.6 | 3701 |
| Range | 1.0–5.6 | 2.0–9.4 | 893–5268 |
| M-1 (n = 4) | |||
| Median | – | – | 4684 |
| Range | – | – | 1258–10,969 |
| 3 PTX (n = 6) | |||
| Median | 0.9 | 1.0 | 7721 |
| Range | 0.5–5.0 | 0.5–2.4 | 1333–13,623 |
| M-1 (n = 6) | |||
| Median | – | – | 5488 |
| Range | – | – | 976–14,238 |
| 4 PTX (n = 4) | |||
| Median | 1.8 | 1.9 | 5263 |
| Range | 0.6–5.5 | 0.9–7.2 | 1510–13,836 |
| M-1 (n = 4) | |||
| Median | – | – | 2879 |
| Range | – | – | 372–8488 |
Cmax = maximum plasma concentration; Tmax = time to Cmax; Cl/FNC = noncompartmental oral clearance; VdNC/F = volume of distribution based on terminal slope (λz); t1/2 = half-life; AUC0–∞ = area under the plasma concentration–time curve from time 0 to infinity; Cl/F1-C = oral clearance from 1-compartment model; Vd1-C/F = volume of distribution from 1-compartment model.
Figure 1.
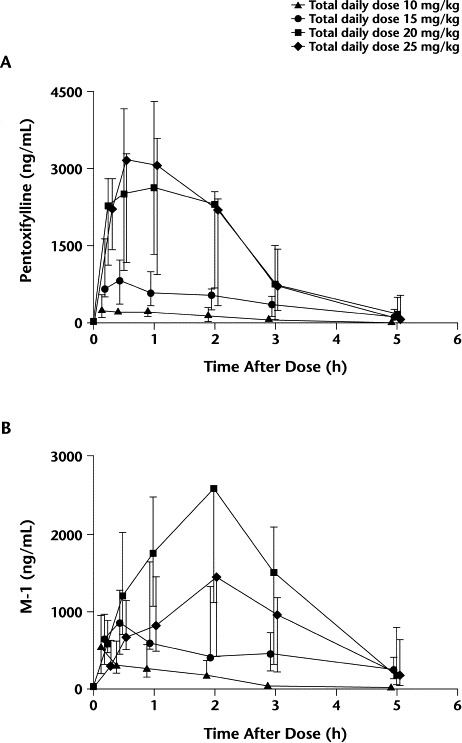
Values are medians with interquartile ranges. (A) Pentoxifylline plasma concentration versus time profiles, and (B) pentoxifylline active metabolite (M-1) plasma concentration versus time profiles.
For pentoxifylline, Tmax and t1/2 were similar across dose levels. The dose-normalized pentoxifylline Cmax showed a numerically higher-than-proportional Cmax in the higher dose levels, but these differences did not reach statistical significance. Significant differences were observed between dose levels for Cl/FNC, VdNC/F, and dose-normalized pentoxifylline AUC0–∞ (P = 0.03, 0.02, and 0.03, respectively). Dose level 3 had significantly higher median AUC0–∞ values and significantly lower Cl/FNC and VdNC/F values than dose level 1 (Figures 2 to 4). In Figure 3, the 1 patient in dose level 2 with a high oral clearance rate of 23.5 L/h had 5 of 7 samples drawn on study day 1, with only the 0.5- and 1.0-hour postdose samples containing measurable pentoxifylline. Consequently, the estimated AUC0–∞ was 16-fold less than the median AUC for that dose level. For M-1, none of the parameters tested were different between groups. Age was explored as a possible determinant of oral clearance rate for pentoxifylline and M-1, with little or no relationship found.
Figure 2.
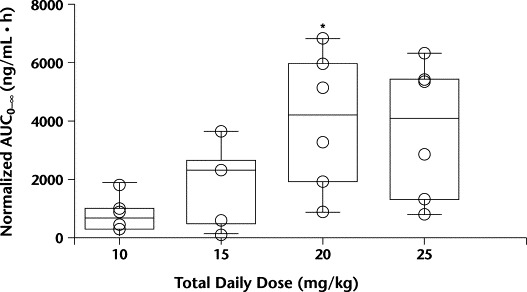
Dose-normalized pentoxifylline area under the plasma concentration–time curve from time 0 to infinity (AUC0–∞) grouped by dose level. ∗P<0.05 versus 10 mg/kg daily.
Figure 3.
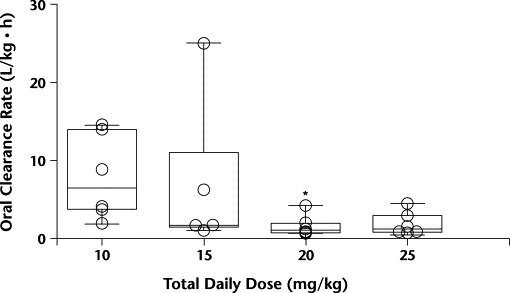
Pentoxifylline noncompartmental oral clearance rate by dose level. ∗P<0.05 versus 10 mg/kg daily.
Figure 4.
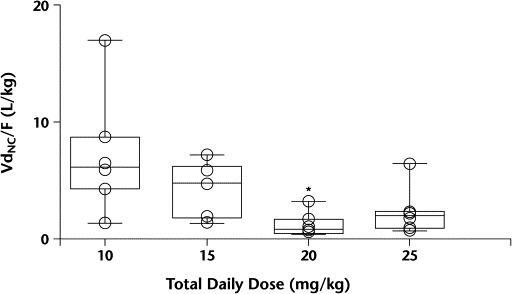
Volume of distribution (VdNC/F) by dose level. ∗P<0.05 versus 10 mg/kg daily.
In the assessment of accumulation with multiple doses, the average ratios of pentoxifylline and M-1 concentrations on study day 6 to study day 1 were 1.18 (95% CI, 0.59–1.77) and 1.06 (95% CI, 0.57–1.55), respectively.
Tolerability Assessment
All patients were included in the tolerability analysis. Parents or legal guardians of 15 of 24 patients (63%) reported mild to moderate AEs that may or may not have been related to the study medication. None of the AEs required discontinuation from the study. No dose-limiting, unusual, or idiosyncratic reactions to pentoxifylline were observed, and the frequency and severity of AEs did not increase with increasing exposure. The most common AEs reported were gastrointestinal (28 episodes in 11 patients), whereas 14 nongastrointestinal events were reported in 8 patients. These AEs included mild cough, inability to walk without help, I/VI systolic flow murmur, anemia, clear rhinorrhea, diaper rash, irritability, and temporary hearing loss. Vomiting was the most common AE (11 episodes in 7 patients), followed by abdominal pain (6 episodes in 3 patients). The reported episodes of vomiting were in part due to patients' spitting out the medication immediately after dosing. The patient who received a total dose of 45 mg/kg daily reported the following AEs: persistent fever (102.3°F) at 48 hours after IVIG administration, mild cough, and occasional vomiting after pentoxifylline administration. A maximum tolerated dose was not identified in this study.
Dose-Response Relationships
For 19 patients (79%) (6, 5, 5, and 3 patients in dose levels 1 to 4, respectively), the findings on echocardiography remained unchanged over the course of the study. Pretreatment and posttreatment echocardiograms were normal for 18 of the study patients (75%) (6, 5, 5, and 2 in dose levels 1 to 4, respectively), and 1 patient (4%) in the dose level 4 group had coronary artery dilatation before and after treatment that resolved 1 month after the end of the study. Four patients (17%) exhibited improvement in their coronary arteries during the study. One patient (4%) in the dose level 3 group and 3 patients (13%) in the dose level 4 group had coronary artery dilatation on admission that normalized over the course of the study. No patients had coronary artery measurements that worsened over the course of the study. Finally, 1 patient (4%) in the dose level 2 group had coronary artery dilatation on the initial echocardiogram, but no follow-up echocardiogram was available.
Body temperatures following pentoxifylline administration were highly variable. Five of 24 patients (21%) received an additional dose(s) of IVIG due to persistent or recrudescent fever at 48 hours after the end of IVIG infusion (1, 3, and 1 patient(s) from dose levels 1, 2, and 3, respectively). The patient in dose level 2 who received 45 mg/kg daily of pentoxifylline was one of the patients requiring additional IVIG. This patient was also the only Asian patient in this study. No patient in the group receiving the highest dose of pentoxifylline required a second IVIG dose.
TNF-alpha levels were assessable in 22 of 24 patients before pentoxifylline administration on study day 1. Follow-up levels were obtained in 12 of 24 patients on study day 6. The TNF-alpha levels did not differ in the 12 paired sera from study days 1 to 6. For the 12 patients with TNF-alpha levels measured on study day 6, the relationship between percentage change in TNF-alpha from study days 1 to 6 and the amount of active drug (pentoxifylline + M-1 AUC0–∞) was examined. An association was observed between active drug exposure and percentage decreases in TNF-alpha (r = −0.53; Figure 5).
Figure 5.
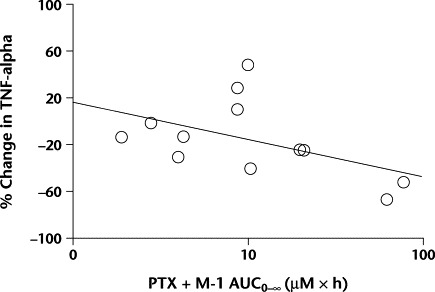
The percentage of change in tumor necrosis factor (TNF)-alpha levels from study days 1 to 6 versus active medication (pentoxifylline [PTX] + its active metabolite [M-1]) area under the plasma concentration–time curve from time 0 to infinity (AUC0–∞). A correlation was found (r = −0.53) but it did not reach statistical significance.
DISCUSSION
The current standard treatment for acute KD in the United States, high-dose IVIG plus high-dose ASA, reduces cardiac sequelae, but 2% to 6% of patients still develop coronary artery abnormalities. Moreover, children without coronary artery abnormalities during the acute phase of KD still may have abnormal test results for ventricular contractility and endothelial cell reactivity decades after the acute illness.20–22 The contribution of KD to cardiovascular disease in adults remains unclear. Additional therapies may be desirable to further reduce the elevated inflammatory cytokines that appear to mediate vascular damage in KD. As a TNF-alpha inhibitor, pentoxifylline is a logical candidate for treatment of this disease.
This study described the pharmacokinetic characteristics of pentoxifylline with escalating doses in children with acute KD. The doses selected for this trial were based on the normal dose of pentoxifylline in adults, 400 mg 3 times daily, or ∼17 mg/kg daily for a 70-kg adult. It is difficult to compare adult and pediatric pentoxifylline pharmacokinetic parameters because the pharmacokinetic characteristics may be dose dependent. Nonetheless, the values observed for the pentoxifylline pharmacokinetic parameters in the children in this study were similar to reported values in adults for the standard 400-mg dose.12–14 Likewise, the range of several M-1 pharmacokinetic-parameter values in this study approximated the reported values in adults (Table II).
Table II.
Pharmacokinetic parameters for pentoxifylline (PTX) and 1-(5′-hydroxyhexyl)-3,7-dimethylxanthine (M-1) in pediatric patients (current study) compared with adults.12–14
| Study | Dose | Tmax, h | Cmax, ng/mL | t1/2, h | AUC0–∞, ng/mL·h | Clearance, L/kg·h | Volume, L/kg |
|---|---|---|---|---|---|---|---|
| PTX | |||||||
| Smith et al,12 mean | 400 mg PO | 0.29 | 1607 | 0.84 | 1229 | – | – |
| Rames et al,13 mean | 100 mg IV | – | – | 0.83 | – | 3.62 | 4.15 |
| Current study, range | 10–25 mg/kg PO daily | 0.3–2.0 | 83–8270 | 0.2–3.5 | 213–15,922 | 0.5–23.5 | 0.6–16.9 |
| M-1 | |||||||
| Smith et al,12 mean | 400 mg PO | 0.83 | 2000 | 0.96 | 5101 | – | – |
| Beermann et al,14 mean | 400 mg PO | 1.80 | 1937 | 0.90 | 5031 | – | – |
| Current study, range | 10–25 mg/kg PO daily | 0.3–3.0 | 192–3391 | 0.4–3.4 | 279–13,235 | – | – |
Tmax = time to Cmax; Cmax = maximum plasma concentration; t1/2 = half-life; AUC0–∞ = area under the plasma concentration–time curve from time 0 to infinity.
In addition to the similar pharmacokinetic-parameter estimates between children and adults, the participants in this study demonstrated nonlinear kinetic characteristics of pentoxifylline, which have been described in the adult population over an oral dosing range of ∼4 to 17 mg/kg daily.12 Our patients with KD exhibited higher-than-proportional increases in AUC0–∞ and decreases in Cl/FNC and VdNC/F between the 10 and 20 mg/kg daily dose levels, whereas t1/2 did not change. Nonlinearity of both Cl/FNC and VdNC/F but not t1/2 in our population could indicate absorption differences with differing doses. In other words, bioavailability of this investigational oral solution may increase as dose increases, causing the higher-than-proportional decreases in Cl/FNC and VdNC/F. Studies of pentoxifylline in other pediatric populations with healthy gastrointestinal tracts would help clarify whether this finding is related to the drug itself or the patient population in this study. In contrast, adults had a higher-than-proportional increase in t1/2, as well as AUC and Cmax. Oral clearance rate and volume of distribution were not reported.12 The nonlinear increases in pentoxifylline t1/2 in adults suggest decreased clearance rate with increased doses.
Higher-than-proportional increases in AUC, Cmax, and t1/2 also were described for M-1 in adults, but were not demonstrated in this study. The M-1 dose-normalized AUC0–∞ in our population approached a significant difference between dose levels 1 and 3, but the high degree of variability in parameters may have prevented the detection of significant nonlinearity of M-1. No evidence of pentoxifylline or M-1 accumulation with multiple doses was noted in this study; these findings are similar to findings in an adult study.12 However, 6 of the 10 patients (60%) for whom pentoxifylline and M-1 concentrations were determined on study day 6 were from dose level 1, the lowest-dose group. Although no evidence of pentoxifylline or M-1 accumulation was noted, these findings are based mainly on a daily dose of 10 mg/kg and may not apply to the higher-dose groups.
Pharmacokinetic parameters of pentoxifylline and M-1 were similar from the noncompartmental and the 1-compartment analyses for dose levels 1, 2, and 3 (Table I). For dose level 4, the 1-compartment median AUC0–∞ for pentoxifylline and M-1 were approximately half and the oral clearance rates were 2- to 3-fold higher than the corresponding parameters estimated by the noncompartmental analysis. These discrepancies were the result of a selection issue related to the 2 patients excluded from the 1-compartment analysis due to the inability to determine the terminal slope of the curve. If these 2 patients are excluded from the noncompartmental analysis, the resultant median parameters from both types of analyses are similar.
The ratios of M-1 to pentoxifylline seen in this study were highly variable. Dose levels 1 and 2 showed a slightly higher M-1 concentration versus parent drug concentrations (1.7 fold). Dose level 3 had equal concentrations of both compounds, and dose level 4 had lower M-1 concentrations (∼50%) compared with the parent drug. In contrast, adults exhibit M-1 concentrations ∼3-fold higher than pentoxifylline concentrations.13 The lower ratios of M-1 to parent drug seen in this study might indicate lower activity of aldo-ketoreductase, the enzyme responsible for M-1 metabolite formation, in children than in adults. Previous evidence of this lower activity in children has been documented by Rady-Pentek et al,23 who found significantly decreased 15-ketoreductase activity in liver cytosol in children aged ≤10 years (n = 15) compared with activity in adolescents and adults aged ≥13 years (n = 22). This decrease in metabolite ratios in our study population also may indicate saturation of aldo-ketoreductase at a pentoxifylline daily dose of 20 mg/kg, with pentoxifylline being shunted to its various other metabolic pathways with higher doses.
Several markers of effect were collected to gain insight into possible dose-response relationships, although the study was not powered to detect significant differences in these biomarkers. In this study, 5 of 24 patients (21%) required IVIG retreatment for persistent or recrudescent fever 48 hours after administration of IVIG. This result is comparable to a previously reported retreatment rate of 15%.6 None of the patients who required retreatment with IVIG had abnormalities of their coronary arteries in the acute or subacute phase of KD, unlike previously reported groups of patients.6 This finding could be due to the small number of patients in this study. Importantly, all patients in this study had improved or unchanged coronary artery assessments after treatment with pentoxifylline, suggesting that pentoxifylline either improves or does not affect coronary artery abnormalities.
Fever was an insensitive marker of pentoxifylline effect in this study. Because these patients received IVIG before and ASA concurrently with pentoxifylline, any effect that pentoxifylline might have had on body temperature could have been masked in this study, especially because the participants may have been afebrile by the time pentoxifylline was administered. TNF-alpha levels were also insensitive markers of pentoxifylline effect in this study. Because of the small number of TNF-alpha samples collected on study day 6 and the large variation in measured levels, an overall decrease in TNF-alpha from study day 1 to study day 6 was difficult to detect. However, when the percentage of decrease in TNF-alpha in each patient was compared with that patient's active drug exposure, a possible relationship emerged. The trend toward decreasing TNF-alpha with increasing active drug exposure suggests a dose-response relationship between active drug (pentoxifylline plus M-1) and decreased circulating TNF-alpha.
In the previous study of pentoxifylline in KD by Furukawa et al,15 trends toward accelerated resolution of fever and normalization of C-reactive protein were noted in children receiving 20 mg/kg daily of pentoxifylline. The relationship seen in this study between decreases in TNF-alpha levels in patients with greater pentoxifylline and M-1 exposure further supports the hypothesis that pentoxifylline may have anti-inflammatory effects in acute KD. In the study from Japan,15 no patients in the group who received 20 mg/kg daily of pentoxifylline developed cardiac abnormalities. In the present study, improvement in coronary artery dimension was noted in 4 patients receiving 20 or 25 mg/kg daily. No patients receiving pentoxifylline in either study developed worsening coronary artery abnormalities.
Evidence of pentoxifylline effect in KD has been difficult to detect, in part because of the unknown cause of KD and the lack of sensitive, reliable biomarkers or surrogate end points for disease progression and/or resolution. Although pentoxifylline efficacy has not been proved in this and another small study15 to date, no harmful effects of pentoxifylline have been noted. Without notable differences in clinical outcomes, dose selection is based on the pharmacokinetic data observed. In this study, dose levels 1 and 2 demonstrated highly variable pharmacokinetic characteristics and low overall exposure. Dose levels 3 and 4 resulted in similar exposure to both pentoxifylline and the active metabolite, M-1. Increasing the daily dose to 25 mg/kg did not increase systemic exposure to either pentoxifylline or its active metabolite over what was seen with the 20-mg/kg daily dose. Therefore, a pentoxifylline daily dose of 20 mg/kg divided into 3 doses is a rational choice for future studies in KD.
Due to its tolerability and theoretic benefits, pentoxifylline is a reasonable candidate for Phase II or III clinical trials in KD. IVIG is not available in many countries because of its high cost. In addition, IVIG is currently in short supply worldwide. Pentoxifylline could be tested in combination with high-dose ASA in countries where IVIG is unavailable in the hopes of identifying a safe, inexpensive, and readily available agent that is effective in preventing coronary artery lesions. In countries where IVIG is available, the use of pentoxifylline may reduce the number of patients in whom coronary artery abnormalities develop. Another therapeutic niche for pentoxifylline would be as adjunctive therapy in the subset of patients with KD who enter the health care system with coronary artery abnormalities already detectable. Increased susceptibility to coronary artery lesions has been associated with increased TNF-alpha levels, so this subgroup of patients may benefit from treatment with pentoxifylline, a TNF-alpha inhibitor.9 In our current study, we chose to delay administration of pentoxifylline until after IVIG was given and tolerated to fully assess the tolerability of pentoxifylline, but the ideal pentoxifylline administration time is not known. Given its tolerability and ease of administration, early pentoxifylline administration may be advantageous. Oral pentoxifylline could be administered while IVIG is being prepared.
CONCLUSIONS
In the children in this study, pentoxifylline was well tolerated at the doses studied. No notable differences in clinical outcomes were observed between dose levels, and dose levels 3 and 4 resulted in similar exposure to both pentoxifylline and M-1. Future efficacy and tolerability studies should use a daily dose of 20 mg/kg of pentoxifylline in acute KD.
Acknowledgements
Members of the Pediatric Pharmacology Research Unit Network include: Arkansas Children's Hospital, Little Rock, Arkansas; Baylor College of Medicine, Houston, Texas; Children's Hospital Medical Center, Cincinnati, Ohio; Children's Hospital of Columbus, Columbus, Ohio; Children's Hospital of Michigan/Wayne State University, Detroit, Michigan; Children's Hospital of Philadelphia, Philadelphia, Pennsylvania; Children's Mercy Hospital, Kansas City, Missouri; Louisiana State University Health Sciences Center, Shreveport, Louisiana; National Jewish Medical and Research Center/University of Colorado Health Science Center, Denver, Colorado; Rainbow Babies and Children's Hospital, Cleveland, Ohio; University of California at San Diego, La Jolla, California; University of Tennessee Health Science Center, Memphis, Tennessee; and Yale University School of Medicine, New Haven, Connecticut.
B.M. Best received salary support from a National Institutes of Health National Research Service Award, grant no. HD 08722. The PPRU Network is funded by the National Institute for Child Health and Human Development, which provided supplemental funds for this study.
Pentoxifylline was provided by Sage Pharmaceuticals, Shreveport, Louisiana.
The authors acknowledge John F. Bastian, MD, Jacob V. Aranda, MD, PhD, and Emily Chou, MD, for their contributions to this study. We also thank Timothy Davis at the Louisiana State University Medical Center (Shreveport, Louisiana) for his assistance with pentoxifylline analytical procedures and Vanessa Herring at the University of Tennessee Health Science Center (Memphis, Tennessee) for her help with TNF-alpha analytical procedures.
Footnotes
A preliminary analysis of the first 2 dose levels was presented at the American College of Clinical Pharmacy Spring Research Forum in Salt Lake City, Utah, April 22–25, 2001. The preliminary results from all dose levels were presented at the 7th International Kawasaki Disease Symposium, Hakone, Japan, December 4–7, 2001.
References
- 1.Taubert K.A., Rowley A.H., Shulman S.T. Seven-year national survey of Kawasaki disease and acute rheumatic fever. Pediatr Inf Dis J. 1994;13:704–708. doi: 10.1097/00006454-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kato H., Sugimura T., Akagi T. Long-term consequences of Kawasaki disease: A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Leung D.Y., Burns J.C., Newburger J.W., Geha R.S. Reversal of lymphocyte activation in vivo in the Kawasaki syndrome by intravenous gammaglobulin. J Clin Invest. 1987;79:468–472. doi: 10.1172/JCI112835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furusho K., Kamiya T., Nakano H. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 5.Newburger J.W., Takahashi M., Burns J.C. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 6.Burns J.C., Capparelli E.V., Brown J.A. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Inf Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Newburger J.W., Takahashi M., Beiser A.S. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 8.Matsubara T., Furukawa S., Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-alpha in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa S., Matsubara T., Umezawa Y. Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J Pediatr. 1994;124:721–725. doi: 10.1016/s0022-3476(05)81361-7. [DOI] [PubMed] [Google Scholar]
- 10.Maury C.P., Salo E., Pelkonen P. Elevated circulating tumor necrosis factor-alpha in patients with Kawasaki disease. J Lab Clin Med. 1989;113:651–654. [PubMed] [Google Scholar]
- 11.Strieter R.M., Remick D.G., Ward P.A. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 12.Smith R.V., Waller E.S., Doluisio J.T. Pharmacokinetics of orally administered pentoxifylline in humans. J Pharm Sci. 1986;75:47–52. doi: 10.1002/jps.2600750111. [DOI] [PubMed] [Google Scholar]
- 13.Rames A., Poirier J.M., LeCoz F. Pharmacokinetics of intravenous and oral pentoxifylline in healthy volunteers and in cirrhotic patients. Clin Pharmacol Ther. 1990;47:354–359. doi: 10.1038/clpt.1990.39. [DOI] [PubMed] [Google Scholar]
- 14.Beermann B., Ings R., Mansby J. Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clin Pharmacol Ther. 1985;37:25–28. doi: 10.1038/clpt.1985.6. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S., Matsubara T., Umezawa Y. Pentoxifylline and intravenous gamma globulin combination therapy for acute Kawasaki disease. Eur J Pediatr. 1994;153:663–667. doi: 10.1007/BF02190688. [DOI] [PubMed] [Google Scholar]
- 16.Koren G., Silverman E., Sundel R. Decreased protein binding of salicylates in Kawasaki disease. J Pediatr. 1991;118:456–459. doi: 10.1016/s0022-3476(05)82168-7. [DOI] [PubMed] [Google Scholar]
- 17.Koren G., Schaffer F., Silverman E. Determinants of low serum concentrations of salicylates in patients with Kawasaki disease. J Pediatr. 1988;112:663–667. doi: 10.1016/s0022-3476(88)80194-x. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics . Kawasaki disease. In: Pickering L.K., editor. 2000 Red Book: Report of the Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; Elk Grove Village, Ill: 2000. p. 360. [Google Scholar]
- 19.de Zorzi A., Colan S.D., Gauvreau K. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 20.Muzik O., Paridon S.M., Singh T.P. Quantification of myocardial blood flow and flow reserve in children with a history of Kawasaki disease and normal coronary arteries using positron emission tomography. J Am Coll Cardiol. 1996;28:757–762. doi: 10.1016/0735-1097(96)00199-4. [DOI] [PubMed] [Google Scholar]
- 21.Anderson T.M., Meyer R.A., Kaplan S. Long-term echocardiographic evaluation of cardiac size and function in patients with Kawasaki disease. Am Heart J. 1985;110:107–115. doi: 10.1016/0002-8703(85)90523-x. [DOI] [PubMed] [Google Scholar]
- 22.Dhillon R., Clarkson P., Donald A.E. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–2106. doi: 10.1161/01.cir.94.9.2103. [DOI] [PubMed] [Google Scholar]
- 23.Rady-Pentek P., Mueller R., Tang B.K., Kalow W. Interindividual variation in the enzymatic 15-keto-reduction of 13, 14-dihydro-15-keto-prostaglandin E1 in human liver and in human erythrocytes. Eur J Clin Pharmacol. 1997;52:147–153. doi: 10.1007/s002280050264. [DOI] [PubMed] [Google Scholar]


