Abstract
Background: In chronic hepatitis C virus (HCV) infection, interferon (IFN) monotherapy usually is carried out at doses of 3 to 6 million units (MU) 3 times per week, but treatment efficacy is low.
Objective: The aim of our study was to assess the efficacy and tolerability of IFN-alfa2b in combination with ribavirin in relapsers and nonresponders to high-dose IFN treatment (5 to 6 MU 3 times per week). We measured the biochemical and virologic responses to treatment and the risk for relapse during the 24 weeks following the end of treatment.
Methods: Patients with chronic HCV infection (relapsers and nonresponders to a previous treatment with high-dose IFN) received IFN-alfa2b, 3 MU 3 times per week, and ribavirin, 1000 or 1200 mg/d for 24 or 48 weeks. The patients were then followed up for an additional 24 weeks. Sustained response was defined as normal serum alanine aminotransferase (ALT) level and undetectable HCV RNA 24 weeks after treatment was stopped.
Results: Forty-three patients (32 men, 11 women; mean [SD] age, 45 [2] years; 10 relapsers, 33 nonresponders) were included in the study. Four patients were withdrawn from the study at week 4 of treatment because of treatment-related adverse events, and 1 dropped out. At the end of the treatment period, normalization of serum ALT levels and undetectable HCV RNA levels were seen in 58.1% and 30.2% of patients, respectively. No significant difference in virologic response at the end of treatment was found between nonresponders (10/33 [30.3%]) to previous IFN therapy and relapsers (3/10 [30.0%]). At the end of follow-up, 3 (7.0%) treated patients had sustained response (2 nonresponders to the first IFN course and 1 relapser). All of the patients with sustained response were treated for 24 weeks.
Conclusion: Based on the results of our study, combination therapy with IFN-alfa and ribavirin may be of value in a limited number of patients with chronic HCV infection who do not respond to, or relapse after, a first course of treatment with high-dose IFN monotherapy.
Keywords: chronic hepatitis C virus, high-dose interferon, ribavirin, combination therapy
INTRODUCTION
Chronic infection with hepatitis C virus (HCV) is estimated to affect >5 million people in Europe, almost 4 million in the United States, and 170 million individuals worldwide.1,2 In ∼15% to 20% of these individuals, infection progresses to chronic hepatitis and cirrhosis.3,4
Interferon (IFN)-alfa monotherapy has been, for a decade, the only effective treatment for chronic HCV infection,5–7 but treatment efficacy is low. Sustained response, defined as viral clearance persisting for >24 weeks after treatment is stopped, occurs in only 30% of treated patients, whereas 15% to 25% of patients relapse during the 24 weeks after treatment is stopped (relapsers) and 25% to 50% of patients do not respond at all during IFN treatment (nonresponders).8
Combination therapy with 2 antiviral drugs has been proposed as a strategy to improve treatment efficacy. The combination of ribavirin, a synthetic nucleoside analogue with in vitro activity against a range of RNA and DNA viruses, plus IFN-alfa has been shown to be more effective than IFN alone.9,10 This combination therapy is currently the standard treatment for HCV infection.11
The probability of biochemical and virologic response after IFN–ribavirin combination therapy has been reported to range from 36% to 43% for treatment-naive patients.9,10 The combination of ribavirin with IFN also has been shown to be effective for relapsers and nonresponders to standard IFN therapy. Efficacy has been reported to range from 29% to 49% in relapsers12,13 and from 9.8% to 40% in nonresponders to IFN14–16; however, in most of these studies, IFN treatment was carried out at doses of 3 million units (MU) 3 times per week. This dose is lower than that currently used in most European countries for IFN monotherapy (5 MU 3 times per week). Therefore, it is unclear whether the combination of ribavirin plus IFN may be effective for treatment of patients who are chronic HCV relapsers or nonresponders to IFN doses higher than 3 MU 3 times per week.
The aim of our study was to assess the efficacy and tolerability of IFN-alfa2b in combination with ribavirin in relapsers and nonresponders to high-dose IFN treatment (5 to 6 MU 3 times per week). We measured the biochemical and virologic responses to treatment and the risk for relapse during the 24 weeks following the end of treatment.
PATIENTS AND METHODS
Patients
Patients with serologic (anti-HCV antibodies and HCV RNA positive) and histologic evidence of chronic hepatitis C at liver biopsy were eligible for the study if they were considered to be relapsers or nonresponders to treatment with recombinant IFN-alfa or with linfoblastoid IFN-alfa2b at a dose of 5 to 6 MU 3 times per week for 24 to 48 weeks. Patients entered the study before pegylated IFN became available as standard treatment in Italy in 2001. At baseline, nonresponders were defined as patients who did not have a biochemical or virologic response, defined as serum alanine aminotransferase (ALT) level above the upper limit of normal (ULN) range and detectable HCV RNA at the end of IFN treatment. Relapsers were defined as patients with initial response (normalization of serum ALT levels and undetectable HCV RNA), followed by subsequent relapse (serum ALT levels higher than ULN and detectable HCV RNA) within 1 year after IFN treatment was stopped.
The exclusion criteria were as follows: age <18 or >65 years; histologic evidence of cirrhosis at liver biopsy or concomitant metabolic, autoimmune, or neoplastic liver disease; severe concomitant disease other than liver disease; cardiomyopathy; previous treatment with immunosuppressive drugs; current IV drug abuse; alcoholism; pregnancy or lack of appropriate contraceptive measures in women of childbearing age; serum-positive hepatitis B surface antigen; human immunodeficiency virus infection; history of depression or other psychiatric disease; serum albumin level <3.0 g/dL; platelet count <70,000 cells/mm3; leukocyte count <3000 cells/mm3; hemoglobin level <10 g/dL; and abnormal serum creatinine level (<0.6 or >1.2 mg/dL).
Study Design
Patients enrolled in this single-center, open-label, uncontrolled study received IM IFN-alfa2b∗ at the standard dose of 3 MU 3 times per week plus oral ribavirin† at a dose of 1000 mg/d for patients weighing ≤75 kg and 1200 mg/d for patients weighing >75 kg, given in 2 divided doses for 48 weeks. All the patients were enrolled at the Gastroenterology Unit, Spedali Civili Hospital, Brescia, Italy.
Clinical and laboratory assessments were carried out every 4 weeks during the 24 to 48 weeks of the study and during the 24 weeks of follow-up after treatment was stopped. Adverse events were noted by the attending physician on history and physical examination. The severity of adverse events was graded as mild, moderate, severe, or life-threatening according to the World Health Organization recommendations.17 IFN or ribavirin was reduced by 25% of patients due to mild to moderate toxicity and by 50% of patients due to severe toxicity; therapy was discontinued after life-threatening events. The full dose could be resumed after the event or discontinued if the effect persisted.
Efficacy was assessed by monitoring serum ALT levels and qualitative plasma HCV RNA using polymerase chain reaction (PCR). We did not measure quantitative HCV RNA. The patients were defined as responders if serum ALT levels were within the normal range and HCV RNA was not detectable at the end of treatment. The response was defined as sustained if serum ALT levels were persistently normal and HCV RNA was undetectable at 24 weeks after stopping treatment; the response was defined as nonsustained either if ALT levels were higher than ULN or HCV RNA became detectable again at week 24. Patients with ALT levels higher than ULN and detectable HCV RNA at the end of the treatment were defined as nonresponders.
Written informed consent was obtained from each patient, and the study was approved by the local ethics committee.
Virology
Serum samples were obtained before treatment, at cessation of treatment, and 6 months after treatment. They were stored at −20°C immediately on collection.
Serum HCV RNA was measured by PCR assay using the Amplicor HCV Assay (Roche Diagnostic Systems, Basel, Switzerland). The results were expressed as HCV RNA copies per milliliter.
For HCV genotyping, the Line Probe Assay (Innolipa, HCV II, Ghent, Belgium) was used, and genotypes were identified using restriction fragment length polymorphism of the noncoding region (5′NCR).
Statistical Analysis
Results are given as mean (SD) and range. Differences were tested for statistical significance using the chi-square test or paired Student t test. A value of P<0.05 was considered statistically significant.
RESULTS
Forty-three patients (32 men, 11 women; mean [SD] age, 45 [2] years) were enrolled. Of these, 33 (76.7%) were nonresponders and 10 (23.3%) were relapsers; 9 (20.9%) had been treated with recombinant IFN-alfa and 34 (79.1%) with linfoblastoid IFN-alfa at a dose of 5 to 6 MU 3 times per week for 24 (n = 12) to 48 (n = 31) weeks. Ten patients ([23.3%] 8 nonresponders and 2 relapsers) were treated for 48 weeks, and 1 (2.3%) nonresponder discontinued therapy at week 32 of treatment because of cutaneous melanoma. Twenty-six patients ([60.5%] 18 nonresponders and 8 relapsers) were treated for 24 weeks because of limitations on the duration of treatment imposed by the Italian Ministry of Health while the study was in progress. Six patients ([14.0%] all responders) were treated for a shorter period; 5 patients (11.6%) discontinued therapy at week 4 (4 [9.3%] because of treatment-related adverse events, 1 patient [2.3%] dropped out), and 1 patient (2.3%) discontinued therapy at week 20 because of myocardial infarction.
As shown in the table, no significant difference in the baseline characteristics was found between nonresponders to previous IFN treatment and relapsers in terms of age, sex, body weight, risk factors for HCV infection, duration of disease, or liver histology. However, the mean (SD)×ULN serum ALT level was significantly higher in nonresponders (2.84 [0.26]; range, 1.22–8.30) than in relapsers (1.73 [0.20]; range, 0.50–2.92) (P<0.001) to previous IFN treatment. The mean (SD)×ULN baseline serum gamma-glutamyl transpeptidase level also was sig-nificantly higher in nonresponders (1.72 [0.31]; range, 0.40–8.90) than in relaps-ers (0.63 [0.15]; range, 0.10–1.82) (P<0.003). Pretreatment viral genotype was assessed in 24 patients ([55.8%] 18 nonresponders and 6 relapsers); the proportion of patients with HCV genotype 1b was similar in the 2 groups.
Table.
Baseline characteristics of study patients (N = 43).
| Characteristic | Nonresponders (n = 33) | Relapsers (n = 10) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 44 (1) | 48 (3) |
| Range | 28–65 | 35–64 |
| Sex, no. (%) | ||
| Men | 26 (78.8) | 6 (60.0) |
| Women | 7 (21.2) | 4 (40.0) |
| Body weight, kg | ||
| Mean (SD) | 72 (1) | 72 (3) |
| Range | 63–88 | 53–115 |
| No. (%) of patients with previous IV drug abuse | 4 (12.1) | 0 (0.0) |
| No. (%) of patients with previous blood transfusion | 7 (21.2) | 3 (30.0) |
| No. (%) of patients with previous surgery | 22 (66.7) | 6 (60.0) |
| Duration of infection, y | ||
| Mean (SD) | 8.5 (1.3) | 9.6 (2.6) |
| Range | 0–29 | 1–24 |
| ALT, ×ULN | ||
| Mean (SD) | 2.84 (0.26) | 1.73 (0.20)∗ |
| Range | 1.22–8.30 | 0.50–2.92 |
| GGT, ×ULN | ||
| Mean (SD) | 1.72 (0.31) | 0.63 (0.15)† |
| Range | 0.40–8.90 | 0.10–1.82 |
| Knodell (histologic activity index) score | ||
| Mean (SD) | 8.00 (0.00) | 8.20 (1.36) |
| Range | 4–14 | 4–14 |
| Necroinflammatory activity score | ||
| Mean (SD) | 6.42 (0.66) | 6.83 (0.90) |
| Range | 3–11 | 3–11 |
| No. (%) of patients with HCV genotype 1b‡ | 14 (77.8) | 5 (83.3) |
ALT = alanine aminotransferase; ULN = upper limit of normal; GGT = gamma-glutamyl transpeptidase; HCV = hepatitis C virus.
P<0.001 versus nonresponders.
P<0.003 versus nonresponders.
Pretreatment viral genotype was assessed in 24 patients ([55.8%] 18 nonresponders and 6 relapsers).
Twenty-five of the 43 treated patients (58.1%) had normal serum ALT levels at the end of treatment (Figure 1). Among these patients, no significant difference in biochemical response at the end of treatment was found between nonresponders (17/33 [51.5%]) to previous IFN treatment and relapsers (8/10 [80.0%]). Sustained response also was similar in the 2 groups (5/33 [15.2%] and 2/10 [20.0%], respectively).
Figure 1.
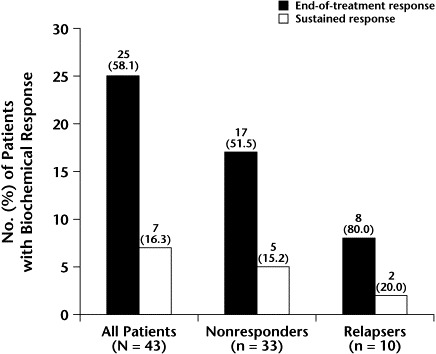
Biochemical response to interferon (IFN) plus ribavirin at the end of treatment and 24 weeks thereafter (sustained response) in patients who were relapsers or nonresponders to a previous course of IFN monotherapy.
At the end of treatment, HCV RNA was undetectable in 13 of 43 treated patients (30.2%; Figure 2). No significant difference in the virologic response at the end of treatment was found between nonresponders (10/33 [30.3%]) to previous IFN treatment and relapsers (3/10 [30.0%]). Sustained response was obtained in 3 patients (7.0%), and the proportion was similar in the 2 groups (2/33 [6.1%] and 1/10 [10.0%], respectively). All 3 patients who had a sustained response were treated for 24 weeks.
Figure 2.
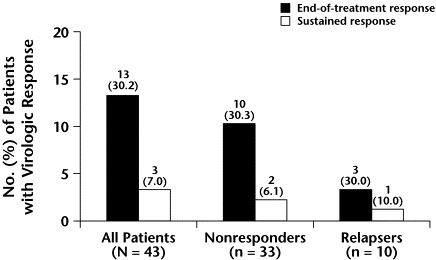
Virologic response to interferon (IFN) plus ribavirin at the end of treatment and 24 weeks thereafter in patients who were relapsers or nonresponders to a previous course of IFN monotherapy.
Four patients (9.3%) discontinued treatment at week 4 due to treatment-related adverse events (2 for severe depression and 2 for vomiting and diarrhea).
Two patients (4.7%) were withdrawn from the study, 1 for myocardial infarction at week 20 and 1 for the detection of cutaneous melanoma at week 32. Hemoglobin level was 11.8 g/dL in the patient with myocardial infarction, indicating that this adverse event was not related to anemia.
Combination therapy was associated with the expected18–20 decrease in hemoglobin level in 9 patients (20.9%); the mean (SD) decrease was 2.9 (0.3) g/dL. In 3 patients (7.0%) the dose of ribavirin had to be reduced by 50% because of a decrease in hemoglobin level to <11.0 g/dL. Following cessation of therapy, hemoglobin returned to pretreatment levels in all 3 patients within 4 to 8 weeks. In 6 patients (14.0%) the decrease in hemoglobin levels was associated with an increase of serum indirect bilirubin level that in no case exceeded 4.0 mg/dL.
A decrease in leukocytes and neutrophils also was observed in 21 (48.8%) and 25 (58.1%) patients, respectively. Only 3 patients (7.0%) showed a decrease in leukocyte count to <2.0×103 cells/μL, and 5 patients (11.6%) showed a decrease in neutrophil count to <1.0×103 cells/μL. A 25% to 50% reduction of the total dose of IFN was necessary in 4 patients (9.3%) because of moderate neutropenia (500 to 1000 cells/μL). No significant changes were observed in platelet counts compared with baseline. No significant changes were observed in levels of serum creatinine; uric acid; alkaline phosphatase; albumin; or alpha1, alpha2, beta, or gamma protein.
No patient developed symptoms or signs of autoimmune disease during treatment. Two patients (4.7%) developed antibodies to antithyroid peroxidase, 1 each during weeks 24 and 48 of treatment, without alterations in thyroid hormone concentrations.
Asthenia was the most predominant treatment-related adverse event (53.5% of patients), but it was not sufficiently severe to require withdrawal from the study.
Influenza-like symptoms, including fever, musculoskeletal pain, myalgia, arthralgia, and fatigue, were noted during the first 4 weeks in 7 of the 43 patients (16.3%) treated. These treatment-related symptoms were controlled using therapy with paracetamol and tended to resolve spontaneously after 3 to 4 weeks of treatment.
During the treatment period, 10 patients (23.3%) experienced a lack of appetite associated with loss of body weight (mean [SD], 4.3 [0.8] kg; range, 2.0–10.0 kg). One of these patients experienced a 10-kg body weight loss during the first 6 months of therapy, although the dose of ribavirin was reduced by 50%. Seven patients (16.3%) had cutaneous symptoms (rash and pruritus), which were controlled by treatment with antihistaminic drugs. Ten patients (23.3%) reported nausea, vomiting, and diarrhea during treatment; however, these symptoms tended to resolve spontaneously in a few weeks. Ten additional patients (23.3%) reported anxiety and depression, and 6 patients (14.0%) reported headache. All of these adverse events were considered treatment related.
DISCUSSION
Our study was designed to assess the effects of combination therapy with IFN plus ribavirin in a group of patients with an extremely poor likelihood of response. Indeed, they had been relapsers or nonresponders to a first antiviral treatment with high-dose IFN (5 to 6 MU 3 times per week for 24 or 48 weeks) and predominantly were infected with HCV genotype 1b.
In our study, the virologic response was 30.2% at the end of treatment and 7.0% 24 weeks after treatment was stopped. This efficacy rate is lower than that reported in the literature for relapsers (29% to 49%)12,13 and nonresponders (9.8% to 40.0%)14,15 to IFN. We believe that the lower response rate observed in our study may be explained by the difference in the dose of IFN given during the previous course of treatment. In our study, the dose initially used by the patients was ∼2-fold (5 to 6 MU 3 times per week) that used in other studies (3 MU 3 times per week).16 Furthermore, all of our patients had been treated for at least 24 weeks during the first course of IFN treatment, and 72.1% of them had been treated for at least 48 weeks. In contrast with our study, in most other studies the duration of IFN treatment ranged from 12 to 24 weeks only, and the duration of treatment was also an important factor in the efficacy of IFN.
The importance of treatment dose and duration are underlined by several studies. In their meta-analysis of 17 trials versus controls and of 16 trials comparing different IFN regimens, Poynard et al21 reported a dose-dependent effect of IFN on sustained response ranging from 28% for 3 MU 3 times per week to 46% for 6 MU 3 times per week for 48 weeks. The duration of treatment also has been reported to affect sustained response in a dose-dependent manner, with maximum efficacy obtained for treatment longer than 48 weeks (46% in the group treated with 6 MU 3 times per week for 48 weeks vs 29% in the group treated with the same IFN dose for only 24 weeks).
Thus, in our study, a high dose given for a long period of time during the first course of IFN treatment may have selected patients resistant to treatment, which may explain the low efficacy reported in our study using IFN plus ribavirin at the standard dose.
Moreover, 76.7% (33/43) of patients enrolled in our study were nonresponders to the first course of IFN therapy. As reported by Alberti et al22 in a meta-analysis of 13 studies on retreatment of nonresponders and 11 studies on relapsers to the first IFN therapy, the most significant variable correlating with the rate of sustained response after retreatment was the type of virologic response achieved during the first cycle. Indeed, the combined analysis showed a sustained response rate of 56% among 145 patients who were HCV RNA negative at the end of the first course of therapy compared with only 2.9% among 206 patients who were HCV RNA positive.
Results similar to the findings in our study have been reported by Younossi et al,23 who found a sustained response rate of 3.9% using IFN plus ribavirin for 24 weeks in nonresponders previously treated with IFN monotherapy. The minimum dose of IFN during the initial treatment ranged from 3 to 6 MU 3 times per week. This range was narrower in our study, with all patients receiving a high IFN dose, which was never <5 MU 3 times per week. In contrast, an additional study24 indicated a sustained response rate of 20.9% in nonresponders to a first cycle of IFN monotherapy, but the percentage of genotype 1 among these patients was lower (70%) and the pretreatment serum ALT level was higher (3.4×ULN) than in the other studies.
In our study, no significant difference in the response to treatment was found between nonresponders and relapsers. This finding may have been the result of the low power of the comparison due to the small number of patients enrolled in the study.
IFN combined with ribavirin was well tolerated by our patients. Hemolytic anemia was the most significant ribavirin-related adverse event, but it was not severe in most cases and required a reduction in the dose of ribavirin in only 3 cases.
CONCLUSION
Based on the results of our study, combination therapy with IFN-alfa and ribavirin may be of value in a limited number of patients with chronic HCV infection who do not respond to, or relapse after, a first course of treatment with high-dose IFN monotherapy.
Footnotes
Trademark: Intron® A Injection (Schering-Plough Corp., Kenilworth, New Jersey).
Trademark: Rebetol® Capsules (Schering-Plough Corp.).
References
- 1.Alter M.J. Epidemiology of hepatitis C. Hepatology. 1997;26(Suppl 1):62–65. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Hepatitis C. Global prevalence. Wkly Epidemiol Rec. 1997;72:341–344. [PubMed] [Google Scholar]
- 3.Seeff L.B, Buskell-Bales Z, Wright E.C. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 4.Koretz R.L, Abbey H, Coleman E, Gitnick G. Non-A, non-B post-transfusion hepatitis: Looking back in the second decade. Ann Intern Med. 1993;119:110–115. doi: 10.7326/0003-4819-119-2-199307150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Davis G.L, Balart L.A, Schiff E.R. for the Hepatitis Interventional Therapy Group. Treatment of chronic hepatitis C with recombinant interferon alfa: A multicenter randomized, controlled trial. N Engl J Med. 1989;321:1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie A.M, Martin P, Kassianides C. Recombinant interferon alfa therapy for chronic hepatitis C: A randomised, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506–1510. doi: 10.1056/NEJM198911303212204. [DOI] [PubMed] [Google Scholar]
- 7.Tine F, Magrin S, Craxi A, Pagliaro L. Interferon for non-A, non-B chronic hepatitis: A meta-analysis of randomised clinical trials. J Hepatol. 1991;13:192–199. doi: 10.1016/0168-8278(91)90814-r. [DOI] [PubMed] [Google Scholar]
- 8.Hoofnagle J.H, Di Bisceglie A.M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 9.Lai M.Y, Kao J.H, Yang P.M. Long-term efficacy of ribavirin plus interferon alfa in the treatment of chronic hepatitis C. Gastroenterology. 1996;111:1307–1312. doi: 10.1053/gast.1996.v111.pm8898645. [DOI] [PubMed] [Google Scholar]
- 10.Reichard O, Norkrans G, Frydèn A, The Swedish Study Group A randomized double-blind, placebo-controlled trial for interferon-α 2b with and without ribavirin for chronic hepatitis C. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 11.Seeff L.B, Hoofnagle J.H. National Institutes of Health Consensus Development Conference: Management of hepatitis C: 2002. Hepatology. 2002;36(Suppl 1):S1–S2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 12.Bell H, Hellum K, Harthug S, CONSTRUCT Group Treatment with interferon-alpha2a alone or interferon-alpha2a plus ribavirin in patients with chronic hepati-tis C previously treated with interferon-alpha2a. Scand J Gastroenterol. 1999;34:194–198. doi: 10.1080/00365529950173087. [DOI] [PubMed] [Google Scholar]
- 13.Davis G.L, Esteban-Mur R, Rustgi V, for the International Hepatitis Interventional Therapy Group Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 14.Pol S, Couzigou P, Bourlière M, for the Multicenter Study Group under the coordination of the Necker Hospital, Paris, France A randomized trial of ribavirin and interferon-α vs. interferon-α alone in patients with chronic hepatitis C who were non-responders to a previous treatment. J Hepatol. 1999;31:1–7. doi: 10.1016/s0168-8278(99)80157-3. [DOI] [PubMed] [Google Scholar]
- 15.Brillanti S, Garson J, Foli M. A pilot study of combination therapy with ribavirin plus interferon alpha for interferon alpha-resistant chronic hepatitis C. Gastroenterology. 1994;107:812–817. doi: 10.1016/0016-5085(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S.J, Bonis P.A, Lau J. Interferon and ribavirin for patients with chronic hepatitis C who did not respond to previous interferon therapy: A meta-analysis of controlled and uncontrolled trials. Hepatology. 2001;33:231–240. doi: 10.1053/jhep.2001.20675. [DOI] [PubMed] [Google Scholar]
- 17.Miller A.B, Hoofstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Di Bisceglie A.M, Conjeevaram H.S, Fried M.W. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Dusheiko G, Main J, Thomas H. Ribavirin treatment for patients with chronic hepatitis C: Results of a placebo-controlled study. J Hepatol. 1996;25:591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 20.Bodenheimer H.C, Jr., Lindsay K.L, Davis G.L. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: A multicenter trial. Hepatology. 1997;26:473–477. doi: 10.1002/hep.510260231. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Leroy V, Cohard M. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: Effects of dose and duration. Hepatology. 1996;24:778–789. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 22.Alberti A, Chemello L, Noventa F. Therapy of hepatitis C: Re-treatment with alpha interferon. Hepatology. 1997;26(Suppl 1):137S–142S. doi: 10.1002/hep.510260724. [DOI] [PubMed] [Google Scholar]
- 23.Younossi Z.M, Mullen K.D, Zakko W. A randomized, double-blind controlled trial of interferon alpha-2b and ribavirin vs. interferon alpha-2b and amantadine for treatment of chronic hepatitis C non-responder to interferon monotherapy. J Hepatol. 2001;34:128–133. doi: 10.1016/s0168-8278(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 24.de Ledinghen V, Trimoulet P, Winnock M. Daily or three times per week interferon α-2b in combination with ribavirin or interferon alone for the treatment of patients with chronic hepatitis C not responding to previous interferon alone. J Hepatol. 2002;36:819–826. doi: 10.1016/s0168-8278(02)00071-5. [DOI] [PubMed] [Google Scholar]


