Abstract
Background: Obesity, which is epidemic in the United States, is associated with increased morbidity and mortality. The combination of diet, exercise, and a behavior-modification program often does not result in ideal body weight.
Objective: The aim of this study was to determine the efficacy of phentermine (Phen) alone compared with phentermine plus fenfluramine (Phen-Fen), when used in combination with a very-low-calorie diet (VLCD) for weight loss in an outpatient obesity center.
Methods: We analyzed data collected at the UCLA outpatient University Obesity Center between 1993 and 1999. Data for patients who attended the center for at least 12 weeks and at least 4 visits, who were taking Phen or Phen-Fen, and whose body mass index (BMI) was ≥30 kg/m2 were included in this retrospective study.
Results: During the study period, 3200 visits were recorded. Of 1133 potential participants, 446 patients were included in the analysis (309 women, 137 men; mean [SD] age, 46.7 [11.4] years; mean [SEM] body weight, 109.6 [26.7] kg; mean [SEM] BMI, 38.0 [7.6] kg/m2). Of these, 128 women and 60 men (mean [SEM] body weight at baseline, 103.4 [24.0] kg and 124.9 [28.2] kg, respectively) received Phen alone; 181 women and 77 men (mean [SEM] body weight at baseline, 102.5 [21.4] kg and 124.9 [30.2] kg, respectively) received Phen-Fen. No statistically significant differences were found between the Phen and Phen-Fen groups in mean age, body weight, or BMI for women or men at baseline. No significant differences in the time of weight loss were found when a VLCD was used with Phen alone compared with the Phen-Fen combination for either sex even at 12 weeks. For women, the mean total body weight loss was 7.4% in the Phen group and 8.7% in the Phen-Fen group, but these differences were not significant. For men, the mean total body weight loss was 7.8% in the Phen group and 8.2% in the Phen-Fen group, but these differences were not significant. No significant differences in BMI, severe adverse events, or dropout rate were found between the 2 treatment groups for men or women.
Conclusions: This outpatient study did not detect any significant difference between adjunctive uses of Phen compared with Phen-Fen pharmacotherapy when used with VLCD over 12 weeks. Phen can be used to achieve significant weight loss when combined with VLCD. The tolerability and positive physical response further suggest that Phen is a valuable medication for obesity management in the outpatient setting.
Keywords: phentermine-fenfluramine, phentermine, very-low-calorie diet, body weight loss
Introduction
Obesity is endemic in the United States, with 1 in 2 Americans being overweight and 1 in 3 being obese (body mass index [BMI] ≥30 kg/m2).1 Obesity is associated with increased morbidity and mortality,2,3 and there is undisputed evidence that obesity increases the risk for hypertension, dyslipidemia, type 2 diabetes mellitus (DM), atherosclerotic cardiovascular disease, cerebrovascular accident, osteoarthritis, sleep apnea, and cancers of the breast, prostate, and colon.4,5 Obesity is a complex disorder resulting from interactions of genetics, metabolism, and nutritional, cultural, and psychosocial factors. In many obese patients, body weight loss can be achieved effectively with adjunctive pharmacotherapy in combination with diet, exercise, and behavioral interventions.6
In 1992, Weintraub7 compared phentermine (Phen) and fenfluramine (Fen) alone and in combination and found modest differences between Phen and the combination of phentermine and fenfluramine (Phen-Fen) in inducing weight loss. This study gained wide acceptance, as many clinicians viewed the combination as a synergistic approach to weight loss by affecting both noradrenergic and serotonergic signaling pathways in the hypothalamus. This combination approach changed the pharmacologic treatment of obesity in the early 1990s.
During this time, the University of California at Los Angeles (UCLA) outpatient University Obesity Center initiated a clinical appetite-suppressant program in which many patients chose to use Phen alone with a very-low-calorie diet (VLCD) rather than the Phen-Fen combination or dexfenfluramine with VLCD. This choice was due to the risk for adverse events (AEs) attributed to dexfenfluramine, including pulmonary hypertension. As a result of reported valvular abnormalities and pulmonary hypertension ascribed to the use of this combination,8 Fen and dexfenfluramine were withdrawn from the market in September 1997.8 However, Phen alone continues to be available because its use as a single agent has not been linked to either valvulopathy or pulmonary hypertension.9–11
The aim of this study was to determine the efficacy of Phen alone compared with Phen-Fen, when used in combination with a VLCD for weight loss in an outpatient obesity center.
Patients and methods
The clinical program was conducted according to the protocol approved by the UCLA Institutional Review Board. All data were collected at the UCLA outpatient University Obesity Center between 1993 and 1999. The center provides a multidisciplinary weight-loss program that patients attend weekly. This program consists of VLCD and group classes on behavior modification, diet, and exercise. Data were included in the study for patients who met the following criteria: (1) they were entering the program for the first time; (2) they attended the clinic for at least 4 visits; (3) they were taking either Phen alone or Phen-Fen and no other appetite suppressants; (4) their chart contained complete baseline information; (5) baseline BMI ≥30 kg/m2; (6) they were in the program for at least 12 weeks; and (7) age ≥18 years.
Patients were excluded if they had any major medical or psychiatric disorder. Pregnant or lactating women also were excluded. Women of childbearing age were required to use an effective method of contraception throughout the study. All patients provided written informed consent to participate.
Patients' identities were confidential in the weight-loss program. A commercially prepared meal-replacement powder was used in an individually adjusted VLCD plan providing 500 to 800 kcal/d.12,13 Each formula packet provided 100 kcal and 15 g of high-biological-value protein; the formula was to be mixed with water before use. Patients were placed on a regimen of either 5 packets/d (a total daily intake of 500 kcal) or 5 packets combined with a defined meal of ∼300 kcal (a total of ∼800 kcal/d).
Patients attended the clinic weekly, and vital signs and body weight were recorded at baseline and at every visit thereafter. Complete blood count, serum electrolyte concentrations, and liver function were determined at baseline and every 2 weeks thereafter. Electrocardiography (ECG) also was performed at baseline and then every 2 weeks.
Patients were prescribed pharmacotherapy (Phen or Phen-Fen) at weekly physician visits. In the Phen group, patients were offered Phen 8-, 10-, or 15-mg once or twice daily orally, and the Phen-Fen group was given Fen 20 mg once daily orally with one of the above dosages of Phen. All patients were started on the lowest dosage of Phen; dosages were adjusted on an individual basis as needed to achieve the desired levels of appetite suppression, as determined by patients' responses to verbal questions from the physician or patients' self-reporting. The choice of Phen or Phen-Fen was made by the individual patients and their physicians. Patients with obesity-related comorbidities, such as DM, dyslipidemia, or hypertension, were continued on their previous medications for each disease. Appropriate adjustments in the dosages of these medications were made only after patients consulted with their primary care physicians. Physicians interviewed participants at each visit to assess their success with the diet program and possible AEs either with the diet or the pharmacotherapy.
In addition to the VLCD, participants were instructed to perform aerobic exercise for 30 minutes and light weight training for 10 to 15 minutes, 3 times a week.14 Dietitians and psychologists were available for consultation at each visit. The program also offered a series of weekly lectures and interactive meetings on diet, exercise, and behavior modification throughout the year. All participants were encouraged to attend these seminars, which were held during clinic hours.
Statistical modeling for weight loss and change in body mass index
A random coefficient model was used to investigate weight loss and changes in BMI following initiation of pharmacotherapy. This model was chosen to analyze the data because this was a longitudinal study with outcome repeatedly measured for the same patient. This model enabled fitting a time-trend curve to the weight data for each study group. Age and race were originally included in the model, but no statistically significant associations with the outcomes were found. Therefore, age and race were subsequently removed from our model.
The final model was:Yijt=αj+β1ijT+β2ij√T+εijt, i=1,…..n, j=1,2where Yijt = weight loss (or change in BMI from baseline) for patient i in treatment group j at time t; αj denotes the intercept for study group j; β1ij and β2ij are the regression coefficients of T and √T for patient i in group j; T is the number of weeks from baseline to date of measurement; [β1ij, β2ij] has a multivariate normal distribution with mean [β1j, β2j] and covariance matrix G (the structure of G is not specified); and ϵijt is the error term; εijt∼MVN(0,R) and R = σ2 In, where In denotes the n×n identity matrix.
The analyses were carried out separately for women and men. Diagnostic tests indicated the model fit the data. Statistical tests were performed to compare the similarity of the time-trend curves for the 2 groups. The model was found to fit the data, and random coefficient analyses were performed to determine whether both groups had significant weight loss during the first 12 weeks. The similarity of the time-trend curves between the 2 treatment groups also was assessed. SAS/STAT version 8 (SAS Institute Inc., Cary, North Carolina) was used to calculate the data. Statistical significance was set at P<0.05.
Results
Data from 1133 patients and 3200 recorded visits were collected. A total of 446 patients met the selection criteria and were included in the study (309 women, 137 men; mean [SD] age, 46.7 [11.4] years; mean [SEM] body weight, 109.6 [26.7] kg; mean [SEM] BMI, 38.0 [7.6] kg/m2). Of these, 128 women and 60 men (mean [SEM] body weight at baseline, 103.4 [24.0] kg and 124.9 [28.2] kg, respectively) received Phen alone; 181 women and 77 men (mean [SEM] body weight at baseline, 102.5 [21.4] kg and 124.9 [30.2] kg, respectively) received Phen-Fen. No significant demographic differences were found between the Phen and Phen-Fen groups (Table I). All patients were moderately obese; mean (SEM) BMI was 38.1 (7.6) kg/m2 and 37.1 (7.1) kg/m2 in the female groups for Phen and Phen-Fen, respectively, and 38.7 (8.0) kg/m2 and 39.1 (8.4) kg/m2 in the male groups for Phen and Phen-Fen, respectively. Of all women in the study, 67 (21.7%) had hypertension and 14 (4.5%) had type 2 DM. Of all men in the study, 129 (94.2%) had hypertension and 13 (9.5%) had type 2 DM. The doses received by patients throughout the study are shown in Table II.
Table I.
Baseline demographic and clinical data in study patients (N = 446). (All values are expressed as no. [%] of patients unless otherwise noted.)
| Phen (n = 188) |
Phen-Fen (n = 258) |
|||
|---|---|---|---|---|
| Characteristic | Women (n = 128) | Men (n = 60) | Women (n = 181) | Men (n = 77) |
| Year of entry∗ | ||||
| 1993 | 5 (3.9) | 2 (3.3) | 18 (9.9) | 6 (7.8) |
| 1994 | 13 (10.2) | 5 (8.3) | 26 (14.4) | 12 (15.6) |
| 1995 | 42 (32.8) | 17 (28.3) | 51 (28.2) | 34 (44.2) |
| 1996 | 40 (31.3) | 20 (33.3) | 74 (40.9) | 24 (31.2) |
| 1997 | 4 (3.1) | 4 (6.7) | 12 (6.6) | 1 (1.3) |
| 1998 | 12 (9.4) | 6 (10.0) | 0 (0.0) | 0 (0.0) |
| 1999 | 12 (9.4) | 6 (10.0) | 0 (0.0) | 0 (0.0) |
| Age, y | ||||
| Mean (SD) | 46.7 (10.8) | 45.5 (10.9) | 46.5 (12.1) | 48.1 (11.0) |
| Range | 18–72 | 20–69 | 18–71 | 27–75 |
| Race | ||||
| White | 101 (78.9) | 58 (96.7) | 138 (76.2) | 63 (81.8) |
| Black | 10 (7.8) | 0 (0.0) | 28 (15.5) | 3 (3.9) |
| Other | 17 (13.3) | 2 (3.3) | 15 (8.3) | 11 (14.3) |
| Marital status∗ | ||||
| Single | 35 (27.3) | 16 (26.7) | 60 (33.1) | 16 (20.8) |
| Married | 72 (56.3) | 39 (65.0) | 95 (52.5) | 49 (63.6) |
| Divorced | 18 (14.1) | 4 (6.7) | 15 (8.3) | 11 (14.3) |
| Other | 3 (2.3) | 1 (1.7) | 11 (6.1) | 1 (1.3) |
| Body weight, kg | ||||
| Mean (SEM) | 103.4 (24.0) | 124.9 (28.2) | 102.5 (21.4) | 124.9 (30.2) |
| Range | 72.7–227.3 | 77.2–232.6 | 71.1–202.7 | 89.3–235.4 |
| BMI, kg/m2 | ||||
| Mean (SEM) | 38.1 (7.6) | 38.7 (8.0) | 37.1 (7.1) | 39.1 (8.4) |
| Range | 30.0–67.9 | 30.2–73.5 | 30.0–67.9 | 30.3–72.3 |
| Hypertension | 33 (25.8) | 27 (45.0) | 34 (18.8) | 30 (39.0) |
| Diabetes | 3 (2.3) | 6 (10.0) | 11 (6.1) | 7 (9.1) |
Phen = phentermine; Phen-Fen = phentermine-fenfluramine; BMI = body mass index.
Percentages may not total 100% due to rounding.
Table II.
Phentermine (Phen) doses received by study patients. (Values are expressed as no. [%] of patients.)
| Phen Dose, mg | Phen (n = 188) | Phen-Fen (n = 258) |
|---|---|---|
| 8 | 48 (25.5) | 121 (46.9) |
| 10 | 5 (2.7) | 2 (0.8) |
| 15 | 56 (29.8) | 145 (56.2) |
| 16 | 9 (4.8) | 21 (8.1) |
| 20 | 8 (4.3) | 15 (5.8) |
| 30 | 11 (5.9) | 5 (1.9) |
Phen-Fen = phentermine-fenfluramine.
Efficacy analysis
Weight loss and change in body mass index
Weight loss following initiation of pharmacotherapy for both groups by sex is summarized in Table III. Weight changes over 12 weeks were separately compared in women and men (Figures 1 and 2). Statistically significant weight loss was seen among women in the Phen group at week 4 (P = 0.042 vs Phen-Fen); no significant difference in the rate of weight loss was found with Phen alone compared with the Phen-Fen combination. For women, weight loss at week 12 was 7.4% in the Phen group and 8.7% in the Phen-Fen group, but these differences were not significant. For men, weight loss was 7.8% in the Phen group and 8.2% in the Phen-Fen group, but these differences were not significant.
Table III.
Mean (SEM) body weight loss (kg) in the phentermine (Phen) group compared with the phentermine-fenfluramine (Phen-Fen) group.
| Sex | Phen | Phen-Fen |
|---|---|---|
| Women | ||
| 4 weeks | 3.6 (2.1)∗ | 4.1 (2.2) |
| 8 weeks | 6.3 (3.4) | 7.1 (5.9) |
| 12 weeks | 8.0 (6.5) | 8.9 (4.6) |
| Men | ||
| 4 weeks | 4.6 (2.4) | 4.5 (0.9) |
| 8 weeks | 7.6 (4.4) | 7.8 (3.5) |
| 12 weeks | 9.7 (5.3) | 10.5 (7.3) |
P=0.042 versus Phen-Fen.
Figure 1.
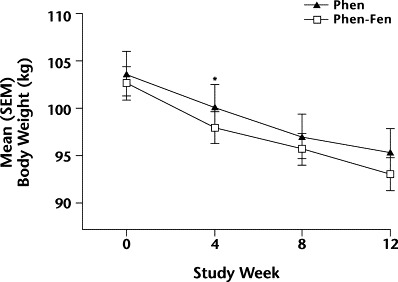
Changes in mean (SEM) body weight over 12 weeks in women taking either phentermine alone (Phen) or phentermine plus fenfluramine (Phen-Fen). ∗P = 0.042 versus Phen-Fen.
Figure 2.
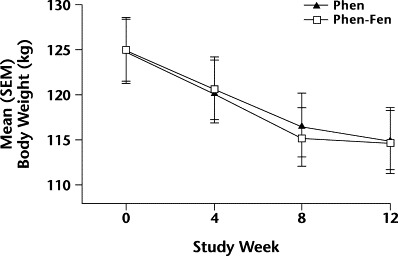
Changes in mean (SEM) body weight over 12 weeks in men taking either phentermine alone (Phen) or phentermine plus fenfluramine (Phen-Fen). No significant differences were found.
No significant differences in BMI were found between the Phen and Phen-Fen groups for women (Figure 3) or men (Figure 4).
Figure 3.
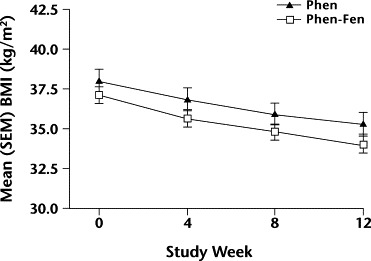
Changes in mean (SEM) body mass index (BMI) over 12 weeks in women taking either phentermine alone (Phen) or phentermine plus fenfluramine (Phen-Fen). No significant differences were found.
Figure 4.
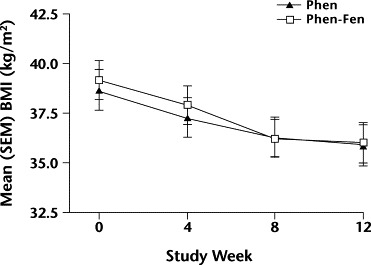
Changes in mean (SEM) body mass index (BMI) over 12 weeks in men taking either phentermine alone (Phen) or phentermine plus fenfluramine (Phen-Fen). No significant differences were found.
The random coefficient analyses showed that both sexes in both groups had significant weight loss during the first 12 weeks (all P<0.001). The rate of weight loss was not significantly different in the 2 treatment groups in either women or men.
Patient dropout rate
Table IV shows the number of patients who remained in the study at each time period. No significant difference in dropout rate was found between the Phen and Phen-Fen groups in men or women. Ten women (7.8%) in the Phen group and 14 (7.7%) in the Phen-Fen group dropped out by the end of week 12. Five men (8.3%) in the Phen group and 6 (7.8%) in the Phen-Fen group dropped out by the end of week 12.
Table IV.
Number (%) of patients in each group throughout the study.
| Phen | Phen-Fen | |||
|---|---|---|---|---|
| Study Week | Women (n = 128) | Men (n = 60) | Women (n = 181) | Men (n = 77) |
| 0 | 128 (100.0) | 60 (100.0) | 181 (100.0) | 77 (100.0) |
| 4 | 123 (96.1) | 60 (100.0) | 177 (97.8) | 75 (97.4) |
| 8 | 118 (92.2) | 55 (91.7) | 173 (95.6) | 73 (94.8) |
| 12 | 118 (92.2) | 55 (91.7) | 167 (92.3) | 71 (92.2) |
Phen = phentermine; Phen-Fen = phentermine-fenfluramine.
Tolerability assessment
A total of 27.7% (52/188) of patients receiving Phen alone and 41.1% (106/258) of those receiving Phen-Fen experienced at least 1 AE during treatment (P<0.004 between groups). The most frequently reported AEs were dry mouth (Phen, 38 patients [20.2%]; Phen-Fen, 98 patients [38.0%]; P<0.001), insomnia (Phen, 18 patients [9.6%]; Phen-Fen, 52 patients [20.2%]; P = 0.002), and constipation (Phen, 22 patients [11.7%]; Phen-Fen, 58 patients [22.5%]; P<0.004) (Table V), which could be related to Phen and/or VLCD. In total, <2% of patients from both groups reported a cardiovascular AE (eg, palpitations, tachycardia). In the patients who experienced these symptoms, the medication was either stopped completely or the dosage was subsequently reduced until symptoms resolved. None of the AEs were severe.
Table V.
Number (%) of patients experiencing ≥1 adverse event (AE), by treatment group.∗
| AE | Phen (n = 188) | Phen-Fen (n = 258) |
|---|---|---|
| CNS | ||
| Insomnia | 18 (9.6) | 52 (20.2)† |
| Depression | 7 (3.7) | 4 (1.6) |
| Anxiety | 6 (3.2) | 12 (4.7) |
| Dizziness | 6 (3.2) | 11 (4.3) |
| Headache | 4 (2.1) | 15 (5.8) |
| Cardiovascular | ||
| Palpitations | 1 (0.5) | 5 (1.9) |
| Tachycardia | 1 (0.5)‡ | 4 (1.6) |
| Other | ||
| Dry mouth | 38 (20.2) | 98 (38.0)§ |
| Constipation | 22 (11.7) | 58 (22.5)‖ |
| Fatigue | 15 (8.0) | 23 (8.9) |
| Urticaria | 3 (1.6) | 2 (0.8) |
| Unpleasant taste | 2 (1.1) | 0 (0.0) |
| Tremor | 2 (1.1) | 5 (1.9) |
| Blurred vision | 0 (0.0) | 1 (0.4) |
| Abdominal pain | 0 (0.0) | 1 (0.4) |
Phen = phentermine; Phen-Fen = phentermine-fenfluramine; CNS = central nervous system.
All AEs were mild or moderate.
P=0.002 versus Phen group.
This patient withdrew due to this AE.
P<0.001 versus Phen group.
P<0.004 versus Phen group.
Overall, a total of 35 (7.8%) patients required medication dosage adjustment secondary to experiencing an AE (Phen, 15 patients [8.0%]; Phen-Fen, 20 patients [7.8%]; P = NS). No deaths or hospitalizations directly related to the study medication were reported. No ventricular dysrhythmia was found on ECG. Of the 6 (1.3%) patients who complained of palpitations (Phen, 1 patient [0.5%]; Phen-Fen, 5 patients [1.9%]; P = NS), only 1 patient in the Phen group (0.5%) had a heart rate >90 beats/min (sinus tachycardia 110 beats/min), which resolved following discontinuation of the medication. In the 5 patients (1.1%) with documented tachycardia (all sinus tachycardia at <120 beats/min; Phen, 1 patient [0.5%]; Phen-Fen, 4 patients [1.6%]; P = NS), the abnormality also had resolved on follow-up ECGs after discontinuation of the medication. No other types of supraventricular arrhythmia or QT prolongation were noted on the ECGs for any patient group.
Discussion
Obesity has increased drastically in recent decades and is now a major public health problem in the United States. Obesity is a complex disorder resulting from genetic and environmental interactions15; no single treatment modality is likely to be successful in all patients.16 The combination of diet, exercise, and a behavior-modification program often does not result in achieving ideal body weight. Even intensive weight-loss programs that include pharmacotherapy are faced with high dropout rates and recidivism. However, even small weight losses of ∼10% of initial body weight have been shown to have significant health benefits by affecting obesity-related comorbid conditions such as hypertension and type 2 DM.17
Pharmacotherapy continues to be investigated as an approach to obesity treatment, but this approach has had varying degrees of success.18 The work of Weintraub et al,19 studying the combination of Phen-Fen for the treatment of obesity, significantly changed medical practice patterns in the 1990s. In this double-blind clinical trial, Phen-Fen 15/60 mg/d resulted in a 14.6-kg (16%) weight loss at 24 weeks compared with the 10.0-kg loss seen with only Phen 15 mg/d and the 7.5-kg loss with Fen 60 mg/d. Many clinicians believed that this combination had synergistic efficacy by affecting both noradrenergic and serotonergic signaling pathways in the hypothalamus.
Phen has been available since the 1960s. Duncan and Munro9 conducted the first long-term, double-blind, placebo-controlled study of Phen in 1968. One hundred eight obese women were assigned to receive placebo, continuous Phen (30 mg), or intermittent Phen (4 weeks with Phen alternating with 4 weeks without Phen) for 36 weeks. Mean body weight loss was significantly greater in patients treated with continuous (12.2 kg) or intermittent Phen (13.0 kg) than with placebo (4.8 kg). AEs were minor, with 8% of the drug-treated patients and 3% of placebo-treated patients dropping out because of perceived stimulant AEs such as agitation or insomnia. Several shorter, double-blind, placebo-controlled studies corroborate the efficacy of Phen observed in the above study. In a 4-month trial by Truant et al,20 mean weight loss in Phen-treated patients (8.8 kg) was consistent with that at 4 months in Phen-treated patients (10.4 kg) found by Duncan and Munro.9 In a study by Willims and Foulsham,11 59 patients with osteoarthritis were treated for 14 weeks with Phen 30 mg or placebo. The patients in the Phen group lost 8.7% of their body weight, compared with 2.0% for the placebo group. Overall, available data suggest that Phen is well tolerated and efficacious.
In our study, Phen was well tolerated, with only mild AEs (eg, insomnia, anxiety) reported. We also found that few patients required dosage adjustment due to AEs. These results are comparable to those seen previously.19
The weakness of our study lies primarily in the design—a nonrandomized, retrospective analysis. There was potential treatment bias because no specific algorithm or clinical criteria were used to guide the decision for therapeutic intervention. Another potential confounder (and weakness) is the concomitant use of VLCD with either Phen or Phen-Fen, which prohibited the direct measurement of the efficacy and impact of the study medications when used alone. VLCD usually is effective in promoting significant short-term weight loss.13,21 Given the effectiveness of VLCD, the ability to detect any differences between Phen and Phen-Fen may have been obscured.
Adherence to dietary treatments such as VLCD is a significant concern,22 and patients in obesity studies have high dropout rates compared with those in studies of other types of clinical interventions. In studies in which adherence is not an important variable, many nonadherent patients can be screened out through run-in periods and other strategies to assess the effectiveness of a given treatment in a compliant patient population. However, the results of such clinical trials cannot necessarily be generalized to the patient population in a typical practice setting.
Our University Obesity Program is an outpatient-based clinical research unit with a free-living population in which patient fees support the clinic. This is the opposite of the usual research study situation, in which research subjects are compensated for their participation. In this sense, it was a selected population, but it is representative of patients participating in many such commercial programs nationally based on VLCD and pharmacotherapy. We had no retention strategies other than offering comprehensive, multidisciplinary treatments for obesity. Thus, this study setting is likely to reflect a typical clinical obesity specialty practice.
Given the recognized potency of VLCD in inducing weight loss,13,21 the present outpatient study did not detect any significant difference between adjunctive uses of Phen pharmacotherapy compared with combination Phen-Fen pharmacotherapy when used with VLCD over 12 weeks. This finding is relevant to present-day practice. Although fenfluramine is no longer available, VLCD and Phen are still available treatment options. This study provides encouraging data with regard to the efficacy of this combination, but longer-term studies are needed to establish this combination as safe and effective for weight loss.
Conclusions
This outpatient study did not detect any significant difference between adjunctive uses of Phen compared with Phen-Fen pharmacotherapy when used with VLCD over 12 weeks. Because we found no significant differences in weight loss with Phen alone compared with Phen-Fen in either men or women, we conclude that Phen can be used to achieve significant weight loss when combined with VLCD. The tolerability and positive physical response further suggest that Phen is a valuable medication for obesity management in the outpatient setting.
Acknowledgements
Morton H. Maxwell, MD (deceased) provided much of the inspiration for this research and established the UCLA outpatient University Obesity Center with one of the authors, David Heber, MD, PhD.
References
- 1.Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Calle E.E., Thun M.J., Petrelli J.M. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 3.Manson J.E., Willett W.C., Stampfer M.J. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer F.X. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 5.National Task Force on the Prevention and Treatment of Obesity Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 6.Bray G.A., Greenway F.L. Current and potential drugs for treatment of obesity. Endocr Rev. 1999;20:805–875. doi: 10.1210/edrv.20.6.0383. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub M. Long-term weight control: The National Heart, Lung, and Blood Institute funded multimodal intervention study. Clin Pharmacol Ther. 1992;51:581–585. doi: 10.1038/clpt.1992.68. [DOI] [PubMed] [Google Scholar]
- 8.Seghatol F.F., Rigolin V.H. Appetite suppressants and valvular heart disease. Curr Opin Cardiol. 2002;17:486–492. doi: 10.1097/00001573-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Duncan L.J., Munro J.F. Current therapeutics. CCXLI. The present status of anorexiant drugs. Practitioner. 1968;200:167–173. [PubMed] [Google Scholar]
- 10.Langlois K.J., Forbes J.A., Bell G.W., Grant G.F., Jr. A double-blind clinical evaluation of the safety and efficacy of phentermine hydrochloride (Fastin) in the treatment of exogenous obesity. Curr Ther Res Clin Exp. 1974;16:289–296. [PubMed] [Google Scholar]
- 11.Willims R.A., Foulsham B.M. Weight reduction in osteoarthritis using phentermine. Practitioner. 1981;225:231–232. [PubMed] [Google Scholar]
- 12.Wadden T.A., Sternberg J.A., Letizia K.A. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: A five-year perspective. Int J Obes. 1989;13(Suppl 2):39–46. [PubMed] [Google Scholar]
- 13.Saris W.H. Very-low-calorie diets and sustained weight loss. Obes Res. 2001;9(Suppl 4):295S–301S. doi: 10.1038/oby.2001.134. [DOI] [PubMed] [Google Scholar]
- 14.Jakicic J.M., Gallagher K.I. Exercise considerations for the sedentary, overweight adult. Exerc Sport Sci Rev. 2003;31:91–95. doi: 10.1097/00003677-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard C. Current understanding of the etiology of obesity: Genetic and nongenetic factors. Am J Clin Nutr. 1991;53(Suppl 6):1561S–1565S. doi: 10.1093/ajcn/53.6.1561S. [DOI] [PubMed] [Google Scholar]
- 16.Van Itallie T.B. “Morbid” obesity: A hazardous disorder that resists conservative treatment. Am J Clin Nutr. 1980;33(Suppl 2):358–363. doi: 10.1093/ajcn/33.2.358. [DOI] [PubMed] [Google Scholar]
- 17.Henry R.R., Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–823. doi: 10.2337/diacare.14.9.802. [DOI] [PubMed] [Google Scholar]
- 18.Glazer G. Long-term pharmacotherapy of obesity 2000: A review of efficacy and safety. Arch Intern Med. 2001;161:1814–1824. doi: 10.1001/archinte.161.15.1814. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub M., Hasday J.D., Mushlin A.I., Lockwood D.H. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch Intern Med. 1984;144:1143–1148. [PubMed] [Google Scholar]
- 20.Truant A.P., Olon L.P., Cobb S. Phentermine resin as an adjunct in medical weight reduction: A controlled, randomized, double-blind prospective study. Curr Ther Res Clin Exp. 1972;14:726–738. [PubMed] [Google Scholar]
- 21.National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health Very low-calorie diets. JAMA. 1993;270:967–974. [PubMed] [Google Scholar]
- 22.Torgerson J.S., Agren L., Sjostrom L. Effects on body weight of strict or liberal adherence to an initial period of VLCD treatment. A randomised, one-year clinical trial of obese subjects. Int J Obes Relat Metab Disord. 1999;23:190–197. doi: 10.1038/sj.ijo.0800816. [DOI] [PubMed] [Google Scholar]


