Abstract
Background
The role of platelets in acute cardiovascular atherothrombotic events has been well established and attention has focused on platelet inhibition therapy. Clopidogrel is a novel thienopyridine inhibitor of adenosine diphosphate–induced platelet activation. Recent studies have shown that in the setting of coronary angioplasty/stenting, a loading dose of 300 mg followed by 75 mg once daily is required for optimum benefit.
Objective
This study assessed the bioequivalence and tolerability of 2 oral formulations of clopidogrel 75-mg tablets.
Methods
This 10-day, open-label, randomized, parallel-group, comparative bioequivalence and tolerability study was carried out in the Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences (Hyderabad, India). Young healthy male volunteers were enrolled. Subjects were randomized to receive one of two 75-mg tablet formulations of clopidogrel (Clopivas® [test formulation] or Plavix® [reference formulation]). Subjects first received a 300-mg loading dose (four 75-mg tablets) on day 1, followed by 75 mg (1 tablet) at 8:00 AM daily for the next 6 days. Inhibition of platelet aggregation, which is the pharmacologic basis for the therapeutic efficacy of antiplatelet agents, and the effect on bleeding time were used as the pharmacodynamic assessment criteria. Pharmacodynamic variables included mean of maximum activity of percentage of inhibition of platelet aggregation (Emax), mean time to reach Emax (tmax), and mean area under the activity–time curve from time 0 to 168 hours (AUC0–168). Tolerability assessments included blood pressure and heart rate measurements before and at regular intervals (every hour for 12 hours and then at 24 hours) over a 24-hour period after drug administration. Clinical tolerability was assessed using adverse effects, platelet count (assessed on days 3, 6, and 10 after first-dose administration), and neutrophil count (assessed on day 10 after first-dose administration).
Results
Twenty subjects were enrolled (mean [SD] age, 26.5 [2.9] years [range, 22–32 years]). Emax, tmax, and AUC0–168 were similar between the 2 groups, as was bleeding time. The 90% CIs were within the bioequivalence acceptance range of 80% to 125%. One subject (10%) in the Plavix group experienced mild headache; no serious adverse effects were reported, and none of the subjects dropped out due to an adverse effect. Platelet and neutrophil counts were found to be within normal limits.
Conclusions
In this study of healthy male volunteers, the 2 tablet preparations of clopidogrel showed bioequivalence. However, the sample size was smaller than that generally recommended for a bioequivalence study, and additional studies with larger sample sizes are needed.
Keywords: ADP-receptor antagonist, clopidogrel, platelet aggregation, bleeding time, bioequivalence
Introduction
The role of platelets in acute cardiovascular atherothrombotic events has been well established and attention has focused on platelet inhibition therapy.1,2 The use of antiplatelet agents in the management of acute atherothrombotic events in cardiovascular disease has become widely accepted.3–5
Clopidogrel is a novel thienopyridine inhibitor of adenosine diphosphate (ADP)–induced platelet activation.6 It blocks the activation of platelets by ADP by selectively and irreversibly inhibiting the binding of the agonist to its receptor on the platelet, thereby affecting ADP-dependent activation of the fibrinogen receptor present on the platelet surface.7 This observation shows the major role of ADP in the platelet aggregation process8–10 and also emphasizes the therapeutic value of ADP-specific antiplatelet drugs.
Studies in healthy subjects11,12 and in patients with atherosclerotic disease13 have shown that clopidogrel at the therapeutic dose of 75 mg is capable of inducing a clinically significant inhibition of ADP-induced platelet aggregation as early as 2 hours after the first dose, while its steady-state effect is achieved only between 3 and 7 days of repeated dosing. However, 2 single-dose studies have shown that levels of inhibition of platelet aggregation, which are similar to those observed at steady state with repeated 75-mg doses of clopidogrel, are reached 2 to 5 hours after a single 400-mg dose.11 Recent studies have demonstrated that in the setting of coronary angioplasty/stenting, a loading dose of 300 mg followed by 75 mg once daily is required for optimum benefit.14,15
The objective of the present study was to assess the bioequivalence and tolerability of 2 oral formulations of clopidogrel 75-mg tablets in young healthy male volunteers.
Subjects and methods
Study design
This 10-day, open-label, randomized, parallel-group, comparative bioequivalence and tolerability study was carried out in the Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences (Hyderabad, India). The study protocol was approved by the institutional ethics committee. Only men were allowed to participate according to the regulatory and institutional review board. The study was conducted in compliance with the principles of the Declaration of Helsinki and its amendments.
Subjects
Healthy male volunteers aged 18 to 40 years were enrolled. All subjects were disease free, had normal resting electrocardiography results, had normal vital signs, did not show any abnormal platelet aggregation or abnormal bleeding time, and had normal results on routine laboratory tests. Laboratory tests included complete hematology, urinalysis, plasma glucose measurement, blood urea nitrogen, serum creatinine, alanine and aspartate aminotransferases, alkaline phosphatase, serum bilirubin, serum cholesterol, HIV, and hepatitis B surface antigen. They had not taken any medication within the 2 weeks preceding the study, and they provided written informed consent before inclusion in the trial.
Methods
After an overnight fast (12 hours), subjects were randomly assigned to 1 of 2 parallel treatment groups (Clopivas®∗ [test formulation] or Plavix®† [reference formulation]). Randomization was done using a block size of 4, in which 2 of the subjects received Clopivas and the other 2 received Plavix. Treatments were given with 240 mL of water per a prerandomization schedule. Both the groups received a loading dose of study drug, 300 mg (4 tablets of 75 mg) on day 1, followed by 75 mg (1 tablet) at 8:00 am daily for the next 6 days. During the 24 hours after drug administration, strenuous physical activity or mental activity (eg, mathematics, accounting, or studying for an examination) was not permitted. The subjects were served standard breakfast, lunch, snacks, and dinner at 4, 6, 9, and 12 hours after drug administration.
Bioequivalence assessment
All bioequivalence parameters were assessed before the first dose and at 2, 5, 12, 24 (prior to dosing), 48 (prior to dosing), 72 (prior to dosing), 120 (prior to dosing), and 168 hours after study drug administration. The laboratory staff estimating platelet aggregation and bleeding time were blinded to treatment assignment.
Percentage of platelet aggregation was assessed within 2 hours of sample collection according to the Born method on platelet-rich plasma prepared from citrated blood samples.16 Maximum percentage of platelet aggregation induced by 5 μmol ADP was recorded. For the purposes of accuracy, all samples were analyzed in duplicate.
The inhibition of platelet aggregation at each time point was expressed as the percentage of change from baseline in maximum percentage of inhibition of platelet aggregation (Emax). The difference in the least squares mean (LSM) ratio between the 2 drugs for Emax and area under the activity–time curve from time 0 to 168 hours (AUC0–168) for Emax was considered bioequivalent if the difference was <20%.
Bleeding time was measured by laboratory technicians using a modified Ivy-Nelson technique.17 The difference in bleeding time was considered significant if a difference of >1.5-fold was found between the 2 formulations.
Tolerability assessment
Tolerability assessments included blood pressure and heart rate measurements recorded by a clinical research assistant before and at regular intervals (every hour for 12 hours and then at 24 hours) over a 24-hour period after drug administration.
Clinical tolerability was monitored by a clinical research assistant using patient interview and an adverse drug reaction checklist throughout the study period, and the incidence of any adverse effects was recorded. Platelet count was assessed on days 3, 6, and 10 after first-dose administration, and neutrophil count was assessed on day 10 after first-dose administration.
Statistical analysis
Analysis of variance and the Student t test were used to evaluate the statistical significance of differences between the 2 formulations. P≤0.05 was considered statistically significant. LSM ratio 90% CIs between 80% and 125% were considered acceptable for bioequivalence.18 Analysis was performed using Prism version 2 (GraphPad Software, Inc., San Diego, California).
Results
Subjects
Twenty subjects were enrolled (10 in each study group) (mean [SD] age, 26.5 [2.9] years [range, 22–32 years]). The mean (SD) age of the subjects was 26.5 (2.9) and 25.6 (4.0) years, mean (SD) height was 160.3 (6.9) and 165.6 (5.5) cm, and mean (SD) body weight was 56.2 (6.1) and 61.5 (7.2) kg in the Clopivas and Plavix groups, respectively (Table I).
Table I.
Demographic characteristics of the study subjects (N = 20).
| Clopivas®∗ |
Plavix®† |
|||||
|---|---|---|---|---|---|---|
| Subject No. | Age, y | Height, cm | BodyWeight, kg | Age, y | Height, cm | BodyWeight, kg |
| 1 | 28 | 171 | 68 | 30 | 166 | 70 |
| 2 | 25 | 168 | 62 | 23 | 170 | 72 |
| 3 | 28 | 168 | 50 | 23 | 175 | 64 |
| 4 | 29 | 154 | 59 | 21 | 159 | 60 |
| 5 | 30 | 154 | 50 | 32 | 165 | 60 |
| 6 | 25 | 156 | 60 | 23 | 164 | 53 |
| 7 | 23 | 158 | 57 | 26 | 161 | 52 |
| 8 | 25 | 153 | 50 | 25 | 172 | 53 |
| 9 | 22 | 165 | 53 | 31 | 158 | 66 |
| 10 | 30 | 156 | 53 | 22 | 166 | 65 |
| Mean | 26.5 | 160.3 | 56.2 | 25.6 | 165.6 | 61.5 |
| SD | 2.9 | 6.9 | 6.1 | 4.0 | 5.5 | 7.2 |
Trademark of Cipla Ltd (Mumbai, India).
Trademark of Sanofi Pharma (Paris, France) and Bristol-Myers Squibb Company (Wallingford, Connecticut).
Bioequivalence
The percentage of inhibition of platelet aggregation that was obtained for each of the 20 subjects with either of the clopidogrel formulations at specified time intervals until the last collection period are shown in Tables II and III and Figure 1. As shown, the mean (SD) percentages of inhibition of platelet aggregation were similar between the groups at 2 hours (34.07 [18.62] and 28.14 [11.47] with Clopivas and Plavix, respectively). At the end of 168 hours, the mean percentages of inhibition of platelet aggregation also were similar between the 2 groups (37.12 [17.65] with Clopivas and 31.78 [18.01] with Plavix).
Table II.
Percentage of inhibition of platelet aggregation with the Clopivas®∗ formulation of clopidogrel (n = 10 subjects).
| Time After Study Drug Administration, h |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject No. | 0 | 2 | 5 | 12 | 24 | 48 | 72 | 120 | 168 |
| 1 | – | 14.1 | 28.9 | 34.2 | 18.4 | 28.9 | 26.3 | 34.2 | 21.0 |
| 2 | – | 25.2 | 42.4 | 34.5 | 20.5 | 29.9 | 39.2 | 34.5 | 46.2 |
| 3 | – | 54.8 | 38.7 | 25.8 | 25.8 | 9.7 | 45.1 | 41.9 | 45.2 |
| 4 | – | 44.8 | 34.4 | 27.6 | 24.1 | 34.5 | 41.3 | 48.3 | 17.2 |
| 5 | – | 25.0 | 37.5 | 27.5 | 50.0 | 45.0 | 45.0 | 40.0 | 45.0 |
| 6 | – | 54.5 | 63.6 | 52.3 | 55.7 | 56.8 | 60.2 | 57.9 | 62.5 |
| 7 | – | 54.8 | 67.7 | 41.9 | 51.6 | 48.4 | 35.5 | 35.5 | 27.4 |
| 8 | – | 0.0 | 15.4 | 0.0 | 7.7 | 28.2 | 43.6 | 20.5 | 7.7 |
| 9 | – | 31.9 | 48.9 | 38.3 | 44.7 | 57.4 | 57.4 | 55.3 | 53.2 |
| 10 | – | 35.6 | 42.4 | 35.6 | 25.4 | 44.1 | 42.4 | 42.4 | 45.8 |
| Mean | – | 34.07 | 41.99 | 31.77 | 32.39 | 38.29 | 43.60 | 41.05 | 37.12 |
| SD | – | 18.62 | 15.43 | 13.64 | 16.61 | 14.84 | 9.78 | 10.99 | 17.65 |
| Minimum | – | 0.00 | 15.40 | 0.00 | 7.70 | 9.70 | 26.30 | 20.50 | 7.70 |
| Maximum | – | 54.80 | 67.70 | 52.30 | 55.70 | 57.40 | 60.20 | 57.90 | 62.50 |
| Median | – | 33.75 | 40.55 | 34.35 | 25.60 | 39.30 | 43.00 | 40.95 | 45.10 |
Trademark of Cipla Ltd. (Mumbai, India).
Table III.
Percentage of inhibition of platelet aggregation with the Plavix®∗ formulation of clopidogrel (n = 10 subjects).
| Time After Study Drug Administration, h |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject No. | 0 | 2 | 5 | 12 | 24 | 48 | 72 | 120 | 168 |
| 1 | – | 36.9 | 39.1 | 30.4 | 30.4 | 41.3 | 30.4 | 36.9 | 56.5 |
| 2 | – | 17.6 | 28.9 | 12.9 | 20.4 | 26.1 | 26.1 | 31.8 | 34.6 |
| 3 | – | 17.4 | 39.1 | 39.1 | 26.0 | 21.7 | 10.9 | 32.6 | 6.5 |
| 4 | – | 39.0 | 47.4 | 50.8 | 35.6 | 44.0 | 47.4 | 42.4 | 33.9 |
| 5 | – | 7.5 | 27.5 | 35.0 | 35.0 | 15.0 | 42.5 | 30.0 | 45.0 |
| 6 | – | 36.9 | 47.8 | 39.1 | 39.1 | 47.8 | 47.8 | 47.8 | 52.2 |
| 7 | – | 28.2 | 23.1 | 28.2 | 30.8 | 28.2 | 30.8 | 15.4 | 2.6 |
| 8 | – | 41.6 | 36.1 | 33.5 | 36.1 | 41.7 | 38.9 | 50.0 | 38.9 |
| 9 | – | 21.9 | 36.6 | 21.9 | 4.9 | 26.8 | 26.8 | 29.3 | 19.5 |
| 10 | – | 34.4 | 25.0 | 9.4 | 28.1 | 31.2 | 47.0 | 31.2 | 28.1 |
| Mean | – | 28.14 | 35.06 | 30.03 | 28.64 | 32.38 | 34.86 | 34.74 | 31.78 |
| SD | – | 11.47 | 8.76 | 12.57 | 10.00 | 10.77 | 12.01 | 10.10 | 18.01 |
| Minimum | – | 7.50 | 23.10 | 9.40 | 4.90 | 15.00 | 10.90 | 15.40 | 2.60 |
| Maximum | – | 41.60 | 47.80 | 50.80 | 39.10 | 47.80 | 47.80 | 50.00 | 56.50 |
| Median | – | 31.30 | 36.35 | 31.95 | 30.60 | 29.70 | 34.85 | 32.20 | 34.25 |
Trademark of Sanofi Pharma (Paris, France) and Bristol-Myers Squibb Company (Wallingford, Connecticut).
Figure 1.
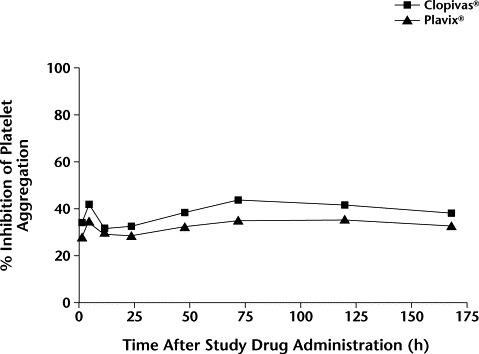
Mean percentage of inhibition of platelet aggregation after administration of Clopivas® (Cipla Ltd., Mumbai, India) and Plavix® (Sanofi Pharma, Paris, France, and Bristol-Myers Squibb Company, Wallingford, Connecticut) (N = 20 subjects). No significant between-group differences were found.
Emax, time to reach Emax (tmax), and AUC0–168 are shown in Table IV. The mean (SD) Emax was 51.2% (9.9%) with Clopivas tablets and 44.3% (8.5%) with Plavix tablets. The mean (SD) tmax was 62.4 (66.8) and 91.0 (74.9) hours with Clopivas and Plavix, respectively. The mean (SD) AUC0–168 with Clopivas and Plavix was 6529.6%/h (1806.7%/h) and 5508.8%/h (1513.0%/h), respectively. None of these differences were statistically significant.
Table IV.
| Emax, % |
tmax, h |
AUC0–168, %/h |
||||
|---|---|---|---|---|---|---|
| Subject No. | Clopivas | Plavix | Clopivas | Plavix | Clopivas | Plavix |
| 1 | 34.2 | 56.5 | 12.0 | 168.0 | 4621.9 | 6336.5 |
| 2 | 46.2 | 34.6 | 168.0 | 168.0 | 5863.0 | 4601.1 |
| 3 | 54.8 | 39.1 | 2.0 | 5.0 | 5992.4 | 3712.5 |
| 4 | 48.3 | 50.8 | 120.0 | 12.0 | 6026.0 | 7069.1 |
| 5 | 50.0 | 45.0 | 24.0 | 168.0 | 7111.3 | 5528.8 |
| 6 | 63.6 | 52.2 | 5.0 | 168.0 | 9763.3 | 7821.7 |
| 7 | 67.7 | 30.8 | 5.0 | 24.0 | 6603.6 | 3595.5 |
| 8 | 43.6 | 50.0 | 72.0 | 120.0 | 3630.8 | 6987.4 |
| 9 | 57.4 | 36.6 | 48.0 | 5.0 | 8867.9 | 4016.4 |
| 10 | 45.8 | 47.0 | 168.0 | 72.0 | 6815.6 | 5418.9 |
| Mean | 51.2 | 44.3 | 62.4 | 91.0 | 6529.6 | 5508.8 |
| SD | 9.9 | 8.5 | 66.8 | 74.9 | 1806.7 | 1513.0 |
| Minimum | 34.2 | 30.8 | 2.0 | 5.0 | 3630.8 | 3595.5 |
| Maximum | 67.7 | 56.5 | 168.0 | 168.0 | 9763.3 | 7821.7 |
| Median | 49.2 | 46.0 | 36.0 | 96.0 | 6314.8 | 5473.8 |
| CV, % | 19.4 | 19.3 | 107.1 | 82.3 | 27.7 | 27.5 |
Emax = maximum percentage of inhibition of platelet aggregation; tmax = time to reach Emax; AUC0–168 = area under the activity–time curve from time 0 to 168 hours; CV = coefficient of variation.
Trademark of Cipla Ltd. (Mumbai, India).
Trademark of Sanofi Pharma (Paris, France) and Bristol-Myers Squibb Company (Wallingford, Connecticut).
No significant between-group differences were found.
The mean values of bleeding time with test and reference clopidogrel formulations are shown in Figure 2. At baseline, the bleeding times in both the groups were found to be comparable. The mean (SD) bleeding times were 139.5 (36.1) and 139.5 (22.4) seconds at the end of 2 hours with Clopivas and Plavix, respectively. A prolongation in bleeding time was observed from 2 to 168 hours. However, the difference in the prolongation of bleeding time between the 2 formulations was nonsignificant.
Figure 2.
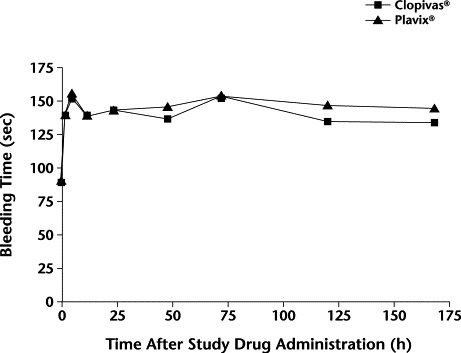
Mean bleeding time after administration of Clopivas® (Cipla Ltd., Mumbai, India) and Plavix® (Sanofi Pharma, Paris, France, and Bristol-Myers Squibb Company, Wallingford, Connecticut) (N = 20 subjects). No significant between-group differences were found.
The LSM ratios for Emax and AUC0–168 for the data were 103.66 and 101.88, respectively, indicating comparable bioavailability of Clopivas and Plavix. The 90% CIs were 98.62 to 109.90 and 99.12 to 105.10, respectively (Table V). These 90% CIs are within the acceptance range for bioequivalence.18
Table V.
Summary statistics data of 2 clopidogrel products in study subjects (N = 20).
| Statistic/Product | Emax, % | tmax, h | AUC0–168, %/h |
|---|---|---|---|
| Geometric mean∗ | |||
| Clopivas®† | 50.28 | 26.47 | 6300 |
| Plavix®‡ | 43.48 | 46.72 | 5318 |
| LSM | |||
| Clopivas | 1.70 | – | 3.80 |
| Plavix | 1.64 | – | 3.73 |
| LSM Clopivas/Plavix ratio, % | 103.66 | – | 101.88 |
| 90% CI, Clopivas vs Plavix | |||
| Lower limit | 98.62 | – | 99.12 |
| Upper limit | 109.90 | – | 105.10 |
| ANOVA summary | NS | – | NS |
Emax = maximum percentage of inhibition of platelet aggregation; tmax = time to reach Emax; AUC0–168 = area under the activity–time curve from time 0 to 168 hours; LSM = least squares mean; ANOVA = analysis of variance.
n=10 for each group.
Trademark of Cipla Ltd. (Mumbai, India).
Trademark of Sanofi Pharma (Paris, France) and Bristol-Myers Squibb Company (Wallingford, Connecticut).
Tolerability
Both formulations were well tolerated by all of the volunteers. The only adverse effect reported was mild and transient headache experienced by 1 subject (10%) in the Plavix group. None of the volunteers experienced serious adverse effects necessitating treatment discontinuation. Platelet and neutrophil counts were found to be within normal limits.
Discussion
The data from this study indicated a significant inhibition of ADP-induced platelet aggregation and prolongation of bleeding time after clopidogrel administration. We observed that a loading dose of 300 mg followed by 75 mg/d of clopidogrel produces rapid onset of pharmacodynamic action, with the level of inhibition of platelet aggregation being close to steady state within 2 hours of clopidogrel dosing. The inhibition of platelet aggregation by clopidogrel is concentration dependent.6 In a similar study in healthy subjects,19 maximum inhibition of 40% to 50% was observed 2 to 5 hours after a single dose of 400 mg clopidogrel. Two previous single-dose studies reported that the inhibition of ADP-induced platelet aggregation by clopidogrel reached a plateau after a dose of 400 mg.11
Bleeding time is a global test for primary hemostasis that involves both vessel wall and platelet function.17 In the absence of vessel wall injury, bleeding time is influenced only by platelet function, and some antiplatelet agents can cause prolongation of bleeding time.16 The Ivy-Nelson technique used in our study has been shown to produce easily quantifiable bleeding times, allowing an accurate assessment of the pharmacologic effect of the drug.17 In the present study, both clopidogrel formulations prolonged bleeding time, and this effect between the 2 treatment groups was comparable. With the 375-mg loading dose regimen of clopidogrel, prolongation of bleeding time was noticed as early as 2 hours.19 The mean bleeding time prolongation factor with respect to baseline did not exceed 2.1, which is in accordance with an earlier report.19
A limitation of the present study was its small sample size, which was smaller than that generally recommended for a bioequivalence study. Hence, the results of the present study require further confirmation by larger studies.
Conclusions
In this study of healthy male volunteers, the 2 tablet preparations of clopidogrel showed bioequivalence. However, the sample size was smaller than that generally recommended for a bioequivalence study, and additional studies with larger sample sizes are needed.
Footnotes
Reproduction in whole or part is not permitted.
Trademark of Cipla Ltd (Mumbai, India).
Trademark of Sanofi Pharma (Paris, France) and Bristol-Myers Squibb Company (Wallingford, Connecticut).
References
- 1.Fuster V., Badimon L., Badimon J.J., Chesebro J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V.M., Badimon L., Badimon J.J., Chesebro J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 3.Antiplatelets Trialists' Collaboration Collaborative overview of randomised trials of antiplatelet therapy—: I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [published correction appears in BMJ. 1994;308:1540] [PMC free article] [PubMed] [Google Scholar]
- 4.Schror K. Antiplatelet drugs. A comparative review. Drugs. 1995;50:7–28. doi: 10.2165/00003495-199550010-00002. [DOI] [PubMed] [Google Scholar]
- 5.CAPRIE Steering Committee A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 6.Mills D.C., Puri R., Hu C.J. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler Thromb. 1992;12:430–436. doi: 10.1161/01.atv.12.4.430. [DOI] [PubMed] [Google Scholar]
- 7.Savi P., Herbert J.M. ADP receptors on platelets and ADP-selective antiaggregating agents. Med Res Rev. 1996;16:159–179. doi: 10.1002/(SICI)1098-1128(199603)16:2<159::AID-MED2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Maffrand J.P., Bernat A., Delebassee D. ADP plays a key role in thrombogenesis in rats. Thromb Haemost. 1988;59:225–230. [PubMed] [Google Scholar]
- 9.Cooper D.R., Lewis G.P., Lieberman G.E. ADP metabolism in vascular tissue, a possible thrombo-regulatory mechanism. Thromb Res. 1979;14:901–914. doi: 10.1016/0049-3848(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 10.Marcus A.J., Safier L.B., Hajjar K.A. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J Clin Invest. 1991;88:1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thebault J.J., Kieffer G., Cariou R. Single-dose pharmacodynamics of clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):3–8. [PubMed] [Google Scholar]
- 12.Thebault J.J., Kieffer G., Lowe G.D. Repeated-dose pharmacodynamics of clopidogrel in healthy subjects. Semin Thromb Hemost. 1999;25(Suppl 2):9–14. [PubMed] [Google Scholar]
- 13.Boneu B. Clopidogrel dose-ranging phase II study in patients with atherosclerotic disease. J Am Coll Cardiol. 1996;27(Suppl A):132A–133A. [Google Scholar]
- 14.Bertrand M.E., Rupprecht H.J., Urban P., the Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS) Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting. Circulation. 2000;102:624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S., Zhao F., Mehta S.R., the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [published correction appears in N Engl J Med. 2001;345:1506] N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 16.Born G.V.P. Quantitative investigations into the aggregation of blood platelets. J Physiol. 1962;162:67–68. [Google Scholar]
- 17.Ivy A.C., Nelson D., Bucher G. The standardization of certain factors in the cutaneous “venostasis” bleeding time technique. J Lab Clin Med. 1941;26:1812–1822. [Google Scholar]
- 18.Guidance for Industry—Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. US Food and Drug Administration, Center for Drug Evaluation and Research; Bethesda, Md: 2002. [Google Scholar]
- 19.Savcic M., Hauert J., Bachmann F. Clopidogrel loading dose regimens: Kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25(Suppl 2):15–19. [PubMed] [Google Scholar]


