Abstract
Background
Amantadine hydrochloride remains an inexpensive means of influenza A prophylaxis, but it is reported to have a high incidence of adverse drug reactions (ADRs) in residents of long-term care facilities (LTCFs) compared with newer, more expensive drugs.
Objective
This study attempted to determine the effects of poor renal function on the rate of ADRs and any other variables on the tolerability of prophylaxis in this population. This would allow a high-risk subset of LTCF residents to be identified before prophylaxis, thus decreasing the risk for ADRs from amantadine.
Methods
In this retrospective case-control study, a course of standardized low-dose (100-mg/d tablets) amantadine prophylaxis was ordered for all 242 residents of Ten Broeck Commons LTCF in Lake Katrine, New York, without influenza A for 14 days. Chart data of residents who developed ADRs (ADR group) were compared with those of a selected group who did not (control group). Residents’ age, sex, renal function (blood urea nitrogen, serum creatinine, and creatinine clearance), dementia diagnosis, and number and classes of medications were compared.
Results
The ADR group comprised 25 residents (21 women, 4 men; mean [SD] age, 84.8 [8.4] years); the control group, 29 residents (23 women, 6 men; mean [SD] age, 85.7 [7.5] years). The development of ADRs was not associated with differences in age, sex, renal function, or any medical condition, including measured, preexisting mental status changes between the groups. The overall risk for ADRs in the 242 residents was highest between days 8 and 14 of prophylaxis (17 residents [7.0%]) compared with the first 7 days (8 residents [3.3%]). Acute confusion was the most common ADR. All ADRs resolved on cessation of treatment.
Conclusions
No preexisting medical condition was statistically associated with an increased risk for ADRs, but an association with the number of days of prophylaxis was observed. By shortening prophylaxis to 7 days, the ADR risk may be lowered to be commensurate with more expensive medications.
Keywords: amantadine, adverse drug reaction, influenza A, nursing home, long-term care facility
Introduction
Eighty percent to 90% of deaths due to influenza A occur in frail individuals aged >65 years, many of whom are residents of long-term care facilities (LTCFs). Vaccination to prevent the disease is strongly recommended for these individuals.1 Although the efficacy of vaccination in preventing influenza A is low (30%–40%), the severity of the illness is decreased with vaccination, with a 50% to 60% reduction in hospitalization and an 85% reduction in mortality rate.2,3
In addition to vaccination, influenza A can be effectively treated with amantadine hydrochloride or rimantadine hydrochloride, and influenza A or B can be treated with zanamivir inhalation or oseltamivir. Prophylaxis of an influenza A outbreak may be achieved with amantadine or rimantadine; use of these antiviral drugs may decrease the incidence of influenza A by 70% to 90% during an epidemic.4 Expert opinion, including that of the American Geriatric Society, recommends all residents be offered one or the other antiviral therapy following an outbreak of influenza A in an LTCF. Furthermore, although the combination of vaccine plus antiviral treatment or prophylaxis is not 100% effective nor always well tolerated, the advantages of therapy outweigh the disadvantages.2,5
Once a case of influenza is identified and confirmed by nasopharyngeal viral culture or rapid viral testing, LTCF residents should be treated with a 3- to 5-day course of antiviral therapy2 or until 1 to 2 days after symptoms have resolved.4 Prophylaxis within that facility should be started within 48 hours; delay of treatment beyond that time reduces the efficacy of therapy. For prophylaxis, between 10 and 14 days2,4,6,7 of treatment is recommended, with 14 days being the standard recommendation.8 Because of the potential for the influenza A virus to develop antiviral drug resistance, 1 author4 recommends either giving a shorter duration of treatment and prophylaxis or giving residents in the same facility rimantadine for 1 purpose and amantadine for the other.
The financial cost of treating LTCF residents who have influenza A ($279.00 per resident in 1997) is substantially higher than that of prophylaxis ($13.75 per resident in 1997),1 with amantadine being the least expensive of the choices ($2.37 per resident for a 7-day course in 1997).7
Adverse drug reactions (ADRs) with amantadine therapy occur in up to 18.6% of LTCF residents compared with <2% with rimantadine.9 The common ADRs are an acute confused state, falls, functional changes, insomnia, dizziness, seizures, and gastrointestinal adverse effects (especially nausea). Despite the tolerability profile, this medication is inexpensive, effective in prophylaxis of influenza A, and generally well tolerated by most LTCF residents. As a result of these ADRs, multiple authors have recommended that the dosage be lowered to ≤100 mg/d, depending on renal function (as measured by diminished creatinine clearance [CrCl]), to decrease central nervous system effects.5,6,9,10 Amantadine is excreted renally, with 90% of it excreted unchanged in the urine.11
Dosage adjustment may be cumbersome and may require regularly updated assessments of CrCl, which may restrict the usefulness of this medication in LTCF practice, where CrCl is often overlooked.12
This study was implemented to determine the effects of poor renal function on the rate of ADRs and any other variables on the tolerability of amantadine prophylaxis in this population. This would allow a high-risk subset of LTCF residents to be identified before prophylaxis, thus decreasing the risk for ADRs from amantadine.
Patients and methods
This retrospective case-control study was performed at Ten Broeck Commons, in a 258-bed LTCF in Lake Katrine, New York. After an outbreak of influenza A in January 2000, all 242 residents without influenza received standardized low-dose prophylaxis with amantadine tablets 100 mg/d for up to 14 days, irrespective of renal function. Data from elderly residents who developed ADRs with amantadine (ADR group) were compared with data from a selected group who did not (control group).
All data (ie, demographic, clinical, and postprophylaxis outcomes) were collected by the author using chart review and a data-collection instrument (Appendix). Data collection was not blinded. Because the dosage was standardized, the variable of interest became renal function. The results of laboratory testing of renal function were recorded if the tests were conducted within 4 weeks of beginning prophylaxis.
Residents with influenza A, confirmed either by laboratory testing or clinical assessment, were not included in the study. Influenza was diagnosed by the presence of a symptom complex of malaise, cough, fever, and coryza and a positive influenza A test. Because this study was a confidential chart review without any requirement for invasive or other tests, informed consent for data inclusion was not considered necessary.
If an ADR was suspected, a registered nurse informed the in-house physician or physician extender. On the clinician’s review of that resident, including the application of a standardized test for likelihood of an ADR (ie, the Naranjo algorithm13), an ADR would be recorded if it met the criteria for a possible or probable ADR.
A sample of residents was selected by choosing every eighth medical record number written on a card from among the record numbers of residents without a suspected ADR. Renal test results (blood urea nitrogen [BUN], serum creatinine concentration, and CrCl) were measured. For each individual, CrCl was calculated from age, sex, body weight, and serum creatinine concentration using the Cockcroft-Gault equation. For both groups, the charts were assessed for numerous variables (ie, age, sex, underlying diagnoses and disabilities, medications, and renal function). Residents with dementia were subclassified into those with mild (Mini Mental State Examination14 [MMSE] score 17–23), moderate (MMSE score 8–16), or severe (MMSE score 0–7) dementia. The MMSE score was documented by a social worker in every chart within 12 months of starting influenza prophylaxis.
Variables between the groups were examined for significant association with the development of ADR with amantadine prophylaxis. These variables were age, sex, renal function (BUN, serum creatinine, and CrCl), dementia diagnosis, and number and classes of medications. The mean time between initiating amantadine prophylaxis and the onset of ADRs was calculated and ADRs were described.
Statistical Analysis
The 2-tailed t test was used for between-group comparisons. The F test was used to determine whether the variances of the 2 populations were equal. All data were analyzed and calculations performed using the Statistical Package for Social Sciences version 10 (SPSS Inc., Chicago, Illinois). P≤0.05 was considered statistically significant.
Results
A total of 242 residents received amantadine, of whom 217 did not develop a suspected ADR. A sample of 29 residents was selected from these 217 (control group; 23 women, 6 men; mean [SD] age, 85.7 [7.5] years). The 25 residents who had ≥1 suspected ADR composed the ADR group (21 women, 4 men; mean [SD] age, 84.8 [8.4] years). No statistically significant between-group differences for age or sex were found. Both groups were distributed throughout the 6 units of the facility.
Renal test results (BUN, serum creatinine, and CrCl) were available for 21 residents (84.0%) in the ADR group and 20 (69.0%) in the control group. When the renal function of both groups was compared using the F test or t test, no significant differences were found between groups (Tables I–III).
Table I.
Demographic and clinical characteristics of the study patients (N = 54).∗ (All values are expressed as mean [SD] unless otherwise indicated.)
| Parameter/Group | n | Mean (SD) | SE |
|---|---|---|---|
| Age, y | |||
| ADR | 25 | 84.8 (8.4) | 1.685 |
| Control | 29 | 85.7 (7.5) | 1.392 |
| Sex, no. (%) of patients | |||
| ADR | 25 | ||
| Women | 21 (84.0) | – | |
| Men | 4 (16.0) | – | |
| Control | 29 | ||
| Women | 23 (79.3) | – | |
| Men | 6 (20.7) | – | |
| White race, no. (%) of patients | |||
| ADR | 25 | 25 (100.0) | – |
| Control | 29 | 29 (100.0) | – |
| Body weight, kg | |||
| ADR | 25 | 63.0 (16.0) | 7.108 |
| Control | 29 | 58.5 (11.2) | 4.611 |
| BUN, mg/dL | |||
| ADR | 21 | 26.6 (13.3) | 2.910 |
| Control | 20 | 27.0 (13.6) | 3.033 |
| Serum creatinine, mg/dL | |||
| ADR | 21 | 1.4 (1.5) | 0.326 |
| Control | 20 | 1.4 (1.5) | 0.343 |
| CrCl, mL/min | |||
| ADR | 21 | 42.0 (20.2) | 4.414 |
| Control | 20 | 42.0 (22.3) | 4.994 |
ADR = adverse drug reaction; BUN = blood urea nitrogen; CrCl = creatinine clearance.
No significant between-group differences were found.
Table II.
F scores∗ and P values between the adverse-drug-reaction and control groups for baseline demographic and clinical characteristics.
| Characteristic | F | P† |
|---|---|---|
| Age | 0.530 | 0.473 |
| Body weight | 1.089 | 0.301 |
| BUN | 0.011 | 0.919 |
| Serum creatinine | 0.008 | 0.927 |
| CrCl | 0.541 | 0.466 |
BUN = blood urea nitrogen; CrCl = creatinine clearance.
The F test identifies equality of variances. The null hypothesis is that there is no difference in variances between the 2 groups.
P<0.05; null hypothesis is accepted.
Table III.
t Test scores and P values between the adverse-drug-reaction (ADR) and control groups for baseline demographic and clinical characteristics.∗
| Characteristic | t | P† |
|---|---|---|
| Age | ||
| ADR | −0.429 | 0.670 |
| Control | −0.425 | 0.672 |
| Body weight | ||
| ADR | 1.212 | 0.231 |
| Control | 1.181 | 0.244 |
| BUN | ||
| ADR | −0.091 | 0.928 |
| Control | −0.091 | 0.928 |
| Serum creatinine | ||
| ADR | −0.014 | 0.989 |
| Control | −0.014 | 0.989 |
| CrCl | ||
| ADR | −0.002 | 0.998 |
| Control | −0.002 | 0.998 |
BUN = blood urea nitrogen; CrCl = creatinine clearance.
The t test identifies a difference in the 2 samples; null hypothesis is that there is no difference between the samples. The 2-tailed test is applied to 2 populations that may differ.
P<0.05; no result approaches this level; null hypothesis is accepted.
Based on the findings using the data-collection instrument, with the exception of age, body weight, BUN, serum creatinine, and ClCr, the other variables (sex, dementia diagnosis, medical diagnoses, number and classes of medications) occurred too infrequently in both groups to warrant further statistical analysis. No significant differences were observed between the groups for the variables reviewed using the data-collection instrument. Differences were observed between the 2 groups for residents receiving antiparkinsonian medications (6 [24.0%] in the ADR group; 2 [6.9%] in the control group). The numbers of residents receiving central nervous system–active medications are shown in Table IV.
Table IV.
No. (%) of patients receiving central nervous system (CNS)–active medications in the adverse-drug-reaction (ADR) and control groups.∗†
| Drug/Class | ADR (n = 25) | Control (n = 29) |
|---|---|---|
| Carbidopa and levodopa | 6 (24.0) | 2 (6.9) |
| Selective serotonin reuptake inhibitors | 4 (16.0) | 3 (10.3) |
| Antipsychotics | 3 (12.0) | 4 (13.8) |
| Tricyclic antidepressants | 2 (8.0) | 2 (6.9) |
| Phenytoin | 1 (4.0) | 2 (6.9) |
| Benzodiazepines | 1 (4.0) | 1 (3.4) |
| Narcotics | 1 (4.0) | 1 (3.4) |
| Gabapentin | 1 (4.0) | 0 (0.0) |
| Valproic acid | 0 (0.0) | 1 (3.4) |
Some patients were receiving >1 CNS drug.
No significant between-group differences were found.
The most common ADRs in the ADR group were an acute confused state (23 patients [92.0%]), hallucinations and delusions (9 patients [36.0%]), and falls (8 patients [32.0%]). Applying the Naranjo algorithm,13 18 residents (72.0%) were categorized as having a possible ADR (score, 1–4) (8 residents [32.0%] scored 2, 1 [4.0%] scored 3, 9 [36.0%] scored 4), and 7 were categorized as having a probable ADR (score, 5–8) (all 7 [28.0%] scored 5). The mean (SD) time to onset of ADRs in this sample was 8.3 (2.5) days (range, 2–13 days) (Figure). The overall risk for ADRs in the 242 LTCF residents was highest between days 8 and 14 of prophylaxis (17 residents [7.0%]) compared with the first 7 days (8 residents [3.3%]). All ADRs resolved on cessation of treatment.
Figure.
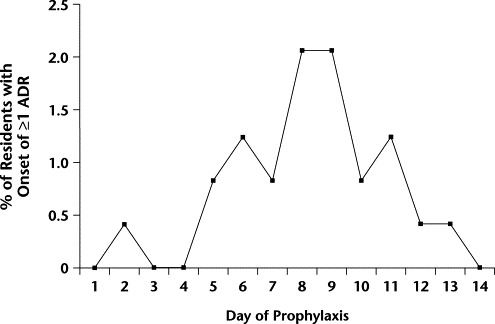
Time to onset of adverse drug reactions (ADRs) (mean [SD], 8.3 [2.5] days; range, 2–13 days) in long-term care facility residents receiving influenza A prophylaxis with amantadine 100 mg/d for up to 14 days.
Discussion
A large number of variables were considered in this study, the majority of which could not be assessed statistically due to the small number of study participants. Doubling the size of the control group, which would have been possible, may have demonstrated the beginning of a clinical trend; however, without a commensurate increase in the size of the ADR group, a more powerful comparison may have been difficult to make.
The observation of no significant differences between the 2 groups with respect to renal function was surprising and contradicts established data.5,6,9–12 Laboratory data obtained at the beginning or during the course of prophylaxis might have revealed more information than the laboratory data used in this study because they were obtained up to 4 weeks before prophylaxis. Unfortunately, laboratory data were acceptable in only a subset of residents in each group. While this highlights the reality of a community LTCF, with its limited medical resources, the lack of more laboratory data weakens the significance of the results. This argument also applies to the MMSE. Aside from the fact that the test was sometimes administered months before the prophylaxis was begun, some residents were unable to complete this test accurately due to visual, auditory, or physical limitations. It is possible that subtle mental status changes due to amantadine prophylaxis were missed.
The emphasis on obtaining renal function prior to starting prophylaxis and adjusting the dose of amantadine accordingly may not be as important as previously realized in this population. If the dose of amantadine is kept low (100 mg/d), other renal factors may contribute to the development of ADRs. Evidence from rat studies15,16 suggests that the renal metabolism of amantadine is dependent on short-term bicarbonate concentrations within the renal tubules. In these studies, with higher concentrations of bicarbonate or lactate, transport inhibition of amantadine occurred, decreasing excretion and increasing the risk for toxicity with this medication.
Of the 8 residents receiving antiparkinsonian medications, 6 had ADRs (primarily falls) to amantadine. Although this evidence is inconclusive, individuals with Parkinson's disease (PD) may be at high risk for ADRs with influenza prophylaxis. Because amantadine is used to treat immobility in patients with PD, these patients may have had more opportunity to fall. A larger study of patients with PD would be necessary to confirm these observations.
The overall incidence of ADRs observed in this study (10.3% of 242 LTCF residents) compares favorably with data in previous studies, which demonstrated a range of 6.3%17 to 18.6%9 in LTCF residents.
In every case in this study, ADRs resolved on cessation of prophylaxis. In keeping with other studies,10 acute confusion and psychosis were the most common ADRs, followed by falls. Had amantadine been reintroduced after the suspected ADRs resolved, higher scores on the Naranjo algorithm might have been achieved, objectively reinforcing the observation that the symptom was an ADR. However, given the high risks associated with confusion and falling and resulting injuries, a reintroduction of prophylaxis was not considered appropriate in this population. Previous observations6 suggest that most adverse effects develop mainly in the first week of treatment, whereas most ADRs in the present study developed in the second week. This observation may be explained by the prolonged half-life of amantadine in elderly people, especially those with impaired renal function. The half-life may be extended from 2 days in patients with mild impairment to 7 to 10 days in those with severe impairment.18 Therefore, steady-state drug levels may not be achieved until late in the course of therapy, at which time ADRs would likely become more common.
Had the course of amantadine been limited to 7 days, we may have seen ADRs in only 8 residents rather than 25 (Figure). In that case, the rate of ADRs would have been only 3.3% (8/242 residents), which is a similar rate to that obtained with rimantadine.9 Lower ADR rates also may have been achieved if dosing intervals had been 48 to 72 hours instead of 24 hours. In this population, adequate antiviral prophylaxis may be achieved either by reducing the dosage or increasing the dosing interval.18 As a result of the prolonged half-life, sufficient drug may remain to provide antiviral prophylaxis for an extended period after the last dose of a 7-day course. To date, no good data are available to substantiate either a 7-day course of prophylaxis or extended dosing intervals.
Multivariate analysis of the results was not possible because of the small number of study participants. Consequently, only univariate analysis could be performed, limiting the results to showing only a potential association. A prospective and substantially larger study is required to confirm the findings of this small retrospective study.
Conclusions
No preexisting medical condition was statistically associated with an increased risk for ADRs, but an association with the number of days of prophylaxis was observed. By shortening prophylaxis to 7 days, the ADR risk may be lowered to be commensurate with more expensive medications.
Acknowledgements
Special thanks to HlaPe Win, MD, MPH, for his guidance with the statistical analysis, and to Angelo Angerame, RPh, for his advice.
Footnotes
Reproduction in whole or part is not permitted.
APPENDIX. Data Collection Table ADR Non-ADR Medical Record #
| Demographic | |
| Age | |
| Sex M/F | |
| Race W/B/other | |
| Comments: | |
| Disease | |
| CVA: L/R | CHF/atrial fibrillation |
| Dehydration | CAD/hypertension |
| Dementia: mild/moderate/severe | IDDM/NIDDM |
| Depression | UTI/pneum |
| Psychosis | COPD/asthma |
| Parkinson’s/MS | GI |
| Thyroid | Hematologic |
| Renal/BPH | Fracture |
| Cancer | OA/RA/other |
| Skin | |
| Medications | |
| Diuretic | Antidiabetic |
| Antihypertensive | Thyroxine |
| Antipsychotic | Antiulcer |
| Antidepressant | Platelet inhibitor |
| Steroid | Antiseizure |
| Antianginal | Antiparkinsonian |
| Laxative | Narcotic |
| Antibiotic | Benzodiazepine |
| Total medications | |
| Laboratory Tests | |
| BUN | |
| Creatinine | |
| CrCl | |
| Body weight | |
| Function | |
| Weight loss 1 mo 6 mo | |
| Visual loss | |
| Hearing loss | |
| Incontinence: urinary/bowel | |
| Dysphagia | |
| Constipation | |
| Pain: mild/moderate/severe | |
| If ADR: | |
| Days to ADR | |
| Which ADR? | |
| Action taken: | |
| Recurrence? |
ADR = adverse drug reaction; M = male; F = female; W = white; B = black; CVA = cerebrovascular accident; L = left; R = right; MS = multiple sclerosis; BPH = benign prostatic hypertrophy; CHF = congestive heart failure; CAD = coronary artery disease; IDDM = insulin-dependent diabetes mellitus; NIDDM = non–insulin-dependent diabetes mellitus; UTI = urinary tract infection; pneum = pneumonia; COPD = chronic obstructive pulmonary disease; GI = gastrointestinal; OA = osteoarthritis; RA = rheumatoid arthritis; BUN = blood urea nitrogen; CrCl = creatinine clearance.
References
- 1.McLure KL. Economic impact of influenza in LTCF. Presented at the 29th American Society of Consultant Pharmacists Annual Meeting; Seattle, Wash; November 11–14, 1999.
- 2.Campbell G, Graham-Robinson N. Amantadine for influenza. Choosing the correct dosage for elderly patients. Pharmacy Connects. 1999:1–7. http://members.pharmacyconnects.com/content/phpractice/1999/01-99/php019905.html Available at: Accessed October 27, 2003. [Google Scholar]
- 3.Kingston B.J, Wright C.V., Jr. Influenza in the nursing home. Am Fam Physician. 2002;65:75–78. [PubMed] [Google Scholar]
- 4.McLure KL. Influenza management: Prevention, prophylaxis and treatment. Presented at the 29th American Society of Consultant Pharmacists Annual Meeting; Seattle, Wash; November 11–14, 1999.
- 5.AGS Position Statement. Prevention and treatment of influenza in the elderly. September 1996. Updated 1999. Available at: http://www.americangeriatrics.org/products/positionpapers/influe96.html. Accessed October 27, 2003.
- 6.Tamblyn S.E. Amantadine use in influenza outbreaks in long-term care facilities. CMAJ. 1997;157:1573–1576. [PMC free article] [PubMed] [Google Scholar]
- 7.Drinka P.J, Gravenstein S, Schilling M. Duration of antiviral prophylaxis during nursing home outbreaks of influenza A: A comparison of 2 protocols. Arch Intern Med. 1998;158:2155–2159. doi: 10.1001/archinte.158.19.2155. [DOI] [PubMed] [Google Scholar]
- 8.Gomolin I.H, Kathpalia R.K. Influenza. How to prevent and control nursing home outbreaks. Geriatrics. 2002;57:28–30. 33–34. [PubMed] [Google Scholar]
- 9.Keyser L.A, Karl M, Nofziger A.N, Bertino J.S., Jr. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med. 2000;160:1485–1488. doi: 10.1001/archinte.160.10.1485. [DOI] [PubMed] [Google Scholar]
- 10.Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2000;49(RR03):1–38. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4903a1.htm. Available at: Accessed October 27, 2003. [Google Scholar]
- 11.Macchio G.J, Ito V, Sahgal V. Amantadine-induced coma. Arch Phys Med Rehabil. 1993;74:1119–1120. doi: 10.1016/0003-9993(93)90072-i. [DOI] [PubMed] [Google Scholar]
- 12.Papaioannou A, Clarke J.A, Campbell G, Bédard M. Assessment of adherence to renal dosing guidelines in long-term care facilities. J Am Geriatr Soc. 2000;48:1470–1473. doi: 10.1111/j.1532-5415.2000.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 13.Naranjo C.A, Busto U, Sellers E.M. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 14.Folstein M.F, Folstein S.E, McHugh P.R. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Bobby B, Sitar D.S. The effect of lactate on sex differences in rat renal tubular energy-dependent transport of the organic cation amantadine. Pharmacology. 2001;62:188–192. doi: 10.1159/000056093. [DOI] [PubMed] [Google Scholar]
- 16.Goralski K.B, Smyth D.D, Sitar D.S. In vivo analysis of amantadine renal clearance in the uninephrectomized rat: Functional significance of in vitro bicarbonate-dependent amantadine renal tubule transport. J Pharmacol Exp Ther. 1999;290:496–504. [PubMed] [Google Scholar]
- 17.Bowles S.K, Kennie N, Ruston L. Influenza outbreak in a long-term care facility: Considerations for pharmacy. Am J Health Syst Pharm. 1999;56:2303–2307. doi: 10.1093/ajhp/56.22.2303. [DOI] [PubMed] [Google Scholar]
- 18.Amantadine. In: USP DI. Drug Information for the Health Care Provider. Rockville, Md: 1999:1–13.


