Abstract
Accumulating evidence indicates that psychological stress can have deleterious influences on cancer development and progression, but the mechanisms responsible remain unclear. One possible mechanism is suggested by emerging evidence that DNA damage is increased by exposure to stress and stress hormones (for example, cortisol, catecholamines). Possible molecular mechanisms for such effects were the subject of a recent paper by Hara and colleagues, which suggests that chronic stress, through β-adrenergic stimulation, can induce two synergistic pathways that result in accumulation of DNA damage. Herein, we discuss the potential implications of these findings for breast cancer etiology, progression, and treatment response.
Background
Evidence that psychological stress can contribute to the development and progression of cancer has accumulated over the past several decades, but the mechanisms responsible remain obscure [1-5]. A comprehensive meta-analysis of 165 longitudinal studies concluded that psychosocial factors are associated with higher incidence, poorer survival and increased mortality [6]. Psychological stress activates the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, resulting in systemic increases in cortisol and catecholamines. The effects of catecholamines are mediated by nine distinct α-adrenergic and β-adrenergic G-protein-coupled receptors, which are present on a wide range of cell types, including cancer cells [7].
One underappreciated possible mechanism for stress effects on cancer is suggested by emerging evidence that DNA damage is increased by exposure to stress and stress hormones [8,9]. Indeed, a recent report has documented that β-adrenergic stimulation can cause DNA damage sufficient to promote transformation and tumorigenicity of mouse 3T3 cells [10]. Currently, however, little is known about the molecular pathways responsible for stress-induced DNA damage. A recent report in Nature has provided evidence that accumulation of DNA damage following chronic adrenergic stimulation (for example, as a result of chronic stress) may be the result of synergistic effects of β-adrenergic stimulation on two molecular pathways - one directly leading to DNA damage, the other leading to a reduction in p53 levels [11].
The article
Using a mouse model, Hara and colleagues simulated chronic stress by prolonged pharmacological stimulation of β2-receptors with the β-adrenergic agonist isoproterenol, a synthetic analogue of adrenaline [11]. The investigators first demonstrated that chronic catecholamine stimulation leads to phosphorylation of histone H2AX, one of the earliest indicators of DNA damage.
Using a specific β2-antagonist, they then determined that the increased DNA damage occurred as a result of nuclear export and p53 degradation through receptor-specific mechanisms. Murine double minute 2 (MDM2) plays an important role in p53 nuclear export and degradation. In light of this fact, the authors were able - using a combination of receptor antagonists and phosphoinositide 3-kinase and AKT inhibitors - to demonstrate that β2-adrenoreceptor activation resulted in MDM2 phosphorylation via the phosphoinositide 3-kinase/AKT cascade and accumulation of DNA damage in wild-type mouse embryonic fibroblasts. They further found that arrestin beta 1 (ARRB1), which functions as an E3 ligase adaptor for MDM2 and p53, facilitated catecholamine-induced p53 degradation by MDM2. β-adrenergic stimulation of the phosphoinositide 3-kinase/AKT cascade can be stimulated by both the Gs-protein kinase A and the β-arrestin-mediated signaling pathways. Through the application of H-89, a protein kinase A inhibitor, and use of ARRB1-knockout or ARRB2-knockout mouse embryonic fibroblasts, the authors demonstrated that chronic catecholamine stimulation leads to accumulation of DNA damage by an ARRB1-dependent and p53-dependent mechanism.
In summary, Hara and colleagues provided evidence that catecholamines can act through two pathways: via Gs-protein kinase A, and via ARRB1 inducing DNA damage [11]. Both pathways are activated as a result of stimulation of β2-adrenoreceptors, with the ARBB1 facilitating AKT-mediated activation of murine double minute, which in turn promotes MDM2 to bind and degrade p53, leading to the accumulation of DNA damage (Figure 1).
Figure 1.
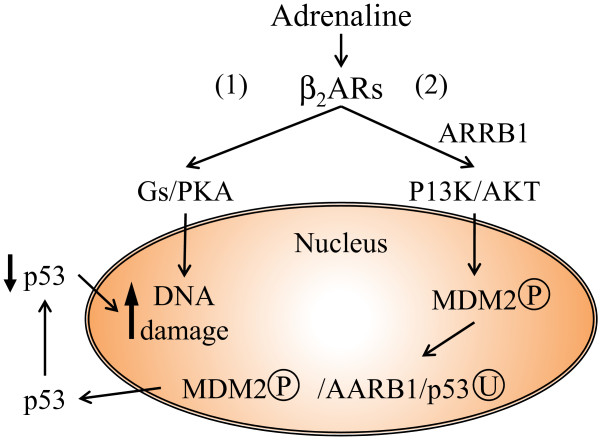
Schematic diagram of catecholamine-induced effects on DNA damage during chronic stress. β2AR, β2-adrenoreceptor; ARRB1, arrestin beta 1; MDM2, murine double minute 2; PKA, protein kinase A; PI3K, phosphoinositide 3-kinase. Based on [11].
The viewpoint
DNA damage triggers a number of cellular responses, including repair mechanisms, cell-cycle checkpoint activity and apoptosis. The tumor suppressor protein that mediates many of these critical cellular functions (p53) is frequently mutated, and has been found to be inactivated or functionally downregulated in breast cancer. The upstream mechanisms that regulate p53 degradation and accumulation of DNA damage, which Hara and colleagues have described, could thus have significant implications. Indeed, in precancerous 3T3 cells, stress hormones can cause induction of the DNA damage sensors Chk1 and Chk2, MDM2, and the protooncogene CDC25A, which is involved in cell-cycle delay following DNA damage, resulting in increased cell transformation [8]. Although the literature has come a long way towards recognizing the potential importance of psychological stress in the initiation and progression of breast cancer [12], few studies have explored the mechanisms through which stress hormones may impact breast cancer initiation and progression. It is tempting to speculate that increases in stress hormones could promote DNA damage and inhibit countervailing processes, and thus lead to a predisposition to breast cancer.
Also, if stress hormones can induce DNA damage in breast cancer, could this damage contribute to differences in the efficacy of chemotherapy drugs across individuals as a result of differences in their stress levels? Such personalized stress effects could have important implications for cancer therapeutic strategies that are based on the induction of DNA damage in rapidly dividing cells. For example, in breast cancer cells treated with anthracyclines - which work through intercalation of DNA and subsequent DNA damage - would heightened levels of catecholamines actually benefit chemotherapy? Would stress-induced DNA damage impact drugs that work through other mechanisms, such as Taxol? Clearly, there is a need for further mechanistic studies to explore the effects of stress hormones on DNA damage pathways involved in breast cancer etiology and in response to chemotherapy treatment.
Abbreviations
ARRB1: arrestin beta 1; MDM2: murine double minute 2.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Melanie S Flint, Email: flintms@upmc.edu.
Dana H Bovbjerg, Email: bovbjergdh@upmc.edu.
References
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;14:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res. 2011;14:208–223. doi: 10.1186/bcr2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;14:331–345. doi: 10.1146/annurev-neuro-062111-150546. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunol Allergy Clin North Am. 2011;14:109–132. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondicova K, Mravec B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010;14:596–601. doi: 10.1016/S1470-2045(09)70337-7. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;14:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Vazquez SM, Mladovan AG, Perez C, Bruzzone A, Baldi A, Luthy IA. Human breast cell lines exhibit functional alpha2-adrenoceptors. Cancer Chemother Pharmacol. 2006;14:50–61. doi: 10.1007/s00280-005-0130-4. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;14:470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol Psychol. 2006;14:291–304. doi: 10.1016/j.biopsycho.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Episcopo B, Knickelbein KZ, Liegey Dougall AJ, Chambers WH, Jenkins FJ. Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress. 2012. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, Shenoy SK, Gregory SG, Ahn S, Duckett DR, Lefkowitz RJ. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature. 2011;14:349–356. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Yang HC, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Young DC, Carson WE. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;14:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]


