Abstract
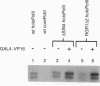
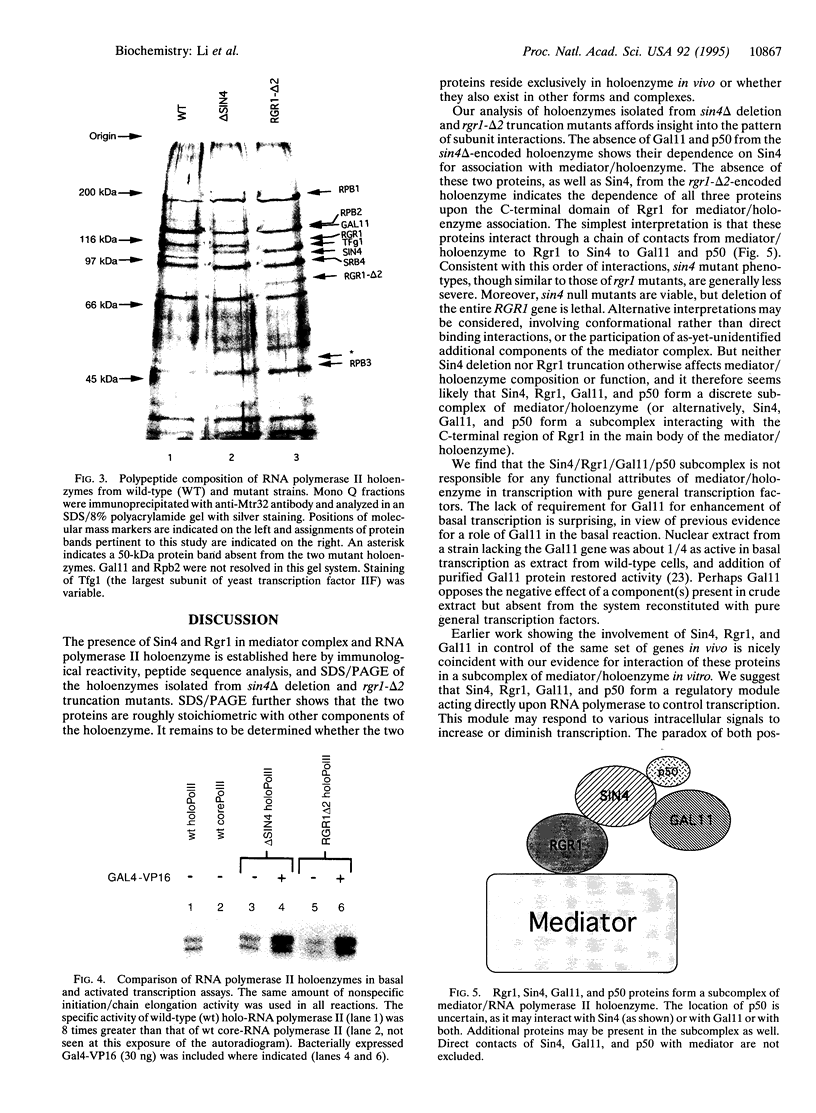
Sin4 and Rgr1 proteins, previously shown by genetic studies to play both positive and negative roles in the transcriptional regulation of many genes, are identified here as components of mediator and RNA polymerase II holoenzyme complexes. Results with Sin4 deletion and Rgr1 truncation strains indicate the association of these proteins in a subcomplex comprising Sin4, Rgr1, Gal11, and a 50-kDa polypeptide. Taken together with the previous genetic evidence, our findings point to a role of the mediator in repression as well as in transcriptional activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S., West R. W., Jr, Johnson S. L., Gans H., Kruger B., Ma J. TSF3, a global regulatory protein that silences transcription of yeast GAL genes, also mediates repression by alpha 2 repressor and is identical to SIN4. Mol Cell Biol. 1993 Feb;13(2):831–840. doi: 10.1128/mcb.13.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covitz P. A., Song W., Mitchell A. P. Requirement for RGR1 and SIN4 in RME1-dependent repression in Saccharomyces cerevisiae. Genetics. 1994 Nov;138(3):577–586. doi: 10.1093/genetics/138.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., Gray W., Lee J. P., Yu G. Y., Gingerich G. The Saccharomyces cerevisiae SPT14 gene is essential for normal expression of the yeast transposon, Ty, as well as for expression of the HIS4 gene and several genes in the mating pathway. Mol Gen Genet. 1991 Nov;230(1-2):310–320. doi: 10.1007/BF00290682. [DOI] [PubMed] [Google Scholar]
- Fernandez J., DeMott M., Atherton D., Mische S. M. Internal protein sequence analysis: enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992 Mar;201(2):255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Flanagan P. M., Kelleher R. J., 3rd, Sayre M. H., Tschochner H., Kornberg R. D. A mediator required for activation of RNA polymerase II transcription in vitro. Nature. 1991 Apr 4;350(6317):436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Hagmann M., Seipel K., Georgiev O., West M. A., Litingtung Y., Schaffner W., Corden J. L. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature. 1995 Apr 13;374(6523):660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- Jiang Y. W., Dohrmann P. R., Stillman D. J. Genetic and physical interactions between yeast RGR1 and SIN4 in chromatin organization and transcriptional regulation. Genetics. 1995 May;140(1):47–54. doi: 10.1093/genetics/140.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. W., Stillman D. J. Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. W., Stillman D. J. Regulation of HIS4 expression by the Saccharomyces cerevisiae SIN4 transcriptional regulator. Genetics. 1995 May;140(1):103–114. doi: 10.1093/genetics/140.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher R. J., 3rd, Flanagan P. M., Kornberg R. D. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990 Jun 29;61(7):1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Björklund S., Li Y., Sayre M. H., Kornberg R. D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994 May 20;77(4):599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Koleske A. J., Young R. A. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994 Mar 31;368(6470):466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- Kuchin S., Yeghiayan P., Carlson M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Ryan F., Swaffield J. C., Johnston S. A., Moore D. D. Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature. 1995 Mar 2;374(6517):91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- Liao S. M., Zhang J., Jeffery D. A., Koleske A. J., Thompson C. M., Chao D. M., Viljoen M., van Vuuren H. J., Young R. A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995 Mar 9;374(6518):193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- Sakai A., Shimizu Y., Kondou S., Chibazakura T., Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H., Hiraoka Y., Fukasawa T. Yeast GAL11 protein is a distinctive type transcription factor that enhances basal transcription in vitro. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8382–8386. doi: 10.1073/pnas.90.18.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surosky R. T., Strich R., Esposito R. E. The yeast UME5 gene regulates the stability of meiotic mRNAs in response to glucose. Mol Cell Biol. 1994 May;14(5):3446–3458. doi: 10.1128/mcb.14.5.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nogi Y., Abe A., Fukasawa T. GAL11 protein, an auxiliary transcription activator for genes encoding galactose-metabolizing enzymes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4991–4999. doi: 10.1128/mcb.8.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup J. Q., Feaver W. J., LaPointe J., Kornberg R. D. RNA polymerase transcription factor IIH holoenzyme from yeast. J Biol Chem. 1994 Nov 11;269(45):28044–28048. [PubMed] [Google Scholar]
- Swaffield J. C., Bromberg J. F., Johnston S. A. Alterations in a yeast protein resembling HIV Tat-binding protein relieve requirement for an acidic activation domain in GAL4. Nature. 1992 Jun 25;357(6380):698–700. doi: 10.1038/357698a0. [DOI] [PubMed] [Google Scholar]
- Swaffield J. C., Melcher K., Johnston S. A. A highly conserved ATPase protein as a mediator between acidic activation domains and the TATA-binding protein. Nature. 1995 Mar 2;374(6517):88–91. doi: 10.1038/374088a0. [DOI] [PubMed] [Google Scholar]
- Wahi M., Johnson A. D. Identification of genes required for alpha 2 repression in Saccharomyces cerevisiae. Genetics. 1995 May;140(1):79–90. doi: 10.1093/genetics/140.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]