Abstract
Introduction
The main prognostic variables in early breast cancer are tumor size, histological grade, estrogen receptor/progesterone receptor (ER/PgR) status, number of positive nodes and human epidermal growth factor receptor 2 (HER2) status. The present study evaluated the prognostic and/or predictive value of vascular endothelial growth factor (VEGF) family members in high-risk early breast cancer patients treated with adjuvant chemo-hormonotherapy.
Methods
RNA was isolated from 308 formalin-fixed paraffin-embedded primary tumor samples from breast cancer patients enrolled in the HE10/97 trial, evaluating adjuvant dose-dense sequential chemotherapy with epirubicin followed by cyclophosphamide, methotrexate, fluorouracil (CMF) with or without paclitaxel (E-T-CMF versus E-CMF). A fully automated method based on magnetic beads was applied for RNA extraction, followed by one-step quantitative RT-PCR for mRNA analysis of VEGF-A, -B, -C and vascular endothelial growth factor receptor (VEGFR) 1, 2, 3.
Results
With a median follow-up of 8 years, 109 patients (35%) developed a relapse and 80 patients (26%) died. In high VEGF-C and VEGFR1 mRNA expressing tumors, ER/PgR-negative tumors (Fisher's exact test, P = 0.001 and P = 0.021, respectively) and HER2-positive tumors (P <0.001 and P = 0.028, respectively) were more frequent than in low VEGF-C and VEGFR1 expressing tumors, respectively. From the VEGF family members evaluated, high VEGFR1 mRNA expression (above the 75th percentile) emerged as a significant negative prognostic factor for overall survival (OS; hazard ratio (HR) = 1.60, 95% confidence interval (CI): 1.01 to 2.55, Wald's P = 0.047) and disease-free survival (DFS; HR = 1.67, 95% CI: 1.13 to 2.48, P = 0.010), when adjusting for treatment group. High VEGF-C mRNA expression was predictive for benefit from adjuvant treatment with paclitaxel (E-T-CMF arm) for OS (test for interaction, Wald's P = 0.038), while in multivariate analysis the interaction of VEGF-C with taxane treatment was significant for both OS (Wald's P = 0.019) and DFS (P = 0.041) and continuous VEGF-B mRNA expression values for OS (P = 0.019).
Conclusions
The present study reports, for the first time, that VEGF-C mRNA overexpression, as assessed by qRT-PCR, has a strong predictive value in high-risk early breast cancer patients undergoing adjuvant paclitaxel-containing treatment. Further studies are warranted to validate the prognostic and/or predictive value of VEGF-B, VEGF-C and VEGFR1 in patients treated with adjuvant therapies and to reveal which members of the VEGF family could possibly be useful markers in identifying patients who will benefit most from anti-VEGF strategies.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR) ACTRN12611000506998
Introduction
The main prognostic variables in early breast cancer are tumor size, grade, estrogen and progesterone receptor (ER/PgR) status, number of positive nodes and human epidermal growth factor receptor 2 (HER2) status [1]. These and other clinicopathological parameters are commonly utilized to identify patients who are more likely to benefit from adjuvant chemotherapy and hormonal therapy. However, a large number of other molecules are being extensively investigated for their predictive and prognostic value, since most existing clinicopathological models have only moderate predictive power and do not account for the molecular diversity of tumors [2].
Recent experimental and clinical studies have suggested the essential role of angiogenesis in breast cancer among many other tumor types. The members of the vascular endothelial growth factor (VEGF) family and their receptors (VEGFRs) have a central function in angiogenesis and the formation of vascular networks. Today, we recognize five VEGFs (VEGF-A, -B, -C, -D, -E), with the first three being better characterized. VEGF-A and -B are considered mainly angiogenic, while VEGF-C is thought to be more lymphangiogenic. Their binding partners are three different tyrosine kinase receptors, VEGFR1 (or Flt-1), VEGFR2 (or KDR/Flk-1) and VEGFR3 (or Flt-4) [3,4]. VEGF-A is expressed at low levels in normal adult life and is over-expressed during wound healing and tissue regeneration. It has two known receptors, VEGFR1 and 2, mainly expressed in endothelial cells. VEGF-B is expressed at higher levels in cardiac and skeletal muscle cells, it forms heterodimers with VEGF-A and has two known binding receptors, VEGFR1 and neuropilin-1. VEGF-C was initially identified as a ligand for the tyrosine kinase receptor VEGFR3, which is associated with the lymphatic vasculature [5]. VEGF-C is also a ligand for VEGFR2, which it shares with VEGF-A and -D. A number of recent studies have investigated the role of VEGF-C in human tumors [6]; however, few have explored its role in human breast cancer. In those, VEGF-C has been proposed to be an inducer of tumor lymphangiogenesis and, therefore, an important promoter of breast cancer metastasis [7-9].
Angiogenesis is of central importance in the growth and metastasis of tumors and in particular of breast cancer [10,11]. Both VEGF-A and -B, and their receptors, have been found to be expressed in several different tumor types, including breast cancer [12]. Recently, VEGF-A has emerged as an important factor for progression in many tumor types and has been the target of bevacizumab [13]. However, its specific role in cancer has not been fully elucidated as yet.
The prognostic and clinicopathological significance of VEGF-A in breast cancer, both in node positive and node negative patients [14,15], has been evaluated by ELISA assays in several studies, less frequently and with controversial results by immunohistochemistry [15] and even less frequently by modern RT-PCR assays [16-18]. The role of VEGF-B is even less studied and understood [19]. Tumor-induced lymphangiogenesis has only recently been described and remains largely unexplored. Recent studies have suggested that it is mainly driven by VEGF-A and VEGF-C [20]. Furthermore, there is very limited information regarding the predictive role of any of the VEGF family members in breast cancer patients undergoing systemic treatment, hormonal therapy and/or chemotherapy.
Although the expression of VEGF family members at the protein level is well studied, the relationship of VEGF family members mRNA expression with various parameters or tumor progression is unclear. Quantitative RT-PCR (qRT-PCR) is a powerful tool that allows the selective measurement of mRNA expression levels in cancer cells, offering accurate relative quantification of mRNA levels of specific biomarkers [21] in formalin-fixed paraffin-embedded (FFPE) tumor tissue samples [22].
We initiated this study, with the aim of evaluating the mRNA expression patterns of VEGF family members in high-risk early breast cancer patients who had participated in a large randomized adjuvant chemo-hormonotherapy trial. We utilized a one-step qRT-PCR technique and correlated VEGF family members' mRNA expression with well-characterized clinicopathological parameters. Last but not least, we sought to explore the prognostic/predictive significance of mRNA expression of the evaluated VEGF family members on disease-free survival (DFS) and overall survival (OS) in high-risk operable breast cancer patients.
Materials and methods
Patient population
Tumor tissue samples were retrospectively obtained from patients with high-risk operable breast cancer, who had participated in a prospective randomized phase III study of dose-dense sequential chemotherapy with epirubicin (E), followed by intensified CMF with or without paclitaxel (T, Taxol®, Bristol Myers-Squibb, Princeton, NJ, USA), by the Hellenic Cooperative Oncology Group (HE10/97). Due to the retrospective nature of the present translational research study, collection of FFPE primary tumor tissue samples was possible in 317 patients only, due to logistical/organizational barriers. The clinical study randomized a total of 595 high-risk (T1-3N1M0 or T3N0M0) breast cancer patients from 1997 to 2000, in order to explore the effect of dose-dense sequential chemotherapy with or without paclitaxel (E-T-CMF versus E-CMF), primarily on DFS and secondarily on OS. The trial was included in the Australian New Zealand Clinical Trials Registry (ANZCTR) and allocated Registration Number ACTRN12611000506998. Chemotherapy cycles were administered every two weeks and patients received granulocyte-colony stimulating factor (G-CSF) support. The present study was approved by the Bioethics Committee of the Aristotle University of Thessaloniki and patients provided written informed consent prior to enrollment. All participating patients also gave written informed consent for research use of their biological material. The results of the HE10/97 study have been previously reported [23].
Data collected for this retrospective experimental study included treatment arm, age, menopausal status, interval from operation, number of positive nodes, tumor size, histological grade and adjuvant radiotherapy/hormonotherapy. Primary tumor diameter and axillary nodal status were obtained from the pathology report. Histological grade was evaluated according to the Scarff, Bloom and Richardson system.
Tissue microarray construction
Representative H & E stained sections from the tissue blocks were reviewed by a pathologist and the most representative tumor areas were marked for the construction of the tissue microarray (TMA) blocks, as previously described [24]. Each case was represented by two tissue cores, 1.5 mm in diameter, with each TMA block also containing cores from various neoplastic, non-neoplastic and reactive tissues serving as assay controls. Cases not represented, damaged or inadequate on the TMA sections were re-cut from the original blocks and these sections were used for protein and gene analysis.
Immunohistochemistry
Immunohistochemistry (IHC) for ER (clone 6F11, Novocastra™, Leica Biosystems, Newcastle, UK), PgR (clone 1A6, Novocastra™, Leica Biosystems) and HER2 (A0485 polyclonal antibody, Dako, Glostrup, Denmark) was performed on serial 2.5 μm thick TMA sections, using a Bond Max™ autostainer (Leica Microsystems, Wetzlar, Germany), as previously described [24]. All cases were also stained for vimentin (clone V9, Dako) and cytokeratin 8/18 (clone 5D3, Novocastra™, Leica Biosystems), which were used as control stains for tissue immunoreactivity and fixation, as well as identification of tumor cells. Tissue samples negative for the above antibodies were excluded from the study. The evaluation of all IHC sections was done by experienced breast cancer pathologists, blinded as to the patients' clinical characteristics and survival data.
Interpretation of the immunohistochemistry results
ER, PgR and HER2 protein expression was evaluated according to established or proposed criteria [25,26]. The ER and PgR immunostaining was scored using the histoscore method. Tissue sections stained for ER/PgR were considered to be positive when ≥1% of the neoplastic cells displayed nuclear immunoreactivity [25]. HER2 protein expression was scored according to the recent guideline recommendations (scores 0 to 3+) [26]. HER2 was considered to be positive in cases with an IHC score of 3+ (uniform, intense membrane staining in >30% of the invasive tumor cells).
Fluorescence in situ hybridization
TMA sections or whole tissue sections (5 μm thick) were used for fluorescence in situ hybridization (FISH) analysis, using the ZytoLight® SPEC HER2/TOP2A/CEN17 triple color probe (ZytoVision, Bremerhaven, Germany), as previously described [27]. Four carcinoma cell lines (MDA-MB-231, MDA-MB-175, MDA-MB-453, and SK-BR-3) from the Oracle HER2 Control Slide (Leica Biosystems), with a known HER2 gene status, were also used as a control of the FISH assays and analyzed for HER2 genomic status. TOP2A gene amplification was not evaluated for the purposes of the present study.
For the evaluation of the HER2 gene status, non-overlapping nuclei from the invasive part of the tumor were randomly selected and scored. The virtual slides of HER2, ER or PgR stains were used for selecting the invasive part of the tumor in each TMA. The virtual slides were created as previously described [28]. Twenty tumor nuclei were counted according to Press et al. [29]. The HER2 gene was considered to be amplified when the ratio of the gene probe/centromere probe was ≥2.2 [26], or the HER2 copy number was >6 [30]. In cases with values at or near the cut-off (1.8 to 2.2), an additional 20 or 40 nuclei were counted and the ratio was recalculated. In cases with a borderline ratio at 60 nuclei, additional FISH assays were performed in whole sections. HER2 was considered to be positive if it was amplified (ratio ≥2.2 or copy number >6) by FISH and/or a HER2 score of 3+ was obtained by IHC.
RNA isolation from formalin-fixed paraffin-embedded tissue and quantitative reverse transcription-polymerase chain reaction assessment
H & E sections from all available FFPE tissue specimens were evaluated histologically by a certified pathologist who recorded percentage of tumor cell content in each one. Prior to RNA isolation, macrodissection of tumor areas was performed in most of the FFPE sections with <50% tumor cell content. The tumor cell content was >30% in practically all (97%) of the samples and >50% in the majority (76%) of the samples. More than one FFPE section was used for RNA extraction when the tumor surface of a given sample was less than 0.25 cm2, in an effort to minimize the rate of technical failures in the RNA extraction.
Sufficient RNA was isolated from 308 FFPE specimens followed by qRT-PCR, as previously described [31]. From each FFPE section or macrodissected tissue fragment (10 μm thick), RNA was isolated using a standardized fully automated isolation method for total RNA from FFPE tissue, based on silica-coated magnetic beads (VERSANT Tissue Preparation Reagents, Siemens Healthcare Diagnostics, Tarrytown, NY, USA) in combination with a liquid handling robot, as previously described in detail [22]. The method involves extraction-integrated deparaffinization and DNase I digestion steps. DNA-free total RNA was eluted with 100 μL elution buffer and stored at -80°C.
One-step qRT-PCR was applied for the relative quantification of VEGF-A, VEGF-B, VEGF-C, VEGFR1, VEGFR2 and VEGFR3 mRNA expression, by using gene-specific TaqMan® based assays. Forty cycles of nucleic acid amplification were applied and the cycle threshold (CT) values of the target genes were identified. CT values were normalized by subtracting the CT value of the housekeeping gene RPL37A (ribosomal protein L37a) from the CT value of the target genes (ΔCT). RNA results were then reported as 40-ΔCT values, which correlate proportionally with the mRNA expression level of the target genes. For assessment of DNA contamination, a qPCR analysis specific for the PAEP gene (progestagen-associated endometrial protein) was performed, without the preceding reverse-transcription step. Samples were considered to be substantially free of DNA when CT values above 38 were detected. In the case of DNA contamination, samples were manually re-digested with DNase I. The quantity of RNA following isolation (yield) was checked by measuring RPL37A expression as a surrogate marker for amplifiable mRNA. Samples with average RPL37A CT values <32 were considered to have sufficient RNA and were eligible for analysis. Only 3 of the 311 extracted samples (1%) had an average RPL37A CT value of ≥32 and were, therefore, excluded from further analysis, resulting in successful RNA extraction from 99% of the samples.
Expression of the target genes, as well as the reference gene RPL37A, was assessed in triplicate by qRT-PCR using the SuperScript III PLATINUM One-Step Quantitative RT-PCR System with ROX (Invitrogen, Karlsruhe, Germany) in an ABI PRISM 7900HT (Applied Biosystems, Darmstadt, Germany) [21]. The lengths of the amplicons detected by the VEGF-A, VEGF-B, VEGF-C, VEGFR1, VEGFR2, VEGFR3 and RPL37A assays were 80 bp, 81 bp, 77 bp, 85 bp, 68 bp, 70 bp and 65 bp, respectively, with PCR efficiencies [E = 1(10-slope)] of 85.5, 110.3, 88.2, 95.7, 94.3, 84.7 and 86.0%, respectively. A commercially available human reference RNA (Stratagene qPCR Human Reference Total RNA, Agilent Technologies, Waldbronn, Germany) was used as positive control. No-template controls were assessed in parallel to exclude contamination.
The Primer/Probe (FAM/TAMRA-labeled) sets used for amplification of the target and reference genes were the following (5' -> 3'):
VEGF-A Probe CACCATGCAGATTATGCGGATCAAACCT
Forward Primer GCCCACTGAGGAGTCCAACA
Reverse Primer TCCTATGTGCTGGCCTTGGT
VEGF-B Probe CACATCTATCCATGACACCACTTTCCTCTGG
Forward Primer TGGCAGGTAGCGCGAGTAT
Reverse Primer CCCTGTCTCCCAGCCTGAT
VEGF-C Probe TTGAGTCATCTCCAGCATCCGAGGAAA
Forward Primer CCACAGATGTCATGGAATCCAT
Reverse Primer TGCCTGGCTCAGGAAGATTT
VEGFR1 Probe TGCTGTCGCCCTGGTAGTCATCAAACA
Forward Primer CATGGGAGAGGCCAACAGA
Reverse Primer AACCTTTGAAGAACTTTTACCGAATG
VEGFR2 Probe TCTTGGCATCGCGAAAGTGTATCCACA
Forward Primer TTCCAAGTGGCTAAGGGCAT
Reverse Primer CGTGCCGCCAGGTCC
VEGFR3 Probe TGCCTGCTTCCCTGGGTAGTCCC
Forward Primer GCACCCACTTACCCCGC
Reverse Primer GAGTTTAACTCAGGTGTCACCTTTGA
RPL37A Probe TGGCTGGCGGTGCCTGGA
Forward Primer TGTGGTTCCTGCATGAAGACA
Reverse Primer GTGACAGCGGAAGTGGTATTGTAC
Statistical analysis
For all VEGF family members the quartiles (first, median and third) were examined as possible thresholds for prognostic significance in terms of OS or DFS. If a cut-off showed prognostic significance it was used to dichotomize the tumors into low and high expressing tumors. Otherwise, only the normalized mRNA expression values were used in the analysis as a continuous variable to evaluate prognostic significance.
OS was measured from the date of randomization until death from any cause. Surviving patients were censored at the date of last contact. DFS was measured from the date of randomization until recurrence of tumor, secondary neoplasm or death from any cause [32]. Time-to-event distributions were estimated using Kaplan-Meier curves. Continuous variables were presented as medians with the corresponding range and categorical variables as frequencies with the respective percentages. Associations of ligands and receptors with basic patient and tumor characteristics were examined using the Fisher's exact test for categorical variables and the Mann-Whitney or the Kruskall-Wallis tests, where appropriate, for continuous variables.
Correlations between the VEGF family ligands and their associated receptors were calculated using the Spearman's rank correlation coefficient (Rho). Cox regression analyses were performed to assess the relationship between markers and OS or DFS. Interactions between markers and treatment group, as well as between ligands and their associated receptors were also explored in the Cox models. In the multivariate Cox regression analysis, a backward selection procedure with a removal criterion of P >0.10 based on the likelihood ratio test was performed to identify significant variables among the following: treatment group (E-CMF versus E-T-CMF), menopausal status (post versus pre), time interval from breast surgery operation (>4 weeks versus 2 to 4 weeks versus <2 weeks), histological grade (III-IV versus I-II), tumor size (>5cm versus 2 to 5cm versus ≤2cm), number of positive axillary nodes (≥4 versus 0 to 3), ER/PgR status (positive versus negative versus missing), HER2 status (negative versus positive versus missing), hormonal therapy (yes versus no), radiotherapy (yes versus no), VEGF-A (continuous mRNA values), VEGF-B (continuous mRNA values), VEGF-C (high versus low at the 75th percentile), VEGFR1 (high versus low at the 75th percentile), VEGFR2 (continuous mRNA values), VEGFR3 (continuous mRNA values).
The design of the study is prospective-retrospective as described in Simon et al. [33]. Results of this study are presented according to reporting recommendations for tumor marker prognostic studies [34]. The SPSS software was used for statistical analysis (SPSS for Windows, version 15.0, SPSS Inc.). No adjustment for multiple comparisons is reported.
Results
Patient and tumor characteristics
A total of 308 primary tumor tissue samples were analyzed as stated in the 'Methods' section. Basic clinical and pathological characteristics of the patients (Table 1) were well balanced according to adjuvant chemotherapy, except for histological grade (P = 0.008), in agreement with the corresponding results presented in the clinical paper [23]. In addition, there were no significant differences in important clinicopathological characteristics between the patients included in the present study and the rest of the HE10/97 randomized patients, for which tissue samples were not available.
Table 1.
Basic patient and tumor characteristics.
| E-T-CMF | E-CMF | All patients | ||||
|---|---|---|---|---|---|---|
| Number | 141 | 167 | 308 | |||
| Age (years) | ||||||
| Median | 50 | 50 | 50 | |||
| Range | 24 to 76 | 22 to 78 | 22 to 78 | |||
| Number of nodes removed | ||||||
| Median | 19 | 20 | 20 | |||
| Range | 5 to 59 | 4 to 53 | 4 to 59 | |||
| Number of positive nodes | ||||||
| Median | 7 | 6 | 6 | |||
| Range | 0 to 54 | 0 to 49 | 0 to 54 | |||
| N | % | N | % | N | % | |
| 0 to 3 nodes | 30 | 21.3 | 45 | 26.9 | 75 | 24.4 |
| ≥4 | 111 | 78.7 | 122 | 73.1 | 233 | 75.6 |
| Menopausal status | ||||||
| Premenopausal | 76 | 53.9 | 89 | 53.3 | 165 | 53.6 |
| Postmenopausal | 65 | 46.1 | 78 | 46.7 | 143 | 46.4 |
| Type of operation | ||||||
| Modified radical mastectomy | 111 | 78.7 | 132 | 79.0 | 243 | 78.9 |
| Breast conserving surgery | 30 | 21.3 | 35 | 21.0 | 65 | 21.1 |
| Interval from operation | ||||||
| <2 weeks | 17 | 12.1 | 24 | 14.4 | 41 | 13.3 |
| 2 to 4 weeks | 72 | 51.1 | 70 | 41.9 | 142 | 46.1 |
| >4 weeks | 52 | 36.9 | 73 | 43.7 | 125 | 40.6 |
| Tumor size | ||||||
| ≤2cm | 40 | 28.4 | 52 | 31.1 | 92 | 29.9 |
| 2 to 5cm | 79 | 56.0 | 83 | 49.7 | 162 | 52.6 |
| >5cm | 22 | 15.6 | 32 | 19.2 | 54 | 17.5 |
| Histological gradea | ||||||
| I-II | 60 | 42.6 | 97 | 58.1 | 157 | 51.0 |
| III-IV | 81 | 57.4 | 70 | 41.9 | 151 | 49.0 |
| ER/PgR status | ||||||
| Negative | 28 | 19.9 | 29 | 17.4 | 57 | 18.5 |
| Positive | 95 | 67.4 | 108 | 64.7 | 203 | 65.9 |
| Missing data | 18 | 12.8 | 30 | 18.0 | 48 | 15.6 |
| HER2 statusb | ||||||
| Negative | 79 | 56.0 | 100 | 59.9 | 179 | 58.1 |
| Positive | 35 | 24.8 | 32 | 19.2 | 67 | 21.8 |
| Missing data | 27 | 19.1 | 35 | 21.0 | 62 | 20.1 |
| Adjuvant RT | ||||||
| No | 21 | 14.9 | 33 | 19.8 | 54 | 17.5 |
| Yes | 119 | 84.4 | 133 | 79.6 | 252 | 81.8 |
| Missing data | 1 | 0.7 | 1 | 0.6 | 2 | 0.6 |
| Adjuvant HT | ||||||
| No | 8 | 5.7 | 18 | 10.8 | 26 | 8.4 |
| Yes | 133 | 94.3 | 149 | 89.2 | 282 | 91.6 |
| Tamoxifen | 120 | 85.1 | 127 | 76.0 | 247 | 80.2 |
| LH-RH agonist | 65 | 46.1 | 62 | 37.1 | 127 | 41.2 |
| Aromatase inhibitors | 5 | 3.5 | 6 | 3.6 | 11 | 3.6 |
| Other | 1 | 0.7 | 2 | 1.2 | 3 | 1.0 |
aThe two treatment arms were not balanced in terms of histological grade (P = 0.008); bpositive HER2 status: HER2 3+ by IHC and/or HER2 amplification by FISH. CMF, cyclophosphamide, methotrexate, fluorouracil; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; HT, hormonal therapy; IHC, immunohistochemistry; LH-RH, luteinizing hormone-releasing hormone; PgR, progesterone receptor; RT, radiation therapy.
The median follow-up period was eight years (range 7 to 126 months). A total of 109 patients developed a relapse (35%) and 80 patients died (26%). Median OS has not been reached yet, while median DFS was 121 months (95% CI: 105 to 138). The five-year OS rate was 83% (95% CI: 79 to 87) and the seven-year OS rate was 77% (95% CI: 72 to 81). The five-year DFS rate was 71% (95% CI: 66 to 76) and the seven-year DFS rate was 66% (95% CI: 60 to 71).
Normalized mRNA expression
The distribution of normalized mRNA expression (40-ΔCT values) of each VEGF family gene is shown in Figure 1. The median value for VEGF-A was 35.0 (range: 28.2 to 38.3), for VEGF-B 35.5 (range: 27.5 to 38.0), for VEGF-C 32.5 (range: 29.3 to 35.3), for VEGFR1 32.2 (range: 29.7 to 34.9), for VEGFR2 32.1 (range: 29.2 to 34.5) and for VEGFR3 32.0 (range: 27.4 to 34.4). All examined genes followed a unimodal distribution pattern.
Figure 1.
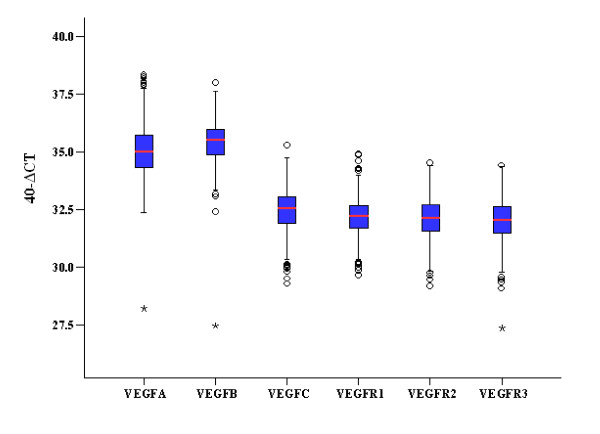
Distribution of mRNA expression values. Normalized mRNA expression values (40-delta CT) of all qRT-PCR evaluated VEGF family members are presented. CT, cycle threshold; VEGF, vascular endothelial growth factor.
Spearman's correlations between ligands and their associated receptors were examined. More specifically, there were statistically significant weak to moderate positive correlations between VEGF-A and receptors VEGFR1 and 2, VEGF-B and VEGFR1, as well as between VEGF-C and receptors VEGFR2 and 3 (Rho ranges from 0.30 to 0.56, P <0.001 in all cases), in agreement with the expected binding of the ligands.
Associations of vascular endothelial growth factor family gene expression with patient and tumor characteristics
The mRNA expression of all VEGF family genes was evaluated for associations with the following patient and tumor characteristics: age, treatment group, menopausal status, ER/PgR status, HER2 status, number of positive nodes, tumor size, histological grade and adjuvant treatment (hormonal and radiation therapy).
Concerning VEGF-A, higher continuous mRNA expression values were associated with higher age (≥50 years, Mann-Whitney test, P = 0.001), postmenopausal status (P = 0.001), negative ER/PgR status (P <0.001), positive HER2 status (P = 0.020), higher grade (III-IV, P = 0.027) and no adjuvant hormonal therapy (P = 0.003). Higher VEGF-B mRNA expression values were associated with higher age (P <0.001), postmenopausal status (P = 0.002), positive ER/PgR status (P = 0.023) and lower grade (I-II, P = 0.024). No statistically significant associations were found for VEGFR2, while higher mRNA expression values of VEGFR3 were associated with higher age (P = 0.030) (Table 2).
Table 2.
Association of VEGF-A, VEGF-B, VEGFR2 and VEGFR3 mRNA expression with basic patient and tumor characteristics.
| VEGF-A mRNA expression | VEGF-B mRNA expression | VEGFR2 mRNA expression | VEGFR3 mRNA expression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Number = 307) | (Number = 304) | (Number = 308) | (Number = 308) | ||||||||||
| Median | Range | P value | Median | Range | P value | Median | Range | P value | Median | Range | P value | ||
| Age | <50 | 34.8 | (32.4-38.3) | 0.001 | 35.3 | (33.1-37.5) | <0.001 | 32.1 | (29.6-34.4) | 0.190 | 31.9 | (27.4-34.2) | 0.030 |
| ≥50 | 35.3 | (28.2-38.3) | 35.7 | (27.5-38.0) | 32.2 | (29.2-34.5) | 32.1 | (29.1-34.4) | |||||
| Treatment group | E-T-CMF | 35.0 | (33.4-38.3) | 0.629 | 35.5 | (27.5-37.4) | 0.13 | 32.1 | (29.5-34.5) | 0.99 | 32.0 | (27.4-34.0) | 0.47 |
| E-CMF | 35.1 | (28.2-38.3) | 35.5 | (32.4-38.0) | 32.1 | (29.2-34.4) | 32.1 | (29.1-34.4) | |||||
| Menopausal status | Premenopausal | 34.8 | (32.4-38.3) | 0.001 | 35.4 | (33.1-37.5) | 0.002 | 32.1 | (29.6-34.4) | 0.17 | 31.9 | (27.4-34.2) | 0.069 |
| Postmenopausal | 35.3 | (28.2-38.3) | 35.7 | (27.5-38.0) | 32.3 | (29.2-34.5) | 32.1 | (29.1-34.4) | |||||
| ER/PgR status | Negative | 35.5 | (32.4-38.3) | <0.001 | 35.4 | (27.5-38.0) | 0.023 | 32.3 | (29.6-34.4) | 0.310 | 32.1 | (27.4-34.4) | 0.288 |
| Positive | 34.9 | (33.1-38.0) | 35.6 | (33.4-37.6) | 32.1 | (29.2-34.5) | 32.0 | (29.1-34.4) | |||||
| HER2 statusa | Negative | 35.0 | (28.2-38.2) | 0.020 | 35.5 | (32.4-37.5) | 0.856 | 32.0 | (29.2-34.5) | 0.088 | 32.0 | (29.1-34.4) | 0.294 |
| Positive | 35.4 | (33.4-38.3) | 35.6 | (27.5-38.0) | 32.4 | (29.5-34.4) | 32.4 | (27.4-34.4) | |||||
| Positive nodes | 0 to 3 | 35.0 | (32.4-38.1) | 0.97 | 35.4 | (33.7-37.2) | 0.51 | 32.0 | (30.3-34.1) | 0.42 | 31.9 | (30.0-34.2) | 0.35 |
| ≥4 | 35.0 | (28.2-38.3) | 35.6 | (27.5-38.0) | 32.1 | (29.2-34.5) | 32.0 | (27.4-34.4) | |||||
| Tumor size | ≤2 | 34.9 | (28.2-38.0) | 0.13 | 35.7 | (33.4-36.9) | 0.140 | 32.2 | (29.6-34.3) | 0.44 | 32.2 | (27.4-34.4) | 0.43 |
| 2-5 | 35.0 | (32.4-38.3) | 35.4 | (27.5-38.0) | 32.0 | (29.2-34.5) | 32.0 | (29.1-34.4) | |||||
| >5 | 35.3 | (33.1-37.9) | 35.5 | (33.1-37.6) | 32.2 | (29.5-34.0) | 32.1 | (29.4-34.1) | |||||
| Histological grade | I-II | 34.9 | (28.2-38.0) | 0.027 | 35.6 | (33.1-37.6) | 0.024 | 32.2 | (29.9-34.4) | 0.25 | 32.1 | (29.5-34.4) | 0.12 |
| III-IV | 35.1 | (32.4-38.3) | 35.4 | (27.5-38.0) | 32.0 | (29.2-34.5) | 32.0 | (27.4-34.4) | |||||
| Adjuvant HT | No | 35.8 | (33.1-37.9) | 0.003 | 35.5 | (33.8-36.8) | 0.59 | 32.1 | (30.1-33.9) | 0.85 | 31.8 | (29.9-33.6) | 0.62 |
| Yes | 34.9 | (28.2-38.3) | 35.5 | (27.5-38.0) | 32.1 | (29.2-34.5) | 32.0 | (27.4-34.4) | |||||
| Adjuvant RT | No | 35.4 | (32.4-37.9) | 0.17 | 35.4 | (33.4-37.2) | 0.72 | 32.0 | (30.1-34.1) | 0.52 | 31.9 | (29.4-33.9) | 0.48 |
| Yes | 35.0 | (28.2-38.3) | 35.5 | (27.5-38.0) | 32.1 | (29.2-34.5) | 32.1 | (27.4-34.4) | |||||
Normalized mRNA expression values (40-delta CT) are presented. Comparisons were made using the Mann-Whitney test, except for tumor size where the Kruskall-Wallis test was used. aPositive HER2 status: HER2 3+ by IHC and/or HER2 amplification by FISH. Significant P values are shown in bold. CMF, cyclophosphamide, methotrexate, fluorouracil; CT, cycle threshold; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; HT, hormonal therapy; PgR, progesterone receptor; RT, radiation therapy; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Associations of VEGF-C and VEGFR1 mRNA status (high versus low at the 75th percentile) with selected clinicopathological factors are shown in Table 3. High mRNA expression of VEGF-C was associated with higher age (≥50 years, Fisher's exact test, P = 0.024), while ER/PgR-negative tumors were more frequent in high VEGF-C expressing tumors (37.7% in high versus 17.3% in low, P = 0.001). Similarly, HER2-positive tumors were more frequent in high VEGF-C expressing tumors (46.6% in high versus 21.5% in low, P <0.001). Overall, high VEGF-C expression was more frequent in ER/PgR-negative and HER2-positive tumors. The number of positive lymph nodes did not seem to be associated with the expression of VEGF-C (P = 0.17). Concerning VEGFR1, ER/PgR-negative tumors and HER2-positive tumors were more frequent in high VEGFR1 expressing tumors (33.3% in high versus 18.8% in low, P = 0.021 and 39.7% in high versus 23.7% in low, P = 0.028, respectively). Finally, high expression of VEGFR1 was associated with adjuvant radiotherapy (P = 0.036).
Table 3.
Association of VEGF-C and VEGFR1 mRNA expression with basic patient and tumor characteristics.
| VEGF-C mRNA expression (Number = 305) |
VEGFR1 mRNA expression (Number = 306) |
||||||
|---|---|---|---|---|---|---|---|
| Low (n = 229) Number (%) |
High (n = 76) Number (%) |
P value | Low (n = 230) Number (%) |
High (n = 76) Number (%) |
P value | ||
| Age | <50 | 123 (53.9) | 29 (38.2) | 0.024 | 118 (51.5) | 35 (46.1) | 0.43 |
| ≥50 | 105 (46.1) | 47 (61.8) | 111 (48.5) | 41 (53.9) | |||
| Treatment group | E-T-CMF | 113 (49.3) | 28 (36.8) | 0.064 | 110 (47.8) | 30 (39.5) | 0.23 |
| E-CMF | 116 (50.7) | 48 (63.2) | 120 (52.2) | 46 (60.5) | |||
| Menopausal status | Premenopausal | 130 (56.8) | 34 (44.7) | 0.084 | 128 (55.7) | 37 (48.7) | 0.35 |
| Postmenopausal | 99 (43.2) | 42 (55.3) | 102 (44.3) | 39 (51.3) | |||
| ER/PgR status | Negative | 34 (17.3) | 23 (37.7) | 0.001 | 37 (18.8) | 20 (33.3) | 0.021 |
| Positive | 162 (82.7) | 38 (62.3) | 160 (81.2) | 40 (66.7) | |||
| HER2 Statusa | Negative | 146 (78.5) | 31 (53.5) | <0.001 | 142 (76.3) | 35 (60.3) | 0.028 |
| Positive | 40 (21.5) | 27 (46.6) | 44 (23.7) | 23 (39.7) | |||
| Positive nodes | 0-3 | 61 (26.6) | 14 (18.4) | 0.17 | 61 (26.5) | 14 (18.4) | 0.17 |
| ≥4 | 168 (73.4) | 62 (81.6) | 169 (73.5) | 62 (81.6) | |||
| Tumor size | ≤2 | 61 (26.6) | 30 (39.5) | 0.093 | 64 (27.8) | 27 (35.5) | 0.40 |
| 2-5 | 127 (55.5) | 33 (43.4) | 123 (53.5) | 38 (50.0) | |||
| >5 | 41 (17.9) | 13 (17.1) | 43 (18.7) | 11 (14.5) | |||
| Histological grade | I-II | 113 (49.3) | 41 (53.9) | 0.51 | 118 (51.3) | 37 (48.7) | 0.79 |
| III-IV | 116 (50.7) | 35 (46.1) | 112 (48.7) | 39 (51.3) | |||
| Adjuvant HT | No | 17 (7.4) | 9 (11.8) | 0.24 | 20 (8.7) | 6 (7.9) | 0.99 |
| Yes | 212 (92.6) | 67 (88.2) | 210 (91.3) | 70 (92.1) | |||
| Adjuvant RT | No | 43 (18.9) | 11 (14.5) | 0.49 | 47 (20.5) | 7 (9.3) | 0.036 |
| Yes | 184 (81.1) | 65 (85.5) | 182 (79.5) | 68 (90.7) | |||
Cut-off values were set at the 75th percentile of the marker's distribution. Comparisons were made using the Fisher's exact test. aPositive HER2 status: HER2 3+ by IHC and/or HER2 amplification by FISH. Significant P values are shown in bold. CMF, cyclophosphamide, methotrexate, fluorouracil; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; HT, hormonal therapy; PgR, progesterone receptor; RT, radiation therapy; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Association of vascular endothelial growth factor ligands with survival
VEGF-A and VEGF-B
VEGF-A and VEGF-B mRNA values did not achieve prognostic significance in any of the distribution cut-offs examined. Cox regression analysis, adjusted for treatment group, for the continuous normalized mRNA expression values of VEGF-A failed to establish a distinct risk for death (HR = 1.14, 95% CI: 0.94 to 1.39, Wald's P = 0.18) or risk for relapse (HR = 1.09, 95% CI: 0.92 to 1.29, P = 0.30). Similarly, normalized mRNA expression values of VEGF-B did not have prognostic significance for OS (HR = 0.86, 95% CI: 0.71 to 1.05, Wald's P = 0.14) or DFS (HR = 0.93, 95% CI: 0.77 to 1.14, P = 0.50) when analyzed as a continuous variable.
Patients were randomized to a taxane-free versus a taxane-containing chemotherapy and, thus, the predictive significance of VEGF markers for the paclitaxel-containing adjuvant chemotherapy treatment was examined as well. There was no significant interaction between VEGF-A and VEGF-B with chemotherapy treatment in terms of OS or DFS (tests for interaction, Wald's P >0.062 in all cases).
VEGF-C
The cut-off for VEGF-C was set at the 75th percentile of the marker's distribution. VEGF-C was predictive for benefit from adjuvant treatment with paclitaxel (E-T-CMF arm) for OS (test for interaction, Wald's P = 0.038), and marginally significant for DFS (test for interaction, Wald's P = 0.055). The impact of VEGF-C expression on OS and DFS in the two treatment groups is shown in Figure 2. Patients with high VEGF-C mRNA expression randomized to the non paclitaxel-containing adjuvant chemotherapy arm (E-CMF) had decreased OS and DFS (log-rank, P = 0.001 and P = 0.005, respectively; HR for OS = 2.57, 95% CI: 1.42 to 4.65; HR for DFS = 2.10, 95% CI: 1.26 to 3.51) compared to the patients with low VEGF-C expression, while no difference in OS or DFS was detected in the E-T-CMF group (log-rank, P = 0.72 and P = 0.67, respectively; HR for OS = 0.84, 95% CI: 0.35 to 2.02 for high VEGF-C expression; HR for DFS = 0.89, 95% CI: 0.44 to 1.80 for high VEGF-C expression).
Figure 2.
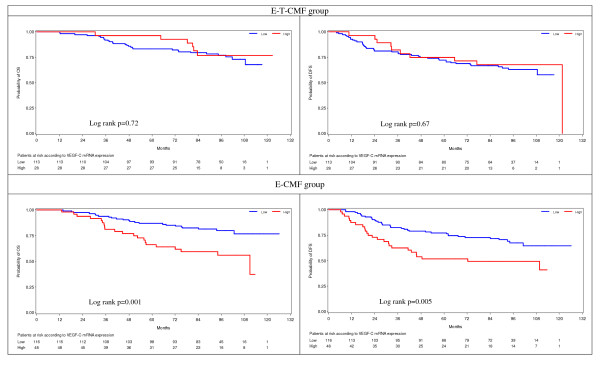
Kaplan-Meier curves according to VEGF-C mRNA expression and treatment. OS and DFS for patients with low VEGF-C mRNA expression (blue line) and high VEGF-C mRNA expression (red line) randomized in the E-T-CMF and E-CMF treatment groups. Interaction between VEGF-C mRNA expression and treatment group was significant for OS (P = 0.019) and DFS (P = 0.041). CMF, cyclophosphamide, methotrexate, fluorouracil; DFS, disease-free survival; OS, overall survival; VEGF-C, vascular endothelial growth factor C.
Association of vascular endothelial growth factor receptors with survival
For the VEGFR1 receptor the 75th percentile was prognostic for both OS and DFS, while for the VEGFR2 and VEGFR3 receptors no prognostic significance was found in the examined cut-offs in terms of OS or DFS. Concerning VEGFR1, 27/76 deaths (36%) and 37/76 relapses (49%) occurred in patients with high expressing tumors, in comparison to 52/230 deaths (23%) and 76/230 relapses (33%) in the low expressing tumors. Moreover, patients with high mRNA expression of VEGFR1 had increased risk for death (HR = 1.60, 95% CI: 1.01 to 2.55, Wald's P = 0.047) and increased risk for relapse (HR = 1.67, 95% CI: 1.13 to 2.48, P = 0.010) compared to patients with low expressing tumors when adjusting for treatment group. Kaplan-Meier curves for OS and DFS according to the mRNA status of VEGFR1 are presented in Figure 3.
Figure 3.
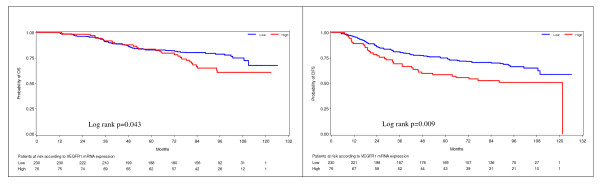
Kaplan-Meier curves according to VEGFR1 mRNA expression. High mRNA expression of VEGFR1 (above the 75th percentile) was associated with significantly reduced OS (left) and DFS (right). DFS, disease-free survival; OS, overall survival; VEGFR1, vascular endothelial growth factor receptor 1.
Examining continuous normalized mRNA expression values of VEGFR2, Cox regression analysis, adjusted for treatment group, did not show any associations with OS (HR = 1.10, 95% CI: 0.88 to 1.38, Wald's P = 0.41), or DFS (HR = 1.12, 95% CI: 0.93 to 1.35, P = 0.23). Similarly, normalized mRNA values of VEGFR3 did not have prognostic significance for OS (HR = 0.97, 95% CI: 0.78 to 1.19, Wald's P = 0.75), or DFS (HR = 1.07, 95% CI: 0.89 to 1.28, P = 0.47) when analyzed as a continuous variable.
Regarding predictive ability, there were no significant interactions between VEGF receptors and adjuvant chemotherapy for either OS or DFS (Wald's P >0.24 in all cases).
Interactions between ligands and receptors
Interactions between all possible combinations of ligands and receptors (VEGF-A*VEGFR1, VEGF-A*VEGFR2, VEGF-B*VEGFR1, VEGF-C*VEGFR2, VEGF-C*VEGFR3) were tested, both for OS and DFS. The interaction between VEGF-A and VEGFR1, adjusted for treatment group, was found to be significant in terms of OS (Wald's P = 0.017). More specifically, for those patients with low expression of VEGFR1 a one unit rise of the VEGF-A mRNA expression value would lead to increased risk for death with an HR of 1.43 (95% CI: 1.11 to 1.83), whereas for patients with high expression of VEGFR1 a one unit rise of the VEGF-A mRNA expression value would lead to an HR for OS of 0.84 (95% CI: 0.59 to 1.20).
Multivariate Cox regression model for overall survival and disease-free survival adjusting for clinical parameters
The Cox multivariate regression analysis for OS (Table 4) revealed that the hazard of death at any time was significantly higher for patients with more than three positive nodes (HR = 2.58, 95% CI: 1.32 to 5.02, Wald's P = 0.005), higher histological grade (HR = 1.94, 95% CI: 1.21 to 3.11, P = 0.006) and no hormonal therapy (HR = 2.86, 95% CI: 1.56 to 5.26, P = 0.001). Among the VEGF family members evaluated, VEGF-B and VEGF-C were associated with risk for death. For a one-unit increase in the mRNA expression of VEGF-B there was an 18% decrease in risk for death (P = 0.019). There was also a statistically significant difference in the treatment effect according to VEGF-C expression (P for interaction 0.019). The same clinicopathological factors had significant prognostic value for DFS: high histological grade (III-IV, P = 0.002), four or more positive nodes (P <0.001) and adjuvant hormonal therapy (P = 0.008), while VEGF-B (P = 0.084), VEGFR1 (P = 0.060) and the change in treatment effect on the hazard for disease progression according to VEGF-C mRNA expression were also statistically significant (P for interaction 0.041). Overall, there was a decreased, but not significant, risk for death in tumors with high VEGF-C expression (HR = 0.74, 95% CI: 0.28 to 1.96, P = 0.547), as well as a non-significant decreased risk for relapse (HR = 0.68, 95% CI: 0.31 to 1.48, P = 0.327) in the E-T-CMF group. Regarding the E-CMF group, high expression of VEGF-C increased the risk for death (HR = 2.85, 95% CI: 1.55 to 5.22, Wald's P <0.001) and the risk for relapse (HR = 1.73, 95% CI: 0.98 to 3.08, P = 0.166).
Table 4.
Multivariate analysis for prognostic significance: parameters in the final Cox model.
| Overall survival | HR | 95% CI | Wald's P |
|---|---|---|---|
| Histological grade | |||
| III-IV versus I-II | 1.94 | 1.21-3.11 | 0.006 |
| Number of positive nodes | |||
| ≥4 versus 0 to 3 | 2.58 | 1.32-5.02 | 0.005 |
| Adjuvant HT | |||
| No versus Yes | 2.86 | 1.56-5.26 | 0.001 |
| VEGF-B | |||
| Continuous mRNA values | 0.82 | 0.69-0.97 | 0.019 |
| VEGF-C/Treatment group Interaction | 3.84 | 1.24-11.84 | 0.019 |
| Treatment group | |||
| E-CMF versus E-T-CMF for VEGF-C low | 0.96 | 0.54-1.70 | 0.885 |
| E-CMF versus E-T-CMF for VEGF-C high | 3.68 | 1.38-9.80 | 0.009 |
| VEGF-C | |||
| High versus Low for E-T-CMF | 0.74 | 0.28-1.96 | 0.547 |
| High versus Low for E-CMF | 2.85 | 1.55-5.22 | <0.001 |
| Disease-free survival | HR | 95% CI | Wald's P |
| Histological grade | |||
| III-IV versus I-II | 1.83 | 1.24-2.71 | 0.002 |
| Number of positive nodes | |||
| ≥4 versus 0 to 3 | 2.80 | 1.58-4.95 | <0.001 |
| Adjuvant HT | |||
| No versus Yes | 2.16 | 1.22-3.84 | 0.008 |
| VEGF-B | |||
| Continuous mRNA values | 0.86 | 0.72-1.02 | 0.084 |
| VEGFR1 | |||
| High versus Low | 1.58 | 0.98-2.55 | 0.060 |
| VEGF-C/Treatment group Interaction | 2.56 | 1.04-6.31 | 0.041 |
| Treatment group | |||
| E-CMF versus E-T-CMF for VEGF-C low | 0.95 | 0.60-1.52 | 0.846 |
| E-CMF versus E-T-CMF for VEGF-C high | 2.44 | 1.12-5.31 | 0.057 |
| VEGF-C | |||
| High versus Low for E-T-CMF | 0.68 | 0.31-1.48 | 0.327 |
| High versus Low for E-CMF | 1.73 | 0.98-3.08 | 0.166 |
CI, confidence interval; CMF, cyclophosphamide, methotrexate, fluorouracil; HR, hazard ratio; HT, hormonal therapy; VEGF, vascular endothelial growth factor.
Discussion
Experimental and clinical evidence is rapidly accumulating regarding the significant role of angiogenesis in breast cancer progression and metastasis. VEGF has emerged as possibly the most essential angiogenic factor, expressed in many tumors including breast cancer, where it has been investigated for more than a decade now for its prognostic significance [35]. In most studies, VEGF expression is measured by IHC [36] or ELISA [37], but recently, PCR-based methods have also been used to assess VEGF mRNA expression in tumor tissues [38]. In general, PCR-based methods have proven to be very effective for the quantitative analysis of gene copy number or mRNA, especially when only a limited amount of tissue is available [39,40], while recent publications have shown that total RNA isolated from FFPE tissue samples can be used for reliable gene expression analysis [22,41]. Furthermore, Oncotype DX is a clinically validated prognostic test for patients with breast cancer, based on a qRT-PCR multigene algorithm [42]. It is worth noting that Oncotype DX does not include angiogenesis markers, but rather proliferation genes and other known prognostic genes, such as ER and HER2; therefore, the identification of useful prognostic indicators among the VEGF family members could have potential applications in similar multigene platforms. Furthermore, evidence is lacking on the ability of VEGF family members to predict benefit from specific treatments, especially on their predictive value for bevacizumab use. Several interesting candidate biomarkers for anti-angiogenic therapies have been evaluated in recent translational research studies and many are currently under investigation in prospective clinical trials. A recent report has shed some light on this issue by exploring biomarkers of the VEGF family for their possible effect on bevacizumab [43]. Results were only indicative that patients with low VEGF-C, among other markers, show trends toward improvement in progression-free survival associated with the addition of bevacizumab to capecitabine. Also, in a recently published biomarker evaluation study from the AVAGAST randomized trial in advanced gastric cancer, plasma VEGF-A and neuropilin-1 emerged as potential predictors of bevacizumab response [44].
In the present study, we analyzed the mRNA expression of well-recognized VEGF family members, including receptors (VEGFR1, 2 and 3) and their ligands (VEGF-A, B and C) in an attempt to identify individual members with prognostic/predictive significance. Our patient cohort included early breast cancer patients with high-risk characteristics: half were premenopausal, the majority had ≥4 positive axillary lymph nodes, large tumor size in most cases, almost half had high grade tumors, while 18.5% had ER/PgR-negative and 21.8% HER2-positive tumors. These patients participated in an adjuvant clinical study and were randomized to receive anthracycline-based chemotherapy with or without a taxane (E-T-CMF versus E-CMF). In this high-risk population, increased levels of VEGF-A mRNA were significantly associated with certain negative prognostic indicators, such as negative ER/PgR status, higher histological grade, positive HER2 status and no adjuvant hormonal therapy. VEGF-A mRNA levels have previously been associated with breast tumor characteristics, such as histological type and grade, albeit with variable results [45,46].
The prognostic value of VEGF family members on survival has been assessed in our patient population. Neither VEGF-A nor -B had prognostic significance for OS; they had no significant interaction with the chemotherapy treatment arm. With regard to receptors, only high expression of VEGFR1 was prognostic for both OS and DFS.
The prognostic value of VEGF-A expression has been assessed by IHC in several studies [15,19,37]. Recent retrospective clinical studies have strengthened the prognostic significance of total VEGF, as assessed by IHC in breast cancer [47,48], and have resulted in the recognition of the importance of VEGF as a possibly predictive biomarker and target for therapy in the more aggressive subcategory of triple-negative breast cancer [49,50]. Recent clinical evidence also strengthens the need for anti-angiogenic treatment in the triple-negative subtype, as bevacizumab added to neoadjuvant chemotherapy significantly increased the pathological complete response among patients with HER-negative early breast cancer, and primarily, those with triple-negative tumors [51]. However, in all of the above-mentioned studies, VEGF expression was assessed with standard IHC methods only [15,19,37,47-51]. In our study population, total VEGF had previously been assessed by IHC together with HER2, and, while HER2 was a negative prognostic indicator, high VEGF protein expression was not significantly associated with either DFS or OS [52].
It is important to note that, in our patient cohort, high mRNA expression of VEGFR1 had prognostic significance and, furthermore, the interaction of VEGF-A with VEGFR1 showed prognostic significance as well, while high expression of the ligand alone did not. This underlines the possible importance of interactions within the VEGF family, rather than that of individual members, and strengthens the need for further investigation. The binding of multiple ligands to individual receptors has previously been described [39]; however, certain interactions appear to be more important than others. According to the findings of our study, the correlation of VEGF-A with tumor profile, namely that higher expression was to be expected when the tumor was more aggressive, is not reflected by a negative prognostic effect on OS or DFS. There is, however, evidence of a negative prognostic role of increased VEGF-A mRNA expression in the low VEGFR1 subgroup with respect to OS. This particular subgroup of patients has a more favorable tumor profile in terms of ER/PgR and HER2 than the subgroup with high VEGFR1 levels. Therefore, a larger study should be conducted to explore whether the strong effect on DFS/OS exhibited by the receptor (VEGFR1) is masking the possible prognostic value of the VEGF-A ligand.
The most significant findings in our study involved VEGF-C; this factor emerged as a very important member of the VEGF family. In agreement with recent evidence from a number of studies, associations were found with VEGF-C and aggressive phenotype characteristics; ER/PgR-negative tumors and HER2-positive tumors had high VEGF-C expression more frequently. It is known that VEGF-C is a potent enhancer of tumor lymphangiogenesis, leading to increased metastatic spread of breast cancer cells to lymph nodes; however, in our study no significant correlation was found between the level of VEGF-C mRNA expression (low/high) and the number of positive lymph nodes (0 to 3 versus ≥4). However, it needs to be noted that the vast majority of patients in our study had large numbers of positive lymph nodes (>75% of the patients had ≥4 positive axillary lymph nodes); therefore, conclusive correlations were not possible. In a previous study, a significant association between increased VEGF-C expression and advanced histological grade was found, suggesting that poorly differentiated tumor cells may be more capable of secreting VEGF-C, which can induce lymphangiogenesis in breast cancer [47], while VEGF-C together with extracellular matrix protein 1 were found overexpressed in breast cancer lymphatic metastases [53]. It is also important to note that in our study, high VEGF-C and VEGFR1 mRNA expression was more frequently seen in HER2-positive tumors, indicating that certain VEGF family members could prove to be even more useful when analyzed in combination with other markers, with potential, for instance, to recognize patients with poor prognosis among the HER2-positive or, more importantly, the HER2-negative populations.
An important finding in our study was the predictive significance of VEGF-C and the impact of the taxane-containing treatment arm. Patients with high VEGF-C expressing tumors benefited more from the addition of paclitaxel in terms of OS. This was also evident in the multivariate analysis: patients with high VEGF-C mRNA expression were those with the worse prognosis, and they appear to benefit more from the taxane-containing treatment, possibly through the potential anti-angiogenic properties of the taxane therapy. Weekly taxane administration is considered very effective, both in the neoadjuvant and metastatic settings [54,55] and recently in the adjuvant setting [56]. Furthermore, there is evidence for anti-angiogenic effects of this schedule in addition to the anti-microtubule properties [57]. In our study, taxane treatment was indeed delivered in a dose-dense manner, every two weeks. The interaction of VEGF-C expression with treatment provides a significant indication for a possible predictive role of mRNA expression of VEGF family members, a role that warrants further evaluation in larger studies.
In our study, VEGF family mRNA expression and, in particular, high VEGF-C and VEGFR1 expression, was able to identify those patients with early breast cancer who have a higher likelihood of recurrence or death than those with low-angiogenic tumors, even if treated with adjuvant chemo-hormonotherapy. The taxane-containing treatment administered in a dose-dense manner, might have offered anti-angiogenic effects, which seem to be of more benefit for those patients with a high expression of angiogenic markers, such as VEGF-C and VEGFR1. The high expression of these factors might reflect subcategories of high-angiogenic tumors. It may be that such patient subsets represent good candidates for testing additional strategies to complement chemotherapy, such as anti-VEGF targeting agents in combination with conventional therapies. The results of our study provide the first evidence toward the identification of relevant angiogenic biomarkers in dose-dense chemotherapy regimens. Recent evidence of the strong predictive value of VEGF in premenopausal early breast cancer patients [58], as well as the predictive significance of tumor angiogenesis in high-risk early breast cancer patients [59], underlines the need for additional studies that could possibly support and/or clarify these findings.
Conclusions
In conclusion, the present study reports, for the first time, that VEGF-C mRNA overexpression, as assessed by qRT-PCR, has a strong predictive value in high-risk early breast cancer patients undergoing adjuvant dose-dense taxane-containing chemotherapy. Further studies are warranted to validate the prognostic and/or predictive value of VEGF-B, VEGF-C and VEGFR1 in patients treated with adjuvant therapies and to reveal which members of the VEGF family might possibly be useful in identifying those patients who will benefit most from anti-VEGF strategies.
Abbreviations
bp: base pair; CI: confidence interval; CMF: cyclophosphamide: methotrexate: fluorouracil; CT: cycle threshold; DFS: disease-free survival; E: epirubicin; ELISA: enzyme-linked immunosorbent assay; ER: estrogen receptor; FFPE: formalin-fixed paraffin-embedded; Flt-1: Fms-related tyrosine kinase 1; Flt-4: Fms-related tyrosine kinase 4; FISH: fluorescence in situ hybridization; G-CSF: granulocyte-colony stimulating factor; H & E: hematoxylin and eosin; HER2: human epidermal growth factor receptor 2; HT: hormonal therapy; HeCOG: Hellenic Cooperative Oncology Group; HR: hazard ratio; IHC: immunohistochemistry; KDR/Flk-1: kinase insert domain receptor/fetal liver kinase; OS: overall survival; PgR: progesterone receptor; qRT-PCR: quantitative reverse transcriptase-polymerase chain reaction; RT: radiation therapy; RT-PCR: reverse transcriptase-polymerase chain reaction; T: taxol (Paclitaxel); TMA: tissue microarray; VEGF (A: B: C): vascular endothelial growth factor (A: B: C); VEGFR (1: 2: 3): vascular endothelial growth factor receptor (1: 2: 3); ΔCT: delta cycle threshold.
Competing interests
On behalf of the Hellenic Foundation for Cancer Research, Athens, Greece, the senior author (GF) has pending patent applications with Siemens Healthcare Diagnostics, Tarrytown, NY. The rest of the authors declare that they have no competing interests.
Authors' contributions
HL conceived of the study, participated in its design and coordination and drafted the manuscript. KTK conceived of the study, participated in its design and coordination and drafted the manuscript. RK carried out the molecular studies and helped to draft the manuscript. GK participated in the design of the study and performed the statistical analysis. RMW carried out the molecular studies and helped to draft the manuscript. FZ participated in the clinical management of the patients and contributed to the collection of the tumor tissue samples analyzed in the study. HG, CC, AKK, ES, DP and DB participated in the clinical management of the patients and contributed to the collection of the tumor tissue samples analyzed in the study. GF conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors' information
Current address for Dr. Ralf Kronenwett: Sividon Diagnostics GmbH, Nattermann Allee 1, D-50829 Cologne, Germany.
Current address for Dr. Ralph M. Wirtz: Stratifyer Molecular Pathology GmbH, Werthmannstrasse 1, D-50935 Cologne, Germany.
Contributor Information
Helena Linardou, Email: elinardou@otenet.gr.
Konstantine T Kalogeras, Email: k_kalogeras@hecog.ondsl.gr.
Ralf Kronenwett, Email: kronenwett@sividon.com.
George Kouvatseas, Email: G.Kouvatseas@heads.gr.
Ralph M Wirtz, Email: ralph.wirtz@stratifyer.de.
Flora Zagouri, Email: florazagouri@yahoo.cc.uk.
Helen Gogas, Email: hgogas@hol.gr.
Christos Christodoulou, Email: c_christodoulou@yahoo.gr.
Angelos K Koutras, Email: angkoutr@otenet.gr.
Epaminondas Samantas, Email: epsam@otenet.gr.
Dimitrios Pectasides, Email: pectasid@otenet.gr.
Dimitrios Bafaloukos, Email: dimmp@otenet.gr.
George Fountzilas, Email: fountzil@auth.gr.
Acknowledgements
The authors wish to thank Evita Fragou and Dimitra Katsala for monitoring the study, Maria Moschoni for coordinating the data management and Thalia Spinari for tissue sample collection. Translational research was supported by a HeCOG research grant: HE TRANS_BR.
The senior investigator (GF) has received Commercial Research Funding by Roche Hellas SA and Genesis Pharma SA, Athens, Greece.
References
- Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;14:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Mazouni C, Anderson K, Wu Y, Symmans F. Molecular classification of breast cancer: limitations and potential. Oncologist. 2006;14:868–898. doi: 10.1634/theoncologist.11-8-868. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;14:11947–11954. [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengzinovitch S, Postorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;14:9–22. [PubMed] [Google Scholar]
- Olofsson B, Jeltsch M, Eriksson U, Alitalo K. Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol. 1999;14:139–147. doi: 10.1016/s0958-1669(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;14:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;14:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;14:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- Hoar FJ, Chaudhri S, Wadley MS, Stonelake PS. Co-expression of vascular endothelial growth factor C (VEGF-C) and c-erbB2 in human breast carcinoma. Eur J Cancer. 2003;14:1698–1703. doi: 10.1016/S0959-8049(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;14:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Toi M, Inada K, Suzuki H, Tominaga T. Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res Treat. 1995;14:193–204. doi: 10.1007/BF00666040. [DOI] [PubMed] [Google Scholar]
- de Jong JS, van Diest PJ, van der Valk P, Baak JPA. Expression of growth factors, growth factor receptors and apoptosis related proteins in invasive breast cancer: relation to apoptotic rate. Breast Cancer Res Treat. 2001;14:201–208. doi: 10.1023/A:1010650305100. [DOI] [PubMed] [Google Scholar]
- Koutras AK, Fountzilas G, Makatsoris T, Peroukides S, Kalofonos HP. Bevacizumab in the treatment of breast cancer. Cancer Treat Rev. 2010;14:75–82. doi: 10.1016/j.ctrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P, Gatti C, Suzuki H, Tominaga T. Prognostic significance of vascular endothelial growth factor protein in node negative breast carcinoma. J Natl Cancer Inst. 1997;14:139–147. doi: 10.1093/jnci/89.2.139. [DOI] [PubMed] [Google Scholar]
- Linderholm B, Lindh B, Tavelin B, Grankvist K, Henriksson R. p53 and vascular endothelial growth factor (VEGF) expression predicts outcome in 833 patients with primary breast cancer. Int J Cancer. 2000;14:51–62. doi: 10.1002/(SICI)1097-0215(20000120)89:1<51::AID-IJC9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bando H, Weich HA, Brokelmann M, Horiguchi S, Funata N, Ogawa T, Toi M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br J Cancer. 2005;14:553–561. doi: 10.1038/sj.bjc.6602374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConmara M, O'Hanlon DM, Kiely MJ, Connolly Y, Jeffers M, Keane FB. An evaluation of the prognostic significance of vascular endothelial growth factor in node positive primary breast carcinoma. Int J Oncol. 2002;14:717–721. [PubMed] [Google Scholar]
- Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, Cohen C, Young AN. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol. 2005;14:143–152. doi: 10.1038/modpathol.3800253. [DOI] [PubMed] [Google Scholar]
- Mylona E, Alexandrou P, Giannopoulou I, Liapis G, Sofia M, Keramopoulos A, Nakopoulou L. The prognostic value of vascular endothelial growth factors (VEGFs)-A and -B and their receptor, VEGFR-1, in invasive breast carcinoma. Gynecol Oncol. 2007;14:557–563. doi: 10.1016/j.ygyno.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Van den Eynden GG, Van der Auwera I, Van Laere SJ, Trinh XB, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Comparison of molecular determinants of angiogenesis and lymphangiogenesis in lymph node metastases and in primary tumors of patients with breast cancer. J Pathol. 2007;14:56–64. doi: 10.1002/path.2211. [DOI] [PubMed] [Google Scholar]
- Mueller BM, Kronenwett R, Hennig G, Euting H, Weber K, Bohmann K, Weichert W, Winzer KJ, Kristiansen G, Petry C, Dietel M, Denkert C. Quantitative determination of estrogen receptor, progesterone receptor and HER2 mRNA in formalin-fixed paraffin-embedded tissue--a new option for predictive biomarker assessment in breast cancer. Diagn Mol Pathol. 2011;14:1–10. doi: 10.1097/PDM.0b013e3181e3630c. [DOI] [PubMed] [Google Scholar]
- Bohmann K, Hennig G, Rogel U, Poremba C, Mueller BM, Fritz P, Stoerkel S, Schaefer K-L. RNA extraction from archival formalin-fixed paraffin-embedded tissue: a comparison of manual, semiautomated, and fully automated purification methods. Clin Chem. 2009;14:1719–1727. doi: 10.1373/clinchem.2008.122572. [DOI] [PubMed] [Google Scholar]
- Fountzilas G, Skarlos D, Dafni U, Gogas H, Briasoulis E, Pectasides D, Papadimitriou C, Markopoulos C, Polychronis A, Kalofonos HP, Siafaka V, Kosmidis P, Timotheadou E, Tsavdaridis D, Bafaloukos D, Papakostas P, Razis E, Makrantonakis P, Aravantinos G, Christodoulou C, Dimopoulos AM. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomised phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;14:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- Skarlos P, Christodoulou C, Kalogeras KT, Eleftheraki AG, Bobos M, Batistatou A, Valavanis C, Tzaida O, Timotheadou E, Kronenwett R, Wirtz RM, Kostopoulos I, Televantou D, Koutselini E, Papaspirou I, Papadimitriou CA, Pectasides D, Gogas H, Aravantinos G, Pavlidis N, Arapantoni P, Skarlos DV, Fountzilas G. Triple negative phenotype is of adverse prognostic value in patients treated with dose-dense sequential adjuvant chemotherapy: a translational research analysis in the context of a Hellenic Cooperative Oncology Group (HeCOG) randomized phase III trial. Cancer Chemother Pharmacol. 2012;14:533–546. doi: 10.1007/s00280-011-1730-9. [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;14:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;14:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- Psyrri A, Kalogeras KT, Kronenwett R, Wirtz RM, Batistatou A, Bournakis E, Timotheadou E, Gogas H, Aravantinos G, Christodoulou C, Makatsoris T, Linardou H, Pectasides D, Pavlidis N, Economopoulos T, Fountzilas G. Prognostic significance of UBE2C mRNA expression in high-risk early breast cancer. A Hellenic Cooperative Oncology Group (HeCOG) Study. Ann Oncol. 2012;14:1422–1427. doi: 10.1093/annonc/mdr527. [DOI] [PubMed] [Google Scholar]
- Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, Zaramboukas T, Nikolaou A, Markou K, Resiga L, Dionysopoulos D, Samantas E, Athanassiou H, Misailidou D, Skarlos D, Ciuleanu T. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012;14:427–435. doi: 10.1093/annonc/mdr116. [DOI] [PubMed] [Google Scholar]
- Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, Villalobos IE, Eiermann W, Pienkowski T, Martin M, Robert N, Crown J, Bee V, Taupin H, Flom KJ, Tabah-Fisch I, Pauletti G, Lindsay MA, Riva A, Slamon DJ. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. 2011;14:859–867. doi: 10.1200/JCO.2009.27.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bempt I, Van Loo P, Drijkoningen M, Neven P, Smeets A, Christiaens MR, Paridaens R, De Wolf-Peeters C. Polysomy 17 in breast cancer: clinicopathologic significance and impact on HER-2 testing. J Clin Oncol. 2008;14:4869–4874. doi: 10.1200/JCO.2007.13.4296. [DOI] [PubMed] [Google Scholar]
- Pentheroudakis G, Batistatou A, Kalogeras KT, Kronenwett R, Wirtz RM, Bournakis E, Eleftheraki AG, Pectasides D, Bobos M, Papaspirou I, Kamina S, Gogas H, Koutras AK, Pavlidis N, Fountzilas G. Prognostic utility of β-tubulin isotype III and correlations with other molecular and clinicopathological variables in patients with early breast cancer: a translational Hellenic Cooperative Oncology Group (HeCOG) study. Breast Cancer Res Treat. 2011;14:179–193. doi: 10.1007/s10549-011-1427-0. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;14:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;14:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;14:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;14:37–44. doi: 10.1634/theoncologist.5-suppl_1-37. [DOI] [PubMed] [Google Scholar]
- Fontanini G, Vignati S, Boldrini L, Chinè S, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;14:861–865. [PubMed] [Google Scholar]
- Chen CA, Cheng WF, Lee CN, Chen TM, Kung CC, Hsieh FJ, Hsieh CY. Serum vascular endothelial growth factor in epithelial ovarian neoplasms: correlation with patient survival. Gynecol Oncol. 1999;14:235–240. doi: 10.1006/gyno.1999.5418. [DOI] [PubMed] [Google Scholar]
- Gu JW, Brady AL, Anand V, Moore MC, Kelly WC, Adair TH. Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol. 1999;14:H595–602. doi: 10.1152/ajpheart.1999.277.2.H595. [DOI] [PubMed] [Google Scholar]
- Clementi M, Menzo S, Bagnarelli P, Manzin A, Valenza A, Varaldo PE. Quantitative PCR and RT-PCR in virology. PCR Methods Appl. 1993;14:191–196. doi: 10.1101/gr.2.3.191. [DOI] [PubMed] [Google Scholar]
- Ferre F. Quantitative or semi-quantitative PCR: reality versus myth. PCR Methods Appl. 1992;14:1–9. doi: 10.1101/gr.2.1.1. [DOI] [PubMed] [Google Scholar]
- Van den Eynden GG, Van der Auwera I, Van Laere SJ, Trinh XB, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Comparison of molecular determinants of angiogenesis and lymphangiogenesis in lymph node metastases and in primary tumours of patients with breast cancer. J Pathol. 2007;14:56–64. doi: 10.1002/path.2211. [DOI] [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;14:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Miller KD, Rugo HS, Harris AL, Chen D, Reimann JD, Cobleigh MA, Schmidt M, Langmuir VK, Hillan KJ, Chen DS, Koeppen H. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;14:372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, Shah MA. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;14:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Tsuda H, Imazeki N, Matsubara O. Vascular endothelial growth factor mRNA levels quantified by reverse transcription-polymerase chain reaction in microdissected breast carcinoma tissues are correlated with histological type and grade of both invasive and intraductal components. Pathol Int. 2005;14:255–263. doi: 10.1111/j.1440-1827.2005.01822.x. [DOI] [PubMed] [Google Scholar]
- Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;14:963–969. [PubMed] [Google Scholar]
- Zhang X-H, Huang D-P, Guo1 G-L, Chen G-R, Zhang H-X, Wan L, Chen S-Y. Co-expression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer. 2008;14:4–12. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Tamandl D, Roberts L, Jomrich G, Schoppmann A, Zwrtek R, Dubsky P, Gnant M, Jakesz R, Birner P. HER2/neu expression correlates with vascular endothelial growth factor-C and lymphangiogenesis in lymph node-positive breast cancer. Ann Oncol. 2010;14:955–960. doi: 10.1093/annonc/mdp532. [DOI] [PubMed] [Google Scholar]
- Rydén L, Jirström K, Haglund M, Stål O, Fernö M. Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Treat. 2010;14:491–498. doi: 10.1007/s10549-010-0758-6. [DOI] [PubMed] [Google Scholar]
- Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, Lewensohn R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;14:1639–1646. doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Müller BM, Denkert C, Loibl S, Nekljudova V, Untch M. German Breast Group; Arbeitsgemeinschaft Gynäkologische Onkologie-Breast Study Groups. Neoadjuvant chemotherapy and bevacizumab for HER-2 negative breast cancer. N Engl J Med. 2012;14:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- Kostopoulos I, Arapantoni-Dadioti P, Gogas H, Papadopoulos S, Malamou-Mitsi V, Scopa CD, Markaki S, Karagianni E, Kyriakou V, Margariti A, Kyrkou E, Pavlakis K, Zaramboukas T, Skordalaki A, Bourli A, Markopoulos C, Pectasides D, Dimopoulos MA, Skarlos D, Fountzilas G. Evaluation of the prognostic value of HER-2 and VEGF in breast cancer patients participating in a randomized study with dose-dense sequential adjuvant chemotherapy. Breast Cancer Res Treat. 2006;14:251–261. doi: 10.1007/s10549-005-9062-2. [DOI] [PubMed] [Google Scholar]
- Wu Q-W, She H-Q, Liang J, Huang Y-F, Yang QM, Yang QL, Zhang ZM. Expression and clinical significance of extracellular matrix protein 1 and vascular endothelial growth factor-C in lymphatic metastasis of human breast cancer. BMC Cancer. 2012;14:47–59. doi: 10.1186/1471-2407-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, Cristofanilli M, Booser DJ, Pusztai L, Rivera E, Theriault RL, Carter C, Frye D, Hunt KK, Symmans WF, Strom EA, Sahin AA, Sikov W, Hortobagyi GN. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;14:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol. 1998;14:3353–3361. doi: 10.1200/JCO.1998.16.10.3353. [DOI] [PubMed] [Google Scholar]
- Loesch D, Greco FA, Senzer NN, Burris HA, Hainsworth JD, Jones S, Vukelja SJ, Sandbach J, Holmes F, Sedlacek S, Pippen J, Lindquist D, McIntyre K, Blum JL, Modiano MR, Boehm KA, Zhan F, Asmar L, Robert N. Phase III multicenter trial of doxorubicin plus cyclophosphamide followed by paclitaxel compared with doxorubicin plus paclitaxel followed by weekly paclitaxel as adjuvant therapy for women with high-risk breast cancer. J Clin Oncol. 2010;14:2958–2965. doi: 10.1200/JCO.2009.24.1000. [DOI] [PubMed] [Google Scholar]
- Ng SSW, Figg WD, Sparreboom A. Taxane mediated angiogenesis in vitro: influence of formulation vehicles and binding proteins. Cancer Res. 2004;14:821–824. doi: 10.1158/0008-5472.CAN-03-3391. [DOI] [PubMed] [Google Scholar]
- Linderholm BK, Gruvbreger-Saal S, Fernö M, Bendahl PO, Malmström P. Vascular endothelial growth factor is a strong predictor of early distant recurrences in a prospective study of premenopausal women with lymph-node negative breast cancer. Breast. 2008;14:484–491. doi: 10.1016/j.breast.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Gluz O, Wild P, Liedtke C, Kates R, Mendrik H, Ehm E, Artinger V, Diallo-Danebrock R, Ting E, Mohrmann S, Poremba C, Harbeck N, Nitz U, Hartmann A, Gaumann A. Tumor angiogenesis as prognostic and predictive marker for chemotherapy dose-intensification efficacy in high-risk breast cancer patients within the WSG AM-01 trial. Breast Cancer Res Treat. 2011;14:643–651. doi: 10.1007/s10549-011-1377-6. [DOI] [PubMed] [Google Scholar]


