Abstract
Introduction
While it has been reported that the risk of contralateral breast cancer in patients from BRCA1 or BRCA2 positive families is elevated, little is known about contralateral breast cancer risk in patients from high risk families that tested negative for BRCA1/2 mutations.
Methods
A retrospective, multicenter cohort study was performed from 1996 to 2011 and comprised 6,235 women with unilateral breast cancer from 6,230 high risk families that had tested positive for BRCA1 (n = 1,154) or BRCA2 (n = 575) mutations or tested negative (n = 4,501). Cumulative contralateral breast cancer risks were calculated using the Kaplan-Meier product-limit method and were compared between groups using the log-rank test. Cox regression analysis was applied to assess the impact of the age at first breast cancer and the familial history stratified by mutation status.
Results
The cumulative risk of contralateral breast cancer 25 years after first breast cancer was 44.1% (95%CI, 37.6% to 50.6%) for patients from BRCA1 positive families, 33.5% (95%CI, 22.4% to 44.7%) for patients from BRCA2 positive families and 17.2% (95%CI, 14.5% to 19.9%) for patients from families that tested negative for BRCA1/2 mutations. Younger age at first breast cancer was associated with a higher risk of contralateral breast cancer. For women who had their first breast cancer before the age of 40 years, the cumulative risk of contralateral breast cancer after 25 years was 55.1% for BRCA1, 38.4% for BRCA2, and 28.4% for patients from BRCA1/2 negative families. If the first breast cancer was diagnosed at the age of 50 or later, 25-year cumulative risks were 21.6% for BRCA1, 15.5% for BRCA2, and 12.9% for BRCA1/2 negative families.
Conclusions
Contralateral breast cancer risk in patients from high risk families that tested negative for BRCA1/2 mutations is similar to the risk in patients with sporadic breast cancer. Thus, the mutation status should guide decision making for contralateral mastectomy.
Introduction
Women carrying a deleterious BRCA1 or BRCA2 mutation not only face a strongly elevated lifetime risk for the development of breast and ovarian cancer but also for a second breast cancer [1,2]. The majority of second primaries develop in the contralateral breast while ipsilateral breast cancer is not significantly increased [3,4]. Estimates for contralateral breast cancer range from 15% to 40% within 10 years [1,3,5-10]. Due to this wide range of risk estimates, it is clinically important to identify predictive factors. In a retrospective cohort study comprising 2,020 women with unilateral breast cancer from 978 families with a BRCA1 or BRCA2 mutation, we could show that contralateral breast cancer depends on the affected BRCA gene and age at onset of the first breast cancer [5]. In a recent retrospective update on 810 breast cancer patients from BRCA1/2 positive families, Metcalfe et al. as well as previous studies also showed that prophylactic bilateral salpingo-oophorectomy (PBSO) under the age of 50 years reduces the risk of contralateral breast cancer by half [9,10]. They further demonstrated that as the number of first degree relatives under the age of 50 years with breast cancer increases the risk of contralateral breast cancer also increases. Data on other factors that may modify contralateral breast cancer risk, for example tamoxifen use, are inconsistent [10,11].
While the risk for second primaries has been studied in BRCA1 or BRCA2 mutation carriers, preliminary data indicate that the risk of contralateral breast cancer is not significantly elevated in patients with familial breast cancer, who tested negative for BRCA1/2 mutations [12,13]. Despite these data and although the latter group accounts for the majority of women with familial breast cancer, there is a rising demand for prophylactic bilateral or prophylactic contralateral mastectomy in these women [14,15]. To question the need for this prophylactic approach, in this article we extended our previously published data on contralateral breast cancer risks and predictive factors in two dimensions. First, we updated the number of analyzed women from families with BRCA1 or BRCA2 mutations. Second, we included women from non-BRCA1/2 high risk families in the analysis for the first time. These risk estimates can be used for counselling in order to allow women to make a non-directive and informed decision on the extent of surgical treatment and secondary prevention.
Methods
German Consortium for Hereditary Breast and Ovarian Cancer
Data were collected within the German Consortium for Hereditary Breast and Ovarian Cancer which comprises 12 university based centers, as previously described [5]. Inclusion criteria and methods for genetic testing are described elsewhere [16,17]. It is worth mentioning that 24% of all families that fulfill the inclusion criteria of the German Consortium for Hereditary Breast and Ovarian Cancer tested positive for a deleterious BRCA1 or BRCA2 mutation which reflects stringent inclusion criteria [16,17]. The study has been approved by the institutional review boards of all participating centers. All patients gave their written informed consent prior to study inclusion.
From 1996 to July 2011 a total of 8,733 families were registered and tested positive for BRCA1 (n = 1,743) or BRCA2 (n = 818) mutations or tested negative (n = 6,172). For this updated retrospective cohort study all index cases and their first- and second-degree relatives with a history of unilateral breast cancer diagnosed after 1960 were selected (12,897 patients from 6,364 families). Individuals were excluded if their first breast cancer diagnosis was bilateral (synchronous bilateral breast cancer). Individuals were only selected from that branch of the family in which the pathogenic mutation was detected or familial clustering of cancers was observed. All index patients, defined as the earliest accessible affected family member and diagnosed with breast or ovarian cancer, were tested for BRCA1/2 mutations. Of the selected relatives of BRCA1/2 mutation carriers, 328 (16%) could be proven to be carriers. Forty relatives who tested negative for the known mutation in the family were considered phenocopies and were excluded. All other relatives, whose mutation status could not be determined, were considered putative carriers. An additional 392 patients (3.0%) were excluded due to insufficient information regarding age at cancer events or surgery (for example, bilateral mastectomy). In summary, 12,465 women with unilateral first breast cancer diagnosis were included in the present analysis, comprising 6,230 index patients and 6,235 relatives. Medical and pathological records could be obtained from 83% of the index patients and 47% of the relatives. For all other individuals information about medical history was obtained through structured interviews. The study was performed retrospectively. Prospective follow-up after recruitment is not considered in this analysis. For the analysis, patients were followed from their first unilateral breast cancer until contralateral breast cancer or censoring.
For the comparison of contralateral breast cancer risk in BRCA1 and 2 mutation carriers and BRCA1/2-negative women at high risk we used data from breast cancer registries [18,19].
Statistical analysis
BRCA1, BRCA2 and non-BRCA1/2 families were analyzed as previously described [5]. In summary, cumulative contralateral breast cancer risks were calculated using the Kaplan-Meier product-limit method and compared between groups using the log-rank test. Cox proportional hazards regression was used to analyze the association with potential risk factors by estimation of hazard ratios (HR) and their 95% CIs. All subjects were censored at the time of second unilateral breast cancer, ovarian cancer, bilateral mastectomy, death, or last observation, whichever occurred first. We censored at unilateral breast cancer and ovarian cancer because an effect of chemotherapy for these cancers on the risk of subsequent contralateral breast cancer can not be excluded. All reported P-values are two-sided. P-values below 0.05 were considered statistically significant. IBM SPSS Statistics 20.0.0.1 was used for all data analyses.
Results
Characteristics of the study population
Basic characteristics of the index patients and their relatives are shown in Table 1. Index patients had a younger median age at first unilateral breast cancer and a considerably higher risk for contralateral breast cancer than their relatives (Figure 1). This was true for the whole study cohort as well as for the three subgroups, that is, BRCA1 positive, BRCA2 positive and BRCA1/2 negative families, and is most likely due to the selection of index patients based on clinical criteria, that is, DNA testing was preferentially performed in those patients with clinical phenotypes that were more indicative of a BRCA mutation. Thus, in order to avoid overestimating the risks of contralateral breast cancer, index patients were excluded from further analyses.
Table 1.
Basic characteristics of relatives and index patients from BRCA positive and negative families.
| Relatives of index patients | Index patients | |
|---|---|---|
| Number of patients | 6,235 | 6,230 |
| Mutation status, number of patients | ||
| BRCA1 pathogenic mutation | 213 | 1,154 |
| BRCA2 pathogenic mutation | 106 | 575 |
| BRCA negative | 4,326 | 4,501 |
| not tested, BRCA1 family | 1,046 | - |
| not tested, BRCA2 family | 544 | - |
| Patients with contralateral breast cancer | ||
| patients from BRCA1 families | 193 | 304 |
| patients from BRCA2 families | 56 | 84 |
| patients from BRCA negative families | 253 | 349 |
| Median year of birth (IQR) | ||
| patients from BRCA1 families | 1,943 (1933-1955) | 1,960 (1952-1968) |
| patients from BRCA2 families | 1,939 (1928-1952) | 1,957 (1945-1965) |
| patients from BRCA negative families | 1,936 (1926-1946) | 1,955 (1944-1964) |
| Median age at first breast cancer (IQR) | ||
| patients from BRCA1 families | 43.5 (37.5-51.5) | 38.2 (32.8-44.4) |
| patients from BRCA2 families | 48.1 (40.4-58.5) | 42.5 (36.4-49.4) |
| patients from BRCA negative families | 53.6 (45.3-63.9) | 44.9 (37.9-51.4) |
| Median age at contralateral breast cancer (IQR) | ||
| patients from BRCA1 families | 47.7 (40.1-55.5) | 43.5 (37.7-50.5) |
| patients from BRCA2 families | 53.1 (44.7-62.6) | 47.9 (42.7-55.7) |
| patients from BRCA negative families | 56.0 (48.5-66.6) | 51.6 (45.3-59.0) |
IQR, interquartile range.
Figure 1.
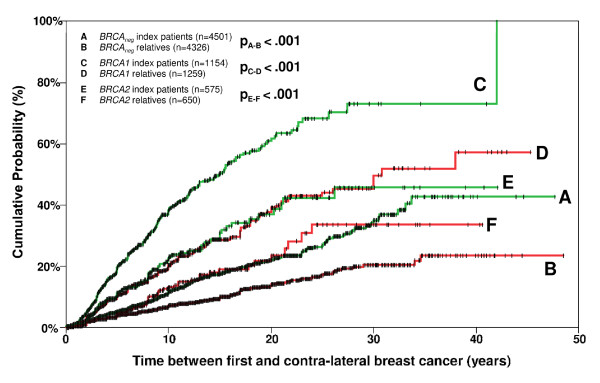
Cumulative risk of contra-lateral breast cancer after first breast cancer in index cases versus relatives from BRCA1/2 positive families and BRCA1/2 negative families.
Risk of contralateral breast cancer depending on mutation status
In 6,235 relatives of index patients, 502 contralateral breast cancers were observed. The total observation time from first breast cancer until contralateral breast cancer or censoring was 48,390 person-years. Figure 2A shows the distribution of age at first breast cancer for relatives of families with BRCA1 and BRCA2 mutations and of BRCA1/2 negative families. Relatives of families with BRCA1 mutations were significantly younger at first breast cancers than those from families with BRCA2 mutations (P < 0.001) and both were significantly younger than patients from BRCA1/2 negative families (P < 0.001 and P < 0.001, respectively). Likewise, the age at contralateral breast cancer was also significantly lower in the BRCA1 group than the BRCA2 group (P < 0.001) and the BRCA1/2 negative group (P < 0.001) (Figure 2B). Analyses of the time from first breast cancer to contralateral breast cancer showed that contralateral breast cancer risk was significantly higher in women from families with a BRCA1 mutation compared to women from families with a BRCA2 mutation and compared to women from families without a BRCA mutation (Figure 2C). Members of families with BRCA1 mutations had a threefold (95%CI, 2.5 to 3.6) higher risk of contralateral breast cancer than members of families without BRCA1/2 mutations. For members of families with BRCA2 mutations the risk was 1.6-fold (95% CI, 1.2 to 2.2) higher than the risk for members of families without BRCA1/2 mutations. The 25-year cumulative risk of contralateral breast cancer after first breast cancer was 44.1% (95%CI, 37.6% to 50.6%) for relatives from families with a BRCA1 mutation, 33.5% (95%CI, 22.4% to 44.7%) for relatives from families with a BRCA2 mutation and 17.2% (95%CI, 14.5% to 19.9%) for relatives from families without a BRCA1/2 mutation.
Figure 2.
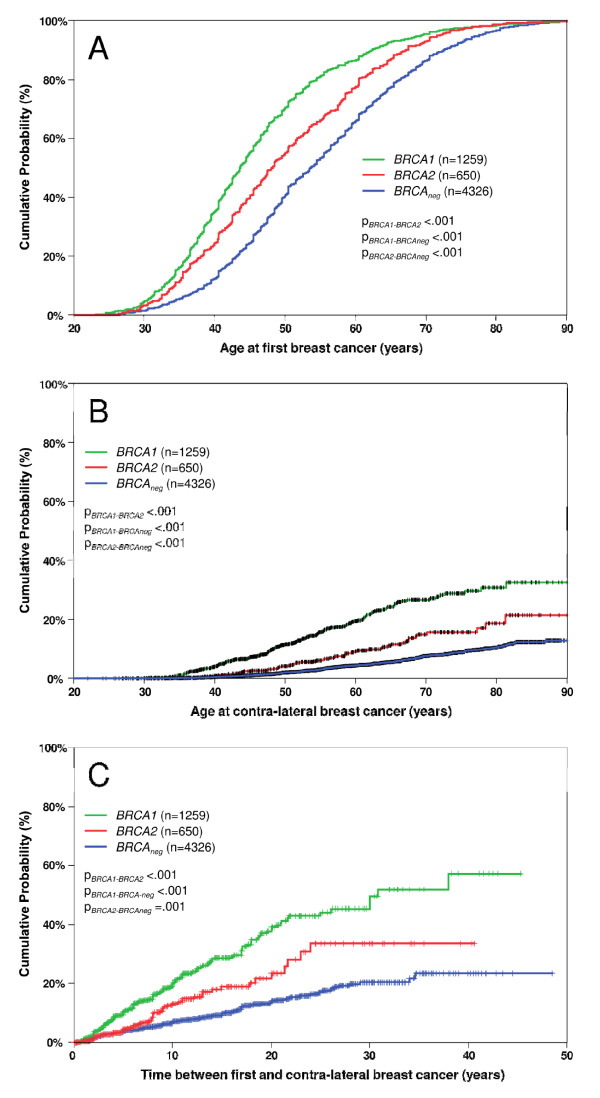
Effect of mutation status on cancer risk. Panel A shows the cumulative distribution of the age of diagnosis at first breast cancer. Panel B depicts the age of diagnosis at contralateral breast cancer. Panel C represents the cumulative risk of contralateral breast cancer after first breast cancer. Index patients were excluded.
Risk of contralateral breast cancer depending on age at first breast cancer
Younger age at first breast cancer was associated with a significantly higher risk of contralateral breast cancer in patients from BRCA1 positive and BRCA1/2 negative families and a trend was observed in patients from BRCA2 positive families (Figures 3A, B and C). For instance, after 25 years 55.1% (95%CI, 45.4% to 64.9%) of patients from BRCA1 positive families, 38.4% (95% CI, 18.5% to 58.2%) of patients from BRCA2 families and 28.4% (95% CI, 20.5% to 36.3%) of patients from non-BRCA1/2 families who were younger than 40 years at first breast cancer developed contralateral breast cancer. Of those who were older than 50 years of age at first breast cancer, 21.6% (95%CI, 12.3% to 30.8%) from families with a BRCA1 mutation, 15.5% (95% CI, 7.8% to 23.3%) from families with a BRCA2 mutation and 12.9% (95% CI 8.9% to 17.0%) from BRCA1/2 negative families developed contralateral breast cancer (Figure 3A, B, C). As expected the highest risks were seen for BRCA1 mutation carriers under the age of 35 years with 15.7% (95% CI, 9.7% to 21.7%) after 5 years, 33.4% (95% CI, 24.6% to 42.1%) after 10 years, 45.3% (95% CI, 34.8% to 55.7%) after 15 years and 61.6% (95% CI, 49.0% to 74.1%) after 25 years. Table 2 depicts the cumulative contralateral breast cancer risk estimates for 5, 10, 15, 20, and 25 years after first breast cancer depending on BRCA1/2 mutation status and age at first breast cancer.
Figure 3.
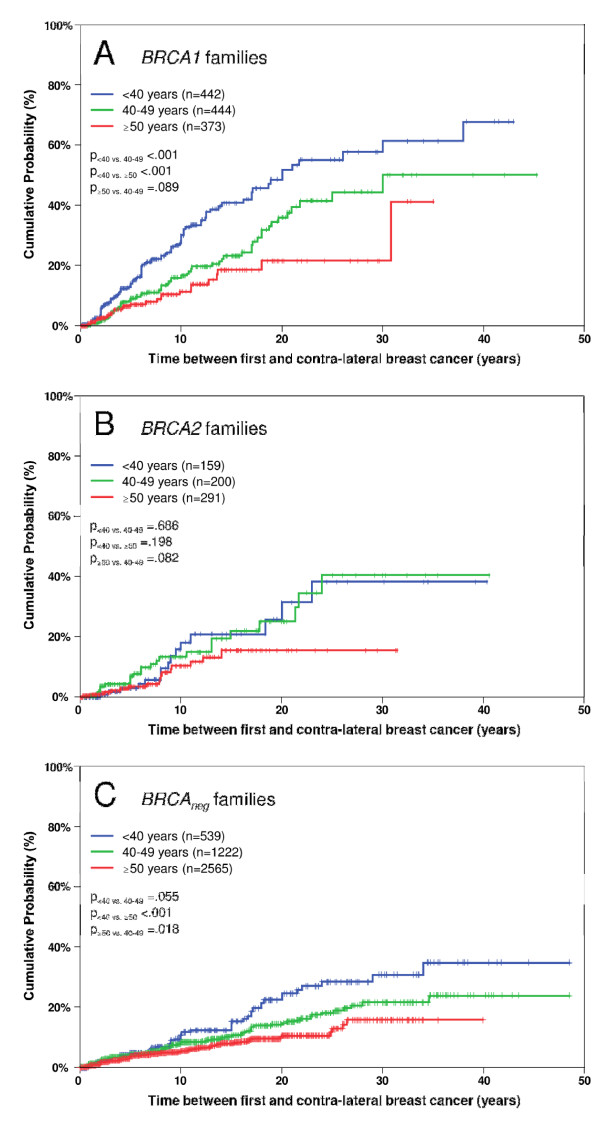
Effect of age at first breast cancer on contralateral breast cancer risk. All panels show the cumulative risk of contralateral breast cancer after first breast cancer. Panel A: relatives from BRCA1 positive families, Panel B: relatives from BRCA2 positive families, Panel C: relatives from BRCA1/2 negative families. Index patients were excluded.
Table 2.
Cumulative risks (in %) and 95% confidence intervals (in parentheses) for contralateral breast cancer depending on age at first breast cancer observed in relatives of index patients.
| BRCA1 | BRCA2 | BRCA negative | |
|---|---|---|---|
| Age at first breast cancer < 40 years | |||
| 5 years after first breast cancer | 14.1 (10.1-18.0) | 2.9 (0.0-6.3) | 4.8 (2.6-6.9) |
| 10 years after first breast cancer | 30.1 (24.0-36.2) | 18.2 (7.9-28.5) | 10.6 (6.8-14.4) |
| 15 years after first breast cancer | 40.8 (33.2-48.3) | 20.9 (9.7-32.1) | 15.3 (10.4-20.3) |
| 25 years after first breast cancer | 55.1 (45.4-64.9) | 38.4 (18.5-58.2) | 28.4 (20.5-36.3) |
| Age at first breast cancer 40-49 years | |||
| 5 years after first breast cancer | 9.2 (5.8-12.5) | 6.9 (2.7-11.1) | 4.2 (2.9-5.5) |
| 10 years after first breast cancer | 16.7 (11.7-21.7) | 13.4 (7.0-19.8) | 8.4 (6.3-10.5) |
| 15 years after first breast cancer | 23.2 (16.9-29.6) | 22.0 (12.1-31.9) | 10.7 (8.1-13.3) |
| 25 years after first breast cancer | 44.5 (33.2-55.7) | 40.5 (22.4-58.6) | 18.1 (13.9-22.3) |
| Age at first breast cancer ≥ 50 years | |||
| 5 years after first breast cancer | 7.1 (3.8-10.5) | 3.5 (0.9-6.1) | 3.6 (2.7-4.5) |
| 10 years after first breast cancer | 11.4 (6.5-16.3) | 10.4 (4.9-16.0) | 5.5 (4.3-6.7) |
| 15 years after first breast cancer | 18.7 (11.0-26.3) | 15.5 (7.8-23.3) | 8.1 (6.3-9.9) |
| 25 years after first breast cancer | 21.6 (12.3-30.8) | 15.5 (7.8-23.3) | 12.9 (8.9-17.0) |
| Total | |||
| 5 years after first breast cancer | 10.4 (8.3-12.5) | 4.5 (2.5-6.5) | 3.9 (3.2-4.6) |
| 10 years after first breast cancer | 20.4 (17.1-23.7) | 13.2 (9.2-17.2) | 7.1 (6.0-8.2) |
| 15 years after first breast cancer | 28.7 (24.4-32.9) | 19.0 (13.5-24.4) | 9.9 (8.5-11.4) |
| 25 years after first breast cancer | 44.1 (37.6-50.6) | 33.5 (22.4-44.7) | 17.2 (14.5-19.9) |
Other potential modifiers of contralateral breast cancer risk
The number of affected family members, the age at onset of the earliest affected family member, the number of bilateral breast cancers in the family and PBSO were not predictive for the occurrence of contralateral breast cancer. The latter may be due to the fact that only 131 patients of our cohort opted for PBSO. Having only limited information on tamoxifen use which was introduced into breast cancer treatment around 1990, we performed a comparison of women who had their first breast cancer before 1990 with women who had their first breast cancer after 1990. We could not find a significant difference in the time from first to contralateral breast cancer between these two groups.
Discussion
Little is known about the contralateral breast cancer risk in women with familial breast cancer that tested negative for BRCA1 or BRCA2 mutations. Despite that lack of knowledge, however, the demand for prophylactic bilateral mastectomy after first breast cancer is increasing not only for mutation carriers but also for women without a BRCA1 or BRCA2 mutation [14,15]. Therefore, there is an urgent need for empirical data that may allow women to base their informed decision regarding prophylactic bilateral mastectomy on reliable risk estimates.
Contralateral breast cancer risk depending on mutation status
Our results are concordant with a previous retrospective study on a cohort of 327 familial non-BRCA1/2 breast cancer cases [12,13]. These authors found incidences of metachronous contralateral breast cancers after 10 years that were similar to those in sporadic breast cancer patients (that is, 6.4% versus 5.4%, respectively). They also provided evidence that previous reports on higher contralateral breast cancer incidence rates may have been due to a selection bias caused by preferential DNA testing in women with bilateral breast cancer [13]. Moreover, our calculated risks are similar to those reported in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database, that is, a contralateral breast cancer risk of approximately 2.5% to 12.6% in 10 years depending on estrogen receptor status and age at onset [18,19]. Our data that we estimated for cases from BRCA1/2 negative families are in the same risk range of 7.1% (95% CI 6.0% to 8.2%) after 10 years.
Therefore, we conclude that contralateral breast cancer risk for familial non-BRCA1/2 breast cancer is essentially in the same range as for women with sporadic breast cancer. However, our risk estimates are in clear contrast with the recently observed increase in prophylactic bilateral mastectomy and prophylactic contralateral mastectomy in women with familial breast cancer and unknown or negative mutation status [14,15,20]. This increase may in part be due to uncertainties and overestimations of the true risks in the case of early age at first breast cancer or due to a positive family history, irrespective of the mutation status [21-23].
The risks that we calculated for relatives of BRCA1 or BRCA2 mutation carriers are in agreement with our previous report on the same but smaller cohort [5]. Our results are further supported by results from the WECARE study, a population-based, nested case-control study on 705 cases with contralateral breast cancer and 1,398 controls with unilateral breast cancer ascertained through US cancer registries that participated in the SEER registry system [24]. In this study, a woman diagnosed with BRCA1 associated breast cancer between the age of 25 to 29 was calculated to have a 5-year and 10-year risk for contralateral breast cancer of 16% and 29%, respectively. This is in line with our results, that is, a risk for contralateral breast cancer of 15.7% (95% CI 9.7% to 21.7%) after 5 years and 33.4% (95% CI 24.6% to 42.1%) after 10 years for women from BRCA1 positive families that developed the first breast cancer before the age of 35 years. However, our risk estimates are lower than those recently reported by Metcalfe et al. in a cohort of 810 mutation carriers [10] where the authors estimated a 15-year risk of 36.1% for BRCA1 and 28.5% for BRCA2 mutation carriers. Pierce et al. also reported higher risk estimates with a 15-year risk of 39% for BRCA1 and BRCA2 mutation carriers [3]. The most likely explanation is that these studies focused on index cases or did not exclude index cases, thereby introducing a recruitment bias.
Modifiers of contralateral breast cancer risk
We present results which highlight that contralateral breast cancer risk in women from BRCA1/2 negative families depends on age at onset of first breast cancer, as is the case for women from BRCA1 or BRCA2 positive families. In a recent paper, Metcalfe et al. also confirmed that the risk of contralateral breast cancer depends on age at first diagnosis in BRCA1/2 mutation carriers. Women older than 50 years at the time of breast cancer experienced a significantly lower risk of contralateral breast cancer than women diagnosed before the age of 40 years (RR 0.47; 95% CI 0.47 to 0.82, P = 0.008) [10]. Metcalfe et al. could further show that increasing numbers of affected first degree relatives under the age of 50 years were associated with an increased risk for contralateral breast cancer in BRCA1 mutation carriers (RR trend 1.40; 95% CI 1.08 to 1.81, P = 0.01) [10]. Despite extensive analyses, we could not confirm this association in our cohort. Moreover, we could not demonstrate an effect of PBSO on contralateral breast cancer risk as described by others [25]. This may be explained by the fact that only 131 women opted for PBSO in this cohort.
Limitations
While the strength of our study is the large sample size, some limitations should be mentioned. First, we cannot rule out that phenocopies might have been included in our analysis, since only 16% of the relatives from BRCA1 or BRCA2 positive families were proven mutation carriers. However, Meijers-Heijboer et al. calculated a phenocopy rate of 5% to 6% [26]. Therefore, it seems unlikely that phenocopies had a large impact on our results. Second, we cannot exclude a survivorship and recruitment bias. This is supported by other studies which provided convincing evidence for such a bias [13,26].. But as we considered only affected relatives, including deceased patients, it is unlikely for the survivorship and recruitment bias to have a negative impact on our results. Besides, we cannot rule out that the exclusion of index patients from the analysis, which was done to avoid overestimation of risks, may have led to an underestimation to some extent. Third, medical reports could only be obtained from 83% of the patients. This could have led to an incomplete ascertainment of contralateral breast cancer. However, results from a recent population-based study are in line with our risk estimates for contralateral breast cancer in BRCA1 and BRCA2 carriers [24]. Therefore, it is unlikely that incomplete reporting of affected family members had a considerable influence on our results. Fourth, the stringent selection of high risk families, as illustrated by a 25% mutation prevalence in our cohort, might influence our risk estimates. In countries where selection criteria are different, the risk estimates for the BRCA1/2 negative families may vary accordingly. Fifth, we could not demonstrate an effect of further modifying factors such as PBSO, tamoxifen, chemo- and radiation therapy on contralateral breast cancer due to the low uptake of these interventions or incomplete reporting in our cohort [3,7,9-11]. Finally, our analysis did not consider competing cancer events. For instance, the group specific risk estimates may be biased to some extent by the different ovarian cancer risks in the three groups.
Conclusions
We calculated long term risk estimates for contralateral breast cancer in the largest cohort of women with familial breast cancer reported so far. We demonstrate: 1) that contralateral breast cancer risk for patients from BRCA1/2 negative families is low and similar to the risk for patients with sporadic breast cancer; and 2) that contralateral breast cancer risk strongly depends on the mutation status and age at onset of the first breast cancer. This strengthens the importance of genetic testing as a prerequisite for risk estimation and, consequently, informed decision making for or against prophylactic bilateral mastectomy or prophylactic contralateral mastectomy.
Abbreviations
BRCA: breast cancer gene; HR: hazard ratio; PBSO: prophylactic bilateral salpingo-oophorectomy.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KR, CE and RS were responsible for the conception and design of the study, acquisition and analysis of data, and drafted the manuscript. SZ, MG, KK, MK, ND, WJ, CM, MG, DV, SP-A, TH, UB, DG, SB and AM acquired data and revised the manuscript critically. All authors read and approved the final manuscript.
See related Letter by Evans et al., http://breast-cancer-research.com/content/15/1/401
Contributor Information
Kerstin Rhiem, Email: kerstin.rhiem@uk-koeln.de.
Christoph Engel, Email: christoph.engel@imise.uni-leipzig.de.
Monika Graeser, Email: monika.graeser@uk-koeln.de.
Silke Zachariae, Email: silke.zachariae@imise.uni-leipzig.de.
Karin Kast, Email: karin.kast@uniklinikum-dresden.de.
Marion Kiechle, Email: marion.kiechle@lrz.tu-muenchen.de.
Nina Ditsch, Email: nina.ditsch@uni-muenchen.de.
Wolfgang Janni, Email: wolfgang.janni@med.uni-duesseldorf.de.
Christoph Mundhenke, Email: cmundhenke@hotmail.com.
Michael Golatta, Email: michael.golatta@med.uni-heidelberg.de.
Dominic Varga, Email: dominic.varga@uniklinik-ulm.de.
Sabine Preisler-Adams, Email: sabine.preisler-adams@ukmuenster.de.
Tilman Heinrich, Email: tilman.heinrich@uk-wuerzburg.de.
Ulrich Bick, Email: ulrich.bick@charite.de.
Dorothea Gadzicki, Email: dorothea.gadzicki@mh-hannover.de.
Susanne Briest, Email: susanne.briest@medizin.uni-leipzig.de.
Alfons Meindl, Email: alfons.meindl@lrz.tu-muenchen.de.
Rita K Schmutzler, Email: rita.schmutzler@uk-koeln.de.
Acknowledgements
We thank the medical data assistants Tim Roenz (Berlin), Kristin Lilpopp (Dresden), Edith Maria Becker (Duesseldorf), Astrid Schmidt (Hannover), Ursula Major (Heidelberg), Manuela Arnold (Kiel), Petra Wihler (Cologne), Sylvia Stark (Leipzig), Ute Enders (Leipzig), Manuela Jähnig (Munich), Andrea Scarlat-Wolkstein (Munich), Anna Baumeister (Muenster), Ute Zemmler (Ulm) and Katharina Höhn (Wuerzburg) for their tremendous and continuous efforts to update the database.
References
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck RT, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M. et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;14:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Gene. 2003;14:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, Harris EE, Gaffney DK, Haffty BG, Dawson LA, Narod SA, Olivotto IA, Eisen A, Whelan TJ, Olopade OI, Isaacs C, Merajver SD, Wong JS, Garber JE, Weber BL. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;14:2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- Pierce LJ, Haffty BG. Radiotherapy in the treatment of hereditary breast cancer. Semin Radiat Oncol. 2011;14:43–50. doi: 10.1016/j.semradonc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, Froster UG, Schlehe B, Bechtold A, Arnold N, Preisler-Adams S, Nestle-Kraemling C, Zaino M, Loeffler M, Kiechle M, Meindl A, Varga D, Schmutzler RK. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation Carriers. J Clin Oncol. 2009;14:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Bégin LR, Offit K, Foulkes WD. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;14:R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod SA, Brunet JS, Ghadirian P, Robson M, Heimdal K, Neuhausen SL, Stoppa-Lyonnet D, Lerman C, Pasini B, de los Rios P, Weber B, Lynch H. Hereditary Breast Cancer Clinical Study Group. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000;14:1876–1881. doi: 10.1016/S0140-6736(00)03258-X. [DOI] [PubMed] [Google Scholar]
- Pierce LJ, Phillips KA, Griffith KA, Buys S, Gaffney DK, Moran MS, Haffty BG, Ben-David M, Kaufman B, Garber JE, Merajver SD, Balmaña J, Meirovitz A, Domchek SM. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;14:389–398. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, Olopade OI, Eisen A, Weber B, McLennan J, Sun P, Foulkes WD, Narod SA. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;14:2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Metcalfe K, Gershman S, Lynch HT, Ghadirian P, Tung N, Kim-Sing C, Olopade OI, Domchek S, McLennan J, Eisen A, Foulkes WD, Rosen B, Sun P, Narod SA. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2011;14:1384–1392. doi: 10.1038/bjc.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, Kim-Sing C, Olsson H, Ainsworth P, Eisen A, Saal H, Friedman E, Olopade O, Osborne M, Weitzel J, Lynch H, Ghadirian P, Lubinski J, Sun P, Narod SA. Hereditary Breast Cancer Clinical Study Group: tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: an update. Int J Cancer. 2006;14:2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- Tilanus-Linthorst MM, Alves C, Seynaeve C, Menke-Pluymers MB, Eggermont AM, Brekelmans CT. Contralateral recurrence and prognostic factors in familial non-BRCA1/2-associated breast cancer. Br J Surg. 2006;14:961–968. doi: 10.1002/bjs.5344. [DOI] [PubMed] [Google Scholar]
- Tilanus-Linthorst MM, Bartels KC, Alves C, Bakri B, Crepin E, van den Ouweland A, Klijn JG, Meijers-Heijboer H, Brekelmans CT. Selection bias influences reported contralateral breast cancer incidence and survival in high risk non-BRCA1/2 patients. Breast Cancer Res Treat. 2006;14:117–123. doi: 10.1007/s10549-005-9054-2. [DOI] [PubMed] [Google Scholar]
- King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, Morrow M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;14:2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- Khan SA. Contralateral prophylactic mastectomy: What do we know and what do our patients know? J Clin Oncol. 2011;14:2132–2135. doi: 10.1200/JCO.2010.33.4482. [DOI] [PubMed] [Google Scholar]
- Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK. Hereditary breast and ovarian cancer: new genes, new treatments, new concepts. Dtsch Arztebl Int. 2011;14:323–330. doi: 10.3238/arztebl.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl A. German Consortium for Hereditary Breast and Ovarian Cancer. Comprehensive analysis of 989 patients with breast and ovarian cancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. Int J Cancer. 2002;14:472–480. doi: 10.1002/ijc.1626. [DOI] [PubMed] [Google Scholar]
- Nichols H, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;14:1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst. 2009;14:1058–1065. doi: 10.1093/jnci/djp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;14:935–941. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichapat V, Garmo H, Holmberg L, Fentiman IS, Tutt A, Gillett C, Lüchtenborg M. Prognosis of metachronous contralateral breast cancer: importance of stage, age and interval time between the two diagnoses. Breast Cancer Res Treat. 2011;14:609–618. doi: 10.1007/s10549-011-1618-8. [DOI] [PubMed] [Google Scholar]
- Vichapat V, Gillett C, Fentiman IS, Tutt A, Holmberg L, Lüchtenborg M. Risk factors for metachronous contralateral breast cancer suggest two aetiological pathways. Eur J Cancer. 2011;14:1919–1927. doi: 10.1016/j.ejca.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Boughey JC, Hoskin TL, Degnim AC, Sellers TA, Johnson JL, Kasner MJ, Hartmann LC, Frost MH. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. 2010;14:2702–2709. doi: 10.1245/s10434-010-1136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M, Reiner AS, Riedel ER, Thomas DC, Mellemkjaer L, Lynch CF, Boice JD Jr, Anton-Culver H, Bernstein JL. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;14:2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t'veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;14:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers-Heijboer H, Brekelmans CT, Menke-Pluymers M, Seynaeve C, Baalbergen A, Burger C, Crepin E, van den Ouweland AW, van Geel B, Klijn JG. Use of genetic testing and prophylactic mastectomy and oophorectomy in women with breast or ovarian cancer from families with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2003;14:1675–1681. doi: 10.1200/JCO.2003.09.052. [DOI] [PubMed] [Google Scholar]


