Abstract
Culex pipiens L. (Diptera: Culicidae) and Culex restuans Theobald are the primary enzootic and bridge vectors of West Nile virus in the eastern United States north of 36° latitude. Recent studies of the natural history of these species have implicated catch basins and underground storm drain systems as important larval development sites in urban and suburban locales. Although the presence of larvae in these habitats is well-documented, the influence of abiotic factors on the ecology of Culex larvae developing in them remains poorly understood. Therefore, we examined the effects of multiple abiotic factors and their interactions on abundance of Culex larvae in catch basins in the Chicago, IL, metropolitan area. Low precipitation and high mean daily temperature were associated with high larval abundance, whereas there was no correlation between catch basin depth or water depth and larval abundance. Rainfall was an especially strong predictor of presence or absence of larvae in the summer of 2010, a season with an unusually high precipitation. Regression tree methods were used to build a schematic decision tree model of the interactions among these factors. This practical, visual representation of key predictors of high larval production may be used by local mosquito abatement districts to target limited resources to treat catch basins when they are particularly likely to produce West Nile virus vectors.
Keywords: Culex pipiens L., Culex restuans Theobald, catch basin, oviposition site, precipitation
Since its introduction in the New York metropolitan area in 1999 (Lanciotti et al. 1999), West Nile virus (family Flaviviridae, genus Flavivirus, WNV) has spread at an unprecedented rate throughout much of North America and is now a serious public health concern. Over the past 11 yr, >30,500 human cases have been clinically confirmed in the United States (Center for Disease Control [CDC], 2011). Locally, Cook County has had consistently high mosquito infection rates and persistent cases of human illness, with 37% of the 1,635 human cases reported in Illinois since 2002 (Cook County Department of Public Health, 2011).
Culex pipiens L. (Diptera: Culicidae) and Culex restuans Theobald have been implicated as the primary enzootic vectors for WNV in the eastern United States north of 36[H11034] latitude (Andreadis et al. 2004, Turell et al. 2005), and also as bridge vectors of WNV to humans (Kilpatrick et al. 2005, Hamer et al. 2008). Recent studies of the natural history of Cx. pipiens and Cx. restuans have identified catch basins and underground storm drain systems as key oviposition and larval development sites in urban and suburban locales (Geery and Holub 1989, Crans 2004, Su et al. 2003, Gu et al. 2006). Although the presence of larvae in these habitats is well-documented, few studies have synthesized fine-scale environmental variation and landscape features to identify characteristics that make some catch basins or time ranges especially productive. The lack of this knowledge has hampered our understanding of the conditions that favor transmission and amplification of WNV in urban environments and reduced the efficiency and effectiveness of interventions.
This exploratory study examines the effects of multiple abiotic factors, including catch basin depth, water depth, temperature, and precipitation, on catch basin larval production in the Chicago suburb of Alsip, IL. We use recursive partitioning Classification and Regression Trees modeling (Breiman et al. 1984) to identify conditions associated with high Culex larval abundances. Our results may be used to guide mosquito control efforts, which often fail to take into account the spatial and temporal heterogeneity in larval habitat productivity in urban areas.
Materials and Methods
Larval sampling was conducted in a residential neighborhood in Alsip, IL. Alsip is a suburban village located 20 km southwest of downtown Chicago, with an area of 16.5 km2 and a population of 18,580 in 2010. WNV became established in the region during the summer of 2002 with 884 human cases state-wide (Watson et al. 2004, Huhn et al. 2005), at the time the largest reported West Nile meningoencephalitis epidemic (CDC, 2002). The greater Chicago area has continuously recorded virus-positive mosquitoes, birds, and humans over the past 8 yr (Bertolotti et al. 2008, Hamer et al. 2008), with peak activity in 2002, 2005, and 2006 (Ruiz et al. 2010). We selected the study area for its history as an important urban WNV hot spot in the United States as well as for its notable concentration of cases of human illness locally (Ruiz et al. 2004).
To estimate the relative abundances of larvae, we selected 19 catch basins within an area with a 1.5 km radius (Fig. 1). Other than depth, the dimensions of the catch basins sampled were relatively uniform and the standard diameter was 60 cm. They all had open grates, were located on the edge of residential suburban streets, and were in neighborhoods of similar age and housing types. The basins were sampled for larvae three times per week from 14 June to 29 September 2010. Basins were sampled up to 45 times during this period with methods described in Hamer et al. (2011). Larvae and pupae were collected using a 10.2 × 10.2-cm aquarium net attached to the end of a conduit pole, 3 m in length and 1.3 cm in diameter. The pole was inserted through the grate and passed over the water surface in a single figure eight. The net was then inverted into a container and flushed out with water from a laboratory wash bottle. Larval abundance per catch basin was classified into four categories as none (no larvae were present), low (1–10 larvae present), intermediate (11–60 larvae present), or high (>60 larvae present). These abundance classes were based on natural demarcations in larvae populations observed in previous years’ sampling in the same neighborhood.
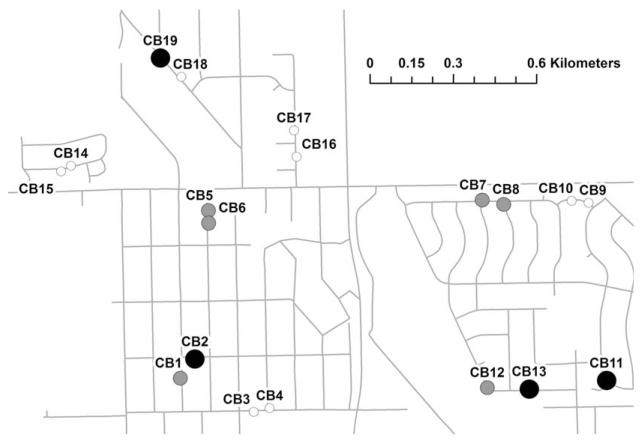
Fig. 1.
Map of catch basin study sites in Alsip, IL, a residential suburban area ≈20 km southwest of downtown Chicago. The 19 catch basins were sampled for larvae and pupae three times per week from 14 June to 29 September 2010. White circles indicate catch basins with intermediate or high larval abundance for <10% of samples. Gray circles indicate basins with intermediate or high abundance for 10–25% of samples. Black circles indicate basins with intermediate or high abundance for >25% of samples.
A subsample of five randomly selected fourth instar larvae per basin were identified to species each day using the key in Andreadis et al. (2005). Cx. pipiens and Cx. restuans were aggregated for analyses because samples with first to third instars were identified to genus but not to species. Larvae of both often share similar habitats (Crans 2004). Cx. restuans occurs at higher densities earlier in the summer while Cx. pipiens is more abundant later, but they commonly overlap seasonally and co-occur locally (Kunkel 2006), allowing comparisons of effects of abiotic factors on both species simultaneously.
To examine the effects of abiotic characteristics of the catch basins on larval production, we measured water depth three times per week on dates coincident with larval sampling. To assess the accuracy of our manual sampling, HOBO pendant data loggers (Onset Computer Corp., Pocasset, MA) were deployed in nine catch basins. The loggers recorded water depth (meters) inside the catch basins at 1-h intervals throughout the 16 wk sampling period. Data were offloaded using HOBOware Pro 3.1.2. Daily precipitation data were obtained from a Cook County Precipitation Network weather station (41.68° N, 87.75° W, Alsip, IL) located within the study area. Temperature data were retrieved from a NOAA National Weather Service station at Midway Airport (41.79° N, 87.75° W, Chicago, IL) located 9.2 miles from the study area. The coordinates of each basin were determined using a Juno handheld GPS unit (Trimble Navigation Ltd., Sunnyvale, CA).
Statistical analyses of collections of larvae were performed according to the following methods. Repeated measures analysis of variance (ANOVA) using SAS 9.2 (SAS Institute Inc., Cary, NC) was conducted using the larval abundance class (0 as no larvae to three as high larval abundance) as the response variable. Independent variables included mean daily temperature, largest precipitation event during the 4 d preceding sampling, water depth, and catch basin depth. The lag for precipitation was determined by logistic regression with the largest precipitation event during 1 to 10 d preceding sampling as the independent variable and larval abundance category as the response variable. The 4 d lag had the strongest correlation (χ2 = 74.04; P < 0.0001). Day of sampling was the repeated variable and the individual catch basins were treated as subjects. The Tukey procedure was used to determine significant differences for multiple comparisons.
Conditional inference tree modeling in R (R Foundation for Statistical Computing, Vienna, Austria; R Development Core Team 2010) with the package party version 1.0 (Hothorn et al. 2011) was used to examine and to visualize the effects of interactions among factors in determining larval abundances within catch basins (Hu et al. 2006). Conditional inference tree models belong to the Classification and Regression Trees family of nonparametric decision tree models described by Breiman et al. (1984). A model is built through recursive partitioning, a process in which an algorithm splits data sets into partitions based on homogeneity of response, then prunes to optimize the tree. Tree-based modeling handles missing covariates, may combine quantitative and qualitative covariates, and doesnot have the assumptions of generalized linear mixed models and neural networks, among other alternatives for quantitative data (Olden et al. 2008).
Results
Factor Effects Summary
In total, 817 larval samples were collected from nineteen catch basins throughout the season. Of these samples, 41 were high larval abundance, 75 were intermediate larval abundance, 184 were low larval abundance, and 517 contained no larvae. Three catch basins (CB2, CB11, and CB19) contained larvae >65% of sampling days. Five catch basins (CB3, CB14, CB15, CB17, and CB18) contained larvae <20% of sampling days (Fig. 1; Table 1). Among the larvae identified to species, 85% were Cx. pipiens, 15% were Cx. restuans, and two individuals sampled from a single basin on a single day were Aedes vexans (Meigen). The instance of Ae. vexans was not included in the analysis. Other invertebrates including some potential mosquito predators such as copepods were collected in the basins, but these were not tabulated.
Table 1. Numbers and percentages of collections of different larval abundances per catch basin from June 14 to Sept. 29.
| Catch basin ID | High abundance | Intermediate abundance | Low abundance | No. larvae | No. samples |
|---|---|---|---|---|---|
| CB1 | 6 (13.6%) | 4 (9.0%) | 8 (18.1%) | 26 (59.0%) | 44 |
| CB2 | 3 (6.8%) | 11 (25.0%) | 16 (36.3%) | 14 (31.8%) | 44 |
| CB3 | 0 (0%) | 1 (2.2%) | 7 (15.9%) | 36 (81.8%) | 44 |
| CB4 | 0 (0%) | 1 (2.2%) | 14 (31.8%) | 29 (65.9%) | 44 |
| CB5 | 0 (0%) | 6 (13.6%) | 13 (29.5%) | 25 (56.8%) | 44 |
| CB6 | 3 (6.8%) | 5 (11.3%) | 14 (31.8%) | 22 (50.0%) | 44 |
| CB7 | 4 (11.4%) | 1 (2.8%) | 6 (17.1%) | 24 (68.5%) | 35 |
| CB8 | 2 (5.0%) | 4 (10.0%) | 10 (25.0%) | 24 (60.0%) | 40 |
| CB9 | 0 (0.0%) | 1 (2.2%) | 10 (22.7%) | 33 (75.0%) | 44 |
| CB10 | 3 (6.8%) | 1 (2.2%) | 7 (15.9%) | 33 (75.0%) | 44 |
| CB11 | 4 (9.0%) | 14 (31.8%) | 12 (27.2%) | 14 (31.8%) | 44 |
| CB12 | 1 (6.8%) | 8 (18.1%) | 9 (20.4%) | 26 (59.0%) | 44 |
| CB13 | 6 (9.0%) | 5 (11.6%) | 9 (20.9%) | 23 (53.4%) | 43 |
| CB14 | 0 (0%) | 0 (0%) | 6 (13.9%) | 37 (86.0%) | 43 |
| CB15 | 0 (0%) | 1 (2.3%) | 6 (13.9%) | 36 (83.7%) | 43 |
| CB16 | 0 (0%) | 0 (0%) | 14 (32.5%) | 29 (67.4%) | 43 |
| CB17 | 0 (0%) | 3 (6.9%) | 2 (4.6%) | 38 (88.3%) | 43 |
| CB18 | 0 (0%) | 0 (0%) | 7 (15.9%) | 37 (84.0%) | 44 |
| CB19 | 9 (20.9%) | 9 (20.9%) | 13 (30.2%) | 12 (27.9%) | 43 |
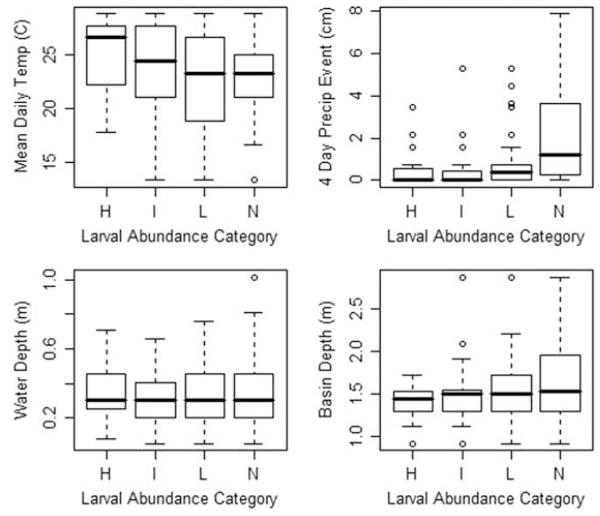
The four abiotic factors considered in our statistical analyses were precipitation (centimeter), mean daily temperature (°C), catch basin depth (meter), and water depth (meter). There was a significant relationship between larval abundance category and temperature (F = 1.93; df = 16, 731; P = 0.0157); and larval abundance category and precipitation (F = 3.72; df = 19, 731; P < 0.001). There were no relationships between larval abundance category and catch basin depth or water depth based on the ANOVA, though larval abundance was generally lower in deeper basins when considered bivariately (Fig. 2). Tukey’s multiple comparisons indicated a positive relationship between temperature and larval abundance category, with the highest mean daily temperatures corresponding to the highest larval abundances and lower temperatures corresponding to lower numbers (Table 2). There was no similar relationship between precipitation and larval abundance category, with equal amounts of precipitation associated with low, intermediate, and high numbers of larvae present.
Fig. 2.
Descriptive summary of relationship between abiotic factors and larval abundance category within 19 catch basins. Larval abundance categories are no larvae (N), low abundance (L), intermediate abundance (I), and high abundance (H).
Table 2. Tukey’s multiple comparisons tests of means for two factors determined significant by max likelihood ANOVA tests with repeated measures.
| Temperature |
Precipitation |
||
|---|---|---|---|
| Contrast | P | Contrast | P |
| 0.0032* | <0.001* | ||
| μN − μL | 0.5429 | μN − μL | <0.001* |
| μN − μI | 0.0610 | μN − μI | <0.001* |
| μN − μH | 0.0064* | μN − μH | <0.001* |
| μL − μI | 0.1909 | μL − μI | 0.1284 |
| μL − μH | 0.0238* | μL − μH | 0.3812 |
| μI − μH | 0.2757 | μI − μH | 0.7681 |
N, larval abundance categories are no larvae: L, low abundance; I. intermediate abundance; H, high abundance.
indicates a statistically significant result at αc = 0.05.
Conditional Inference Tree Analysis
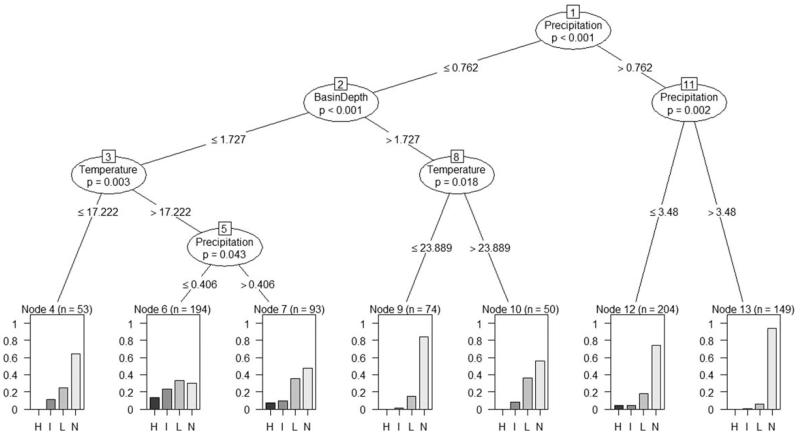
The results of the tree-based analysis supported the ANOVA tests of main effects. Precipitation, temperature, and catch basin depth were included as factors in the conditional inference tree, while water depth was dropped (Fig. 3). The path that favored highest catch basin larval abundance was precipitation <0.406 cm, catch basin depth <1.727 m, and temperature >17.22°C. The path that favored lowest larval abundance was a precipitation event >3.48 cm at least once during the 4 d preceding collection.
Fig. 3.
Conditional inference tree model of interactions between multiple abiotic factors in determining the relative larval productivity of catch basins. The path to the left at each node represent the conditions that lead to higher abundance of larvae compared with the path to the right at that node. The graphs for each terminal node indicate the percentage of the collections in each larval class given the conditions represented by that node.
Precipitation Effect
Both ANOVA comparisons of means and conditional inference tree analysis indicated that precipitation was a key factor in differentiating between the presence and absence of larvae in catch basins. Conditional inference tree modeling predicted that a rainfall event (two or more hours of continuous precipitation) above 3.48 cm reduced the number of catch basins producing larvae to near zero. To assess possible causation of this pattern we examined the interactions between precipitation and other abiotic factors measured in the current study.
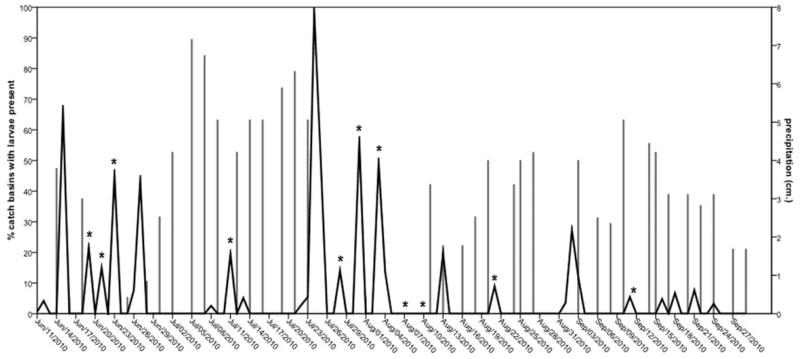
During the 4 d after rainfall events exceeding 3.48 cm, the percentage of catch basins sampled with larvae present (2.63% of catch basins containing larvae) was significantly smaller (χ2 = 285.83; df = 1; P < 0.001) than 5 d after the same events (42.11% of catch basins containing larvae) (Fig. 4). This result demonstrates the temporal lag for recolonization of catch basins through oviposition of female Culex mosquitoes (Chaves and Kitron 2011). The duration of the recovery period may be related to the positive correlation between precipitation and catch basin water depth. As demonstrated by water level data collected by the HOBO data loggers in unique catch basins, it typically takes 4 d for the water depth to drop from flooding conditions to normal levels after a large precipitation event.
Fig. 4.
Daily precipitation (peaks) and percentage of sampled catch basins with larvae present (lines) from 4 d before the beginning of collections to the end of the sampling period (11 June to 29 September). Asterisks indicate dates when no larvae were observed in any catch basins.
Discussion
The summer of 2010 was among the hottest and wettest in Illinois history (Illinois State Water Survey 2011), and our observations of catch basin larval production may have reflected this extreme weather pattern. There was a strong association between precipitation and larval production. Low rainfall favored high larval abundance across all catch basins sampled in our current study while high rainfall was associated with absence of larvae. A possible mechanistic explanation for this trend is that high rainfall flushed immature Culex out of catch basins, preventing adult females from laying egg rafts until water flow slowed. This relationship was emphasized in our conditional inference tree model of abiotic factors affecting larval abundance, where precipitation was the factor differentiating between productive and unproductive catch basins at the primary node.
Significant prior research has demonstrated that precipitation is an important variable in determining rates of both larval emergence and WNV activity. Because Culex oviposit egg rafts directly on the surface of the water (Hinton 1968), as opposed to floodwater mosquitoes that lay eggs above the water line, some precipitation is necessary to create the artificial standing water habitats preferred by these species (Means 1979). Several studies of WNV and other vector-borne disease systems have found positive associations between precipitation and human cases (Takeda et al. 2003, Landesman et al. 2007). However, conflicting recent work has indicated that an excess of rainfall may actually limit WNV incidence and vector production, with no suggested ecological mechanism for this trend (Mogi and Okazawa 1990, Ruiz et al. 2010). Our current results are measured in days rather than weeks or longer and suggest that this shorter time period is more closely linked to the mosquito life cycle.
Our study quantitatively supported the hypothesis that while moderate rainfall is necessary to provide oviposition and larval development habitats for egg raft laying Culex, an excess of precipitation precludes successful pupation and adult emergence. Conditional inference tree analysis indicated that a single multihour rainfall event exceeding 3.48 cm will remove almost all larvae from an underground catch basin system, in contrast to a prior study that reported a much higher amount of rainfall (10 cm) required to dramatically reduce the abundance of Culex in catch basins (Geery and Holub 1989). After the precipitation event, it takes approximately 4 d for larval production to return to preceding levels. Both this rainfall effect and its duration likely can be attributed to the positive correlation between precipitation and catch basin water depth. When the water level rises above the catch basin sump, the water may flood within the storm drain system and no longer offer a stagnant breeding habitat hospitable (Means 1979) to Culex larvae. If the immature Culex development time at the observed temperatures in this study is 10 d (Madder et al. 1983), adult emergence would not be possible until 13 or 14 d after a large rain event.
High temperatures also appeared to favor high mosquito larval abundance. Culex larvae develop more quickly when both ambient and aquatic temperatures are higher (Hagstrum and Workman 1971, Rueda et al. 1990). The faster larvae hatch and develop, the higher the probability of obtaining samples with larvae. This result may have significant implications for WNV activity because prior research suggests that heat also increases Culex species’ competence as vectors (Dohm et al. 2002).
This exploratory analysis should serve as a foundation for future studies of catch basin ecology in heterogeneous urban environments. Catch basin larval productivity may be affected by a multiplicity of additional abiotic and biotic factors, potentially including water chemistry (Chaves et al. 2011), depth and content of detritus within the basin (David et al. 2003), street chemicals and fertiliziers, and surrounding vegetation characteristics (Muturi et al. 2007). While wide scale environmental effects including temperature and precipitation may explain temporal variation in larval productivity throughout the season, further examination of these fine scale landscape features may assist in differentiating between the relative productivity of catch basins. Weather variability likely does not affect all basins equally. Some basins are sheltered from precipitation and heat by trees, and relative permeability of the surface surrounding basins (e.g., concrete versus grass) may amplify or mitigate the effects of rain. Therefore, it seems improbable that temperature and volume of precipitation are the sole determinants of larval production in catch basins. Finescale variables beyond the scope of the current study should be considered.
In addition, our results may be used to guide mosquito abatement districts’ protocols for controlling larval populations and mosquito-borne diseases. Although catch basins may be chemically treated to eliminate larvae or inhibit larval development and thus reduce adult emergence rates (Knepper et al. 1992), many public health departments lack the supplies and personnel to sample catch basins continuously throughout the summer. As a result, many highly productive catch basins are treated less frequently than the 30–90 d typically specified by larvicide product guidelines while resources may be wasted on treating consistently unproductive basins.
Our conditional inference tree model, based on easily approximated and publicly accessible catch basin characteristics, may aid insect pest management programs in focusing on treating basins when they are especially likely to produce WNV vectors. While previous studies of storm drain system ecology have identified factors influencing the relative numbers of mosquito larvae produced by individual catch basins (Rey et al. 2006), none have quantitatively examined different effects of broad and fine scale environmental variation and their interactions. The decision tree provides a practical, schematic visual representation of these interactions that may be used to inform larval control decisions and resource allocation in the field.
Acknowledgments
We thank Tavis Anderson, William Brown, Danielle Donovan, Mike Glester, Diane Godhe, Patrick Kelly, and Timothy Thompson for assistance in collecting larval mosquitoes and microhabitat data and Ronald Weigel for advice on the statistical analysis. We also thank the Village of Alsip, the City of Chicago, and private landowners in these municipalities for allowing us to conduct this research, as well as the Village of Oak Lawn for providing field laboratory facilities and support. This material is based upon work supported by the National Science Foundation/National Institutes of Health Ecology of Infectious Disease program under Award No. 0840403 and through funding from the University of Illinois Adaptive Infrastructure Information Systems Initiative.
References Cited
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut, USA: a five year analysis of mosquito data, 1999– 2003. Vector-borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. The Connecticut Agricultural Experiment Station; New Haven, CT: 2005. [Google Scholar]
- Bertolotti L, Kitron UD, Walker ED, Ruiz MO, Brawn JD, Loss SR, Hamer GL, Goldberg TL. Fine-scale genetic variation and evolution of West Nile virus in a transmission “hot spot” in suburban Chicago, USA. Virology. 2008;374:381–389. doi: 10.1016/j.virol.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. Chapman & Hall/CRC; New York: 1984. [Google Scholar]
- (CDC) Center for Disease Control Update: West Nile virus activity in the United States. 2011 http://www.cdc.gov/ncidod/dvbid/westnile.
- (CDC) Center for Disease Control Provisional surveillance summary of the West Nile virus epidemic: United States, January–November 2002. Morbid. Mortal. Wkly. Rep. 2002;51:1129–1133. [PubMed] [Google Scholar]
- Chaves LF, Keogh CL, Nguyen AM, Decker GM, Vazquez-Prokopecl GM, Kitron UD. Combined sewage overflow accelerates immature development and increases body size in the urban mosquito Culex quinquefasciatus. J. Appl. Entomol. 2011;135:611–620. [Google Scholar]
- Chaves LF, Kitron UD. Weather variability impacts on oviposition dynamics of the southern house mosquito at intermediate time scales. Bull. Entomol. Res. 2011;101:633–641. doi: 10.1017/S0007485310000519. [DOI] [PubMed] [Google Scholar]
- Cook County Department of Public Health West Nile virus homepage. 2011 http://www.cookcountypublichealth.org/west-nile-virus.
- Crans WJ. A classification system for mosquito life cycles: life cycle traps for mosquitoes of the northeastern United States. J. Vector Ecol. 2004:1–10. [PubMed] [Google Scholar]
- David JP, Tilquin M, Rey D, Ravanel P, Meyran JC. Mosquito larval consumption of toxic arborescent leaf-litter, and its biocontrol potential. Med. Vet. Entomol. 2003;17:151–157. doi: 10.1046/j.1365-2915.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Geery PR, Holub RE. Seasonal abundance and control of Culex spp. in catch basins in Illinois. J. Am. Mosq. Control Assoc. 1989;5:537–540. [PubMed] [Google Scholar]
- Gu W, Lampman R, Krasavin N, Berry R, Novak R. Spatio-temporal analyses of West Nile virus transmission in Culex mosquitoes in northern Illinois, USA, 2004. Vector-borne Zoonotic Dis. 2006;6:91–98. doi: 10.1089/vbz.2006.6.91. [DOI] [PubMed] [Google Scholar]
- Hagstrum DW, Workman EB. Interaction of temperature and feeding rate in determining the rate of development of larval Culex tarsalis (Diptera: Culicidae) Ann. Entomol. Soc. Am. 1971;64:668–671. [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J. Med. Entomol. 2008;25:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kelly PH, Focks DA, Goldberg TL, Walker ED. Evaluation of a novel mosquito emergence trap to study Culex mosquitoes in urban catch basins. J. Am. Mosq. Control Assoc. 2011;27:142–147. doi: 10.2987/10-6090.1. [DOI] [PubMed] [Google Scholar]
- Hinton HE. Structure and protective devices of the egg of the mosquito Culex pipiens. J. Insect Physiol. 1968;14:145–148. doi: 10.1016/0022-1910(68)90027-9. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Hornik K, Strobl C, Zeileis A. Party: a laboratory for recursive partytioning version 1.0. 2011 http://cran.r-project.org/web/packages/party/index.html.
- Hu W, Tong S, Mengersen K, Oldenburg B, Dale P. Mosquito species (Diptera: Culicidae) and the transmission of Ross River virus in Brisbane, Australia. J. Med. Entomol. 2006;43:375–381. doi: 10.1603/0022-2585(2006)043[0375:msdcat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Huhn GD, Austin C, Langkop C, Kelly K, Lucht R, Lampman R, Novak R, Haramis L, Boker R, Smith S, et al. The emergence of West Nile virus during a large outbreak in Illinois in 2002. Am. J. Trop. Med. Hyg. 2005;72:768–776. [PubMed] [Google Scholar]
- Illinois State Water Survey Water and atmospheric resources monitoring program (WARM) 2011 http://www.isws.illinois.edu/warm/weatherdata.asp
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper RG, LeClair AD, Strickler JD, Walker ED. Evaluation of methoprene (Altosid XR) sustained-release briquets for control of Culex mosquitoes in urban catch basins. J. Am. Mosq. Control Assoc. 1992;8:228–230. [PubMed] [Google Scholar]
- Kunkel KE, Novak RJ, Lampman RL, Gu W. Modeling the impact of variable climatic factors on the crossover of Culex restuans and Culex pipiens (Diptera: Culicidae), vectors of West Nile virus in Illinois. Am. J. Trop. Med Hyg. 2006;74:168–173. [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Landesman WJ, Allan BF, Langerhans RB, Knight TM, Chase JM. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector-borne Zoonotic Dis. 2007;7:337–343. doi: 10.1089/vbz.2006.0590. [DOI] [PubMed] [Google Scholar]
- Madder DJ, Surgeoner GA, Helson BV. Number of generations, egg production, and developmental time of Culex pipiens and Culex restuans (Diptera: Culicidae) in southern Ontario. J. Med. Entomol. 1983;20:275–287. doi: 10.1093/jmedent/20.3.275. [DOI] [PubMed] [Google Scholar]
- Means RG. Mosquitoes of New York: part II. Genera of Culicidae other than Aedes Occurring in New York. The University of the State of New York State Education Department Press; Albany, NY: 1979. [Google Scholar]
- Mogi M, Okazawa T. Factors influencing development and survival of Culex pipiens pallens larvae (Diptera: Culicidae) in polluted urban creeks. Res. Popul. Ecol. 1990;32:135–149. [Google Scholar]
- Muturi EJ, Shililu JI, Gu W, Jacob BG, Githure JI, Novak RJ. Larval habitat dynamics and diversity of Culex mosquitoes in rice agro-ecosystem in Mwea, Kenya. Am. J. Trop. Med. Hyg. 2007;76:95–102. [PubMed] [Google Scholar]
- Olden JD, Lawler JJ, LeRoy-Poff N. Machine learning methods without tears: a primer for ecologists. Q. Rev. Biol. 2008;83:171–193. doi: 10.1086/587826. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- Rey JR, O’Meara GF, O’Connell SM, Cutwa-Francis MM. Factors affecting mosquito production from stormwater drains and catch basins in two Florida cities. J. Vector Ecol. 2006;31:334–343. doi: 10.3376/1081-1710(2006)31[334:fampfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC, Stinner RE. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, Haramis L, Goldberg TL, Kitron UD. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit. Vectors. 2010;3:19. doi: 10.1186/1756-3305-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int. J. Health Geogr. 2004;3:8. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Webb JP, Meyer RP, Mulla MS. Spatial and temporal distribution of mosquitoes in underground storm drain systems in Orange County, California. J. Vector Ecol. 2003;28:79–89. [PubMed] [Google Scholar]
- Takeda T, Whitehouse CA, Brewer M, Gettman AD. Arbovirus surveillance in Rhode Island: assessing potential ecological and climatic correlates. J. Am. Mosq. Control Assoc. 2003;19:179–189. [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, Guinn MLO, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Watson JT, Jones RC, Gibbs K, Paul W. Dead crow reports and location of human West Nile virus cases, Chicago, 2002. Emerg. Infect. Dis. 2004;10:938–940. doi: 10.3201/eid1005.030603. [DOI] [PMC free article] [PubMed] [Google Scholar]