Abstract
Recent models of hippocampal function emphasize the potential role of this brain structure in encoding and retrieving sequences of events that compose episodic memories. Here we show that hippocampal lesions produce a severe and selective impairment in the capacity of rats to remember the sequential ordering of a series of odors, despite an intact capacity to recognize odors that recently occurred. These findings support the hypothesis that hippocampal networks mediate associations between sequential events that constitute elements of an episodic memory.
In humans, hippocampal function underlies the ability to recall specific personal experiences1,2. Does this fundamental role of the hippocampus in human episodic memory extend to animals as well? Because animals cannot provide explicit reports of their experiences, this question is difficult to address experimentally. Some features of episodic memory may be assessed in animals, however, including the rich temporal, spatial and situational context of episodic memories3. In particular, recent theoretical analyses have emphasized the potential role of hippocampal circuitry in representing the sequential ordering of events that compose a unique behavioral episode4–7.
Hippocampal damage impairs memory for the order of a series of recently visited spatial locations8,9. Because the hippocampus is important to spatial learning and memory in a variety of protocols10, however, there has not been conclusive evidence for a role in sequence memory specifically. It has been suggested that recognition and working memory for nonspatial stimuli also depend on associating events across time11. Selective hippocampal damage, however, has only a modest12 or no13 impairment in recognition or working memory for nonspatial cues, even when animals are required to remember long lists of single stimuli14,15. Furthermore, several recent studies have suggested a distinction between the capacity to recollect the spatial and temporal context of episodic memories, mediated by the hippocampus, and a separate capacity to remember independent events based on the familiarity of recently experienced stimuli16–20. Here, we directly compared memory for sequential order of events with memory for prior occurrence of events independent of their order.
Results
Memory for the sequential order of events
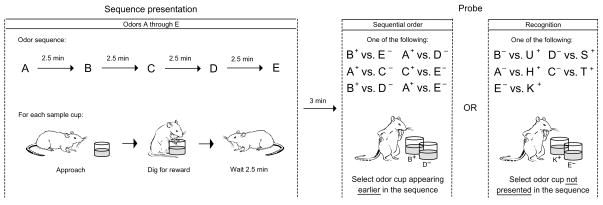
To test for sequential order memory, rats were presented with a series of five randomly selected odors. Memory for each series was subsequently probed in a single-choice test in which the animal was rewarded for selecting the odor that had appeared earlier in the series (Fig. 1). Sessions included six different types of probes in a random order. Before hippocampal surgery, rats required an average of 21–38 trials to reach a criterion of 80% correct over 10 consecutive trials on each type of probe. Subjects were then divided into two groups, a control group and a hippocampal lesion group, matched for average number of trials needed to reach criterion among the different probes. We report the ranges of average trial number ± s.e.m. for each group (control, 22.9 ± 4.9 –31.9 ± 7.8 trials per probe, n = 7; lesioned, 19.25 ± 3.8 – 37.9 ± 9.9 trials per probe, n = 7; F1,14 = 0.056, P = 0.816).
Fig. 1.
Sequential order and recognition tasks. Top, sequence of events in each trial. Bottom, an example trial for the sequential order and recognition probes. A–E designates the order of presentation for odors in each series, with odor A presented first and odor E presented last (see Methods). +, reinforced odor; −, nonreinforced odor.
Postoperatively, animals were tested for 15 trials on each probe, and scores on the last 10 trials were compared. Control rats performed well on all types of probes, whereas animals with hippocampal lesions performed at near-chance levels (Fig. 2). In addition, whereas performance on probe trials was dependent on the temporal separation (lag) between items during presentation of the series for both groups, rats with hippocampal damage were impaired at all lags (Table 1).
Fig 2.
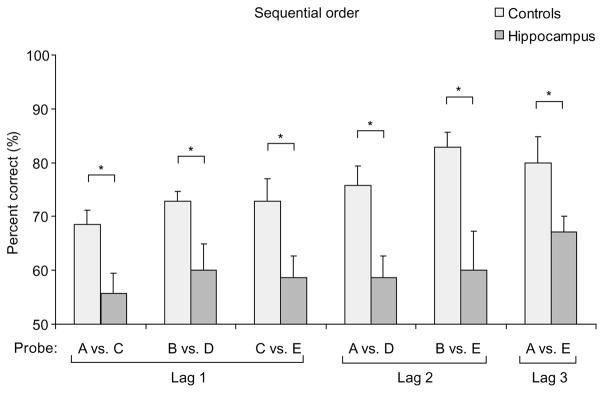
Performance (mean percent correct ± s.e.m.) of control rats and rats with hippocampal lesions on sequential order memory task. Rats with hippocampal lesions were reliably impaired (repeated-measures ANOVA, F1,12 = 39.609, P < 0.0001) and the severity of impairment did not vary reliably among the probe types (Group × Probe interaction, F5,60 = 0.472, P = 0.795). Post-hoc tests revealed significant impairment on every type of probe (all t-tests > critical t12 = 2.119; *, P < 0.05). Controls performed significantly above chance on every probe (all one-sample t-tests > critical t6 = 2.447), whereas rats with hippocampal lesions performed better than chance only on the probe that involved the first versus the last odor in the sequence (t6 = 6.000; for all other probes, t < 2.447). Performances on different probes are grouped according to the lag (number of intervening elements) between items in the probe test.
Table 1.
Performance (mean percent correct ± s.e.m.) on sequential order judgments involving different lags.
| Lag 3 | Lag 2 | Lag 1 | |
|---|---|---|---|
| Controls | 80.0 ± 4.9 | 79.3 ± 2.8 | 71.4 ± 1.6 |
| Hippocampus | 67.1 ± 2.9 | 59.3 ± 5.2 | 58.1 ± 1.4 |
Scores of each group were combined across lags of 1, 2 or 3 intervening elements between the two probed items (Fig. 1). In general, scores were higher for larger lags (F2,24 = 3.787, P = 0.037), indicating superior performance when probe items were more separated in the presentation series. Nevertheless, lesioned rats were significantly impaired overall (F1,12 = 23.769, P < 0.001) and at each lag (lag 3, t12 = 2.274, P = 0.042; lag 2, t12 = 3.412, P = 0.005; lag 1, t12 = 6.208, P < 0.0001), and the magnitude of the deficit did not differ between lags (F2,24 = 0.778, P = 0.471).
We also examined whether the animals had spatial biases that could have influenced their performance on this task and whether performance was dependent on the spatial positions of the probe items. Control and lesioned rats showed no preference, measured as the percentage of responses to each of six odor-presentation locations (control, 14.4 ± 0.9%– 19.2 ±1.1%; lesioned, 15.5 ±2.1%– 18.0 ±1.8%; F5,60 = 1.239, P = 0.42). Though accuracy did not differ among the locations where the rewarded choice was presented (control, 70.6 ± 6.8% – 79.7 ± 4.1% correct; lesioned, 57.6 ± 7.2%– 64.5 ± 6.5% correct; F5,60 = 1.111, P = 0.36), it did significantly differ between the two groups of rats (F1,12 = 16.571, P = 0.002).
Memory for recent occurrence of events
After completion of sequential order tests, the same rats were tested on their ability to recognize the recent occurrence of odors presented in the series (Fig. 1). On each trial, a series of five odors was presented in a format identical to that used in the sequential order task. Then recognition was probed using a choice test in which the animal was rewarded for selecting the odor that was not presented in the previous series. We examined learning of the recognition task by comparing accuracy in 15 sessions of 6 trials each, segmented into 3 blocks. Performance improved from 70.9 ± 4.2% in controls and 74.3 ± 4.4% in lesioned rats in the first block, to 82.3 ± 2.7% and 81.7 ± 2.9%, respectively, in the second block, to 89.6 ± 2.5% and 91.4 ± 3.0%, respectively, in the third block (ANOVA, F2,24 = 13.636, P < 0.001). There was no overall performance difference between the groups (F1,12 = 0.356, P = 0.561) and no significant difference between groups in acquisition rate (F2,24 = 0.171, P = 0.844).
Subsequent analyses for the individual probes focused on the last 10 sessions of testing, where overall performance was above 80% for all control subjects. Rats with hippocampal lesions performed as well as normal rats in recognition throughout the series (Fig. 3). In both groups, recognition scores were consistently superior on probes involving odors that appeared later in the series, suggesting items were more difficult to remember for longer periods or through more intervening items.
Fig. 3.
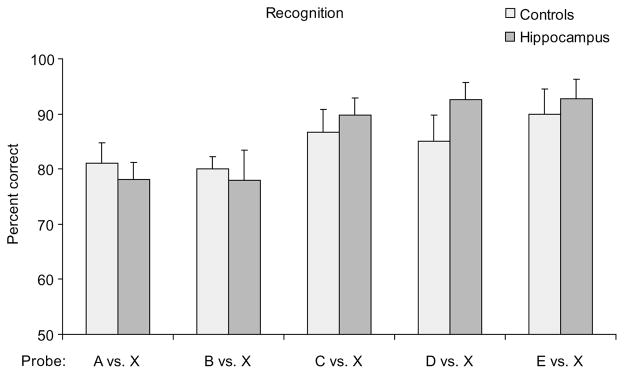
Performance (mean percent correct ± s.e.m.) of control rats and rats with hippocampal lesions on recognition for each of the different types of probes. ‘X’ designates a randomly selected odor that was not presented in the series and used as the alternative choice. Control rats and rats with hippocampal lesions performed equally well on odor recognition (repeated-measures ANOVA, F1,12 = 0.328, P = 0.577). In addition, recognition scores were higher for more recently presented items (main effect of probe type, F4,48 = 4.778, P = 0.003), and there was no significant difference between groups in the effect of item recency (Group × Probe interaction, F4,48 = 0.664, P = 0.620).
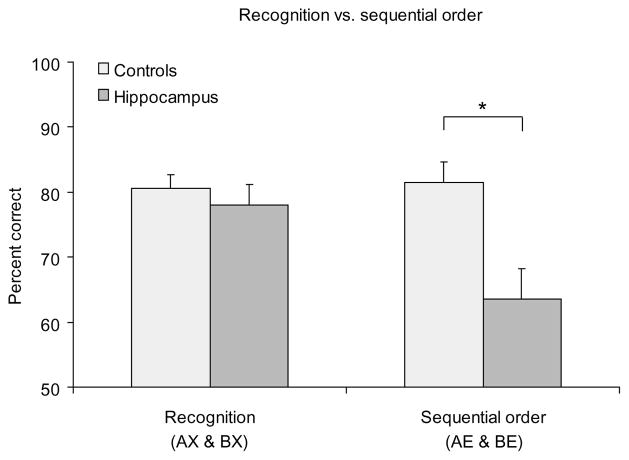
Performance on recognition versus sequential order
Control rats performed somewhat better overall on the recognition task than on the sequential order task (t6 = −3.292, P = 0.017), raising the possibility that recognition performance was spared in the hippocampal group because the problem was too easy. However, Dudchenko et al.15, using a similar odor recognition task, reported normal performance by hippocampal rats on lists of up to 25 items that strongly challenged normal subjects. The issue of task difficulty was directly addressed within this experiment by comparing the average scores on a subset of the probes in each task where performance was equivalent for control rats, specifically the two easiest probes of the sequential order task (BE and AE; Fig. 2) and the two most difficult probes of the recognition task (AX and BX; Fig. 3). Rats with hippocampal lesions performed as well as normal rats on the recognition probes, but were impaired on the equally difficult sequential order probes (Fig. 4). Thus, we conclude that animals with hippocampal damage had a fully intact capacity to recognize the odors even though they could not remember the order of their appearance.
Fig. 4.
Comparison of performance on recognition and sequential order tests. Control and lesioned rats performed differently between the two tasks (Group × Problem interaction, F1,12 = 7.385, P = 0.019). Post-hoc tests confirmed that controls did not perform differently on these probes (t6 = 0.241, P = 0.818). In contrast, rats with hippocampal lesions were impaired on the sequential order probes (t12 = 3.125; *,P = 0.009) but not on the recognition probes (t12 = 0.661, P = 0.521).
Discussion
The present results show that the hippocampus is essential to memory for sequences of events. The hippocampus is not required to recognize items that occur in a unique series of events, but is necessary to remember their order in the series. This combination of findings supports the view that the hippocampus plays a specific and fundamental role in memory for sequences of events that compose unique episodes. Notably, the present tasks are specifically designed to prevent the use of spatial cues, indicating that the hippocampus makes its contribution to sequence memory independent of any requirement to remember the spatial context of the events.
Could normal animals be using differences in the relative strengths of memory traces for the odors to judge their sequential order? The observation of a temporal gradient in recognition performance by normal animals suggests that memories were in fact stronger for the more recently presented items in each sequence. These differences in trace strength could potentially be used to judge the order of presentation. Our analyses indicate, however, that rats with hippocampal lesions had normal access to the differences in trace strengths for the odors, yet these intact trace-strength differences were not sufficient for above-chance discrimination on any sequential order probe (with the exception of slightly above-chance performance on the furthest separated items). These findings strongly suggest that normal rats do not use the relative strengths of memories for sequential order judgments.
Animal models of amnesia have been used to characterize the fundamental features of declarative memory and to identify the specific roles of brain structures within the medial temporal region. Much initial progress in the development of animal models came with the use of the delayed non-match to sample (DNMS) task, a simple test of recognition memory for once-experienced objects21. Performance on this task is insensitive to the effects of selective damage to the hippocampus, however, even with a long list of items, when animals are preoperatively trained on the task14,15. Other studies have shown that hippocampal damage can result in severe impairments on a recognition task that differs from DNMS in the types of visual stimuli and response demands22. Recent reviews indicate that the data on the role of the hippocampus in recognition memory in humans, monkeys, and rats are quite mixed, suggesting that subtle procedural differences can invoke requisite hippocampal function in recognition memory16,18,23. However, none of these analyses has yet identified the elements of simple recognition tasks that differentially demand hippocampal function. Instead, specific efforts at modeling episodic memory in monkeys and birds suggest that more powerful tests should include demands for memory of events within their spatial and temporal context24–26. Whereas the importance of spatial context is well-established in rodent models of amnesia and has been shown to be important in studies on the effects of hippocampal damage in monkeys24, the findings on temporal context to date come from studies on serial ordering of spatial memories8,9,27,28. The present results highlight the critical role of the hippocampus in memory for sequential order, independent of familiarity and spatial representation.
It has long been thought that a fundamental role of the hippocampus is the capacity to bridge discontinuities in time11. Several recent computational models have highlighted the representation of event sequences as a defining feature of episodic memory. According to one model, the capacity to integrate a series of events in time that compose an episodic memory is dependent on special architectural features of the hippocampus4. Particularly well-suited to the coding of sequential patterns are three characteristics of area CA3: local associative synaptic modification, recurrent activation, and sparse random connectivity. Central to this model are ‘local context’ neurons, that is, hippocampal cells that represent events in relation to the preceding and following events. It has also been shown that local context neurons could link sequential events to compose a network of related episodic memories7. These mechanisms could provide a framework for a ‘cognitive mapping’ of navigational episodes, as well as of multiple nonspatial experiences, and thereby support the flexibility of hippocampus-dependent memories7. Another recent model offers an elaborate mechanism of coordination between networks in the dentate gyrus and CA3 that produces storage and recall of event sequences6. It has also been suggested, based on the firing properties of hippocampal neurons, that they encode events and the places where they occur, and that hippocampal ensembles associate sequential codings as episodic memories10. Furthermore, these episodic representations could be linked within a network of memories that supports inferences across separate experiences. Each of these models proposes that hippocampal sequence representation underlies a range of memory performance capacities, including episodic recall, cognitive mapping, sequence prediction, and inferential memory expression. The present findings confirm that the hippocampus plays an essential role in memory for sequences of events.
Methods
Animals
All surgical and behavioral methods were approved by the Boston University Animal Care and Use Committee. Sixteen Long-Evans rats weighed approximately 225–250 g at the beginning of the experiment. They were kept mildly food deprived to maintain at least 85% of free-feeding body weight with ad libitum access to water throughout testing. Testing occurred during the middle of the light phase of a 12 h:12 h illumination cycle.
Behavioral tasks
The odor stimuli were 20 common spices: allspice (0.8% by weight), anise (1%), basil (1%), celery (1%), cinnamon (0.8%), clove (0.5%), cocoa (1%), coffee (1%), cumin (0.5%), dill (1%), fennel (0.8%), lemon (1%), marjoram (1%), mint (1%), nutmeg (0.8%), orange (1%), paprika (1%), parsley (1%), tarragon (1%), thyme (1%). The spices were mixed in playground sand to a total weight of 100 g in clear plastic cups (70 mm diameter × 63 mm high). On each trial, the odors and their ordering were randomly selected, excepting odors used in the preceding three trials. Rewards were Froot Loop cereal (Kellogg’s, Battle Creek, Michigan) buried at the bottom of the odor cup, and the rat was required to dig in the sand to obtain the reward.
During exposure to the sequences, odor cups were presented singly mounted on a plastic platform placed in the middle of one end of the home cage. During probe tests, two odor cups mounted 1 cm apart on a plastic platform were presented. To prevent the adoption of spatial biases and use of spatial cues during probe tests, the left–right positions of the odors on the platform were randomized and the platform was presented in any of three different locations, randomly selected on each trial. To ensure that animals were not using the smell of the reward to guide their choices, on some trials the food reward was withheld until after the choice was made; accuracy on these trials was equivalent to that on trials where the reward was buried. A choice response was scored when the animal touched the sand in one of the stimulus cups with its paw. These responses were scored by observers blind to the group assignment of subjects and provide a well-established measure of choice performance15,29,30.
After 3 days of food deprivation, rats were trained to dig for rewards buried in cups of unscented sand. Subsequently, they were trained on the sequential order task, then operated, then tested on the sequential order task, and finally trained and tested on the recognition task. For each trial in both the sequential order and recognition tests, 5 odors were randomly selected from the pool, and these odors were presented singly with 1/4 Froot Loop buried at the bottom of the cup, centered at the front wall of the home cage. A variable (average 2.5-min) inter-stimulus interval was interposed between stimulus presentations, and an additional 3-min memory delay was interposed before the probe test. The inter-trial interval was 30–60 min. Sequential order probes were composed as a choice between two odors that had appeared in non-adjacent positions in the series (Fig. 1). Recognition probes were composed as a choice between one odor that appeared in the series and another odor from the pool (that did not appear in the series). The reward for a correct choice was one whole Froot Loop. Animals received 6 trials per day.
Surgery
After preoperative training on the sequential order task, animals were matched for performance and randomly assigned to either the control or the hippocampal lesion group. Anesthesia was administered using 1% halothane plus 0.081 mg of atropine sulfate to prevent respiratory difficulties, and body temperature was maintained using a 37°C heating pad. At each of 12 sites bilaterally within the dentate gyrus and Ammon’s horn, the dura was pierced using a small syringe tip and a 100-μm nichrome electrode (0.7-mm uninsulated tip) was lowered into the brain. Radiofrequency lesions were made by passing an 8–11 mA radiofrequency current (Radionics RFG-4A, Burlington, MA) for 1 min. Sham controls underwent the same surgical treatment, except that the electrode was not lowered in the brain after puncturing the dura. One animal did not recover from surgery, and another developed an unrelated skin disease postoperatively; data from both animals were excluded from the analyses. All other animals recovered for two weeks after surgery and regained their preoperative weights.
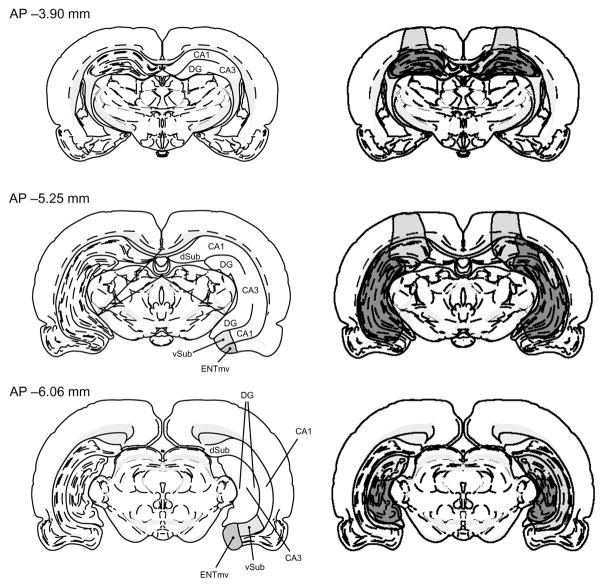
This procedure resulted in loss of 55–77% of the total volume of all hippocampal subdivisions (dentate gyrus, Ammon’s horn and subiculum; Fig. 5). Radiofrequency lesions are nonspecific to fibers of passage, so it is likely that connections to all subdivisions of the hippocampus were severed. However, neurotoxic hippocampal lesions of similar size result in a more severe deficit than do radiofrequency lesions in an odor sequence disambiguation task (N.G.A., K.L.F. and H.B.E., unpublished data), and cause a more severe deficit than do equivalent aspiration lesions on nonspatial working memory31. Therefore, the current approach using multiple small, tissue-damaging lesions may effectively result in more limited and selective damage than the infusion of excitatory neurotoxins. The cortex overlying the hippocampus was slightly damaged either unilaterally or bilaterally in some animals. Some rats showed minute damage to the most posterior aspect of the ventral subiculum, though the medial entorhinal cortex was spared in its entirety. Three animals had superficial damage to the medial geniculate nucleus of the thalamus. Damage to these ancillary areas was unrelated to the magnitude of the observed deficits.
Fig. 5.
Hippocampal subdivisions affected by the lesions at three anterior–posterior levels. Left, anatomically labeled coronal sections adapted from Swanson32. Right, reconstructions of the smallest (dark shading) and largest (light shading) hippocampal lesions. CA1 and CA3, components of Ammon’s horn; DG, dentate gyrus; dSub and vSub, dorsal and ventral components of the subiculum; ENTmv, medial–ventral entorhinal cortex).
Acknowledgments
We thank S. Rubin, J. Tourigny, S. Wright, L. Griffith, A. Cahill and A. Ceriales for help with behavioral testing and histological work. Supported by NIMH MH52090 (H.B.E.), NIA AG09973 (H.B.E.), and NSERC (N.J.F.).
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 2.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Phil Trans R Soc Lond B Biol Sci. 1997;352:1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Sohal VS, Hasselmo ME. Changes in GABAB modulation during a theta cycle may be analogous to the fall of temperature during annealing. Neural Comput. 1998;10:889–902. doi: 10.1162/089976698300017539. [DOI] [PubMed] [Google Scholar]
- 6.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate–CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 7.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 8.Kesner RP, Novak JM. Serial position curve in rats: role of the dorsal hippocampus. Science. 1982;218:173–175. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- 9.Chiba AA, Kesner RP, Reynolds AM. Memory for spatial location as a function of temporal lag in rats: role of hippocampus and medial prefrontal cortex. Behav Neural Biol. 1994;61:123–131. doi: 10.1016/s0163-1047(05)80065-2. [DOI] [PubMed] [Google Scholar]
- 10.Eichenbaum H, Dudchenko PA, Wood ER, Shapiro ML, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 11.Rawlins JNP. Associations across time: the hippocampus as a temporary memory store. Behav Brain Sci. 1985;8:479–496. [Google Scholar]
- 12.Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]
- 13.Murray EA. What have ablation studies told us about the neural substrates of stimulus memory? Semin Neurosci. 1996;8:13–22. [Google Scholar]
- 14.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. discussion 444–489. [PubMed] [Google Scholar]
- 17.Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–472. discussion 472–518. [Google Scholar]
- 18.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 19.Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- 20.Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- 21.Mishkin M. Memory in monkeys severely impaired by combined but not separate removal of the amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 22.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies on rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 24.Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- 25.Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- 26.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 27.Kesner RP. In: Neurobiology of Comparative Cognition. Kesner RP, Olton DS, editors. Lawrence Erlbaum; Mahwah, New Jersey: 1990. pp. 179–204. [Google Scholar]
- 28.Shapiro ML, Olton DS. In: Memory Systems. Schacter DL, Tulving E, editors. MIT Press; Cambridge, Massachusetts: 1994. pp. 87–117. [Google Scholar]
- 29.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 30.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarrard LE. In: The Hippocampus. 4. Isaacson RL, Pribram KH, editors. Plenum; New York: 1986. pp. 93–126. [Google Scholar]
- 32.Swanson LW. Slides 32, 37 & 39. Elsevier; Amsterdam: 1992. Brain Maps: Structure of the Rat Brain. [Google Scholar]