Abstract
Background/Aims
Pancreatic adenocarcinoma (PC) harbors frequent alterations of p16, resulting in cell cycle dysregulation. A phase I study of docetaxel and flavopiridol, a pan-cyclin dependent kinase inhibitor, demonstrated encouraging clinical activity in PC. This phase II study was designed to further define the efficacy and toxicity of this regimen in patients with previously treated PC.
Methods
Patients with gemcitabine-refractory, metastatic PC were treated with docetaxel 35 mg/m2 followed by flavopiridol 80 mg/m2 on days 1, 8, and 15 of a 28 day cycle. Tumor measurements were performed every two cycles. A Simon two-stage design was used to evaluate the primary endpoint of response.
Results
Ten patients were enrolled; nine were evaluable for response. No objective responses were observed; however, three patients (33%) achieved transient stable disease, with one of these patients achieving a 20% reduction in tumor size. Median survival was 4.2 months, with no patients alive at the time of analysis. Adverse events were significant, with seven patients (78%) requiring ≥1 dose reduction for transaminitis (11%), grade 4 neutropenia (33%), grade 3 fatigue (44%), and grade 3 diarrhea (22%)
Conclusions
The combination of flavopiridol and docetaxel has minimal activity and significant toxicity in this patient population. These results reflect the challenges of treating patients with PC in a second-line setting where the risk/benefit equation is tightly balanced.
Keywords: Docetaxel, Flavopiridol, Pancreatic adenocarcinoma
Introduction
Adenocarcinoma of the pancreas (PC) is the fourth leading cause of cancer death in the United States, with an estimated 37,680 new cases and 34,290 deaths in 2008.[1-3] The diagnosis of advanced PC is associated with poor outcomes, and the median survival is less than 6 months for patients treated with single agent gemcitabine.[4] The combination of gemcitabine and erlotinib results in a modest improvement in one-year survival when compared with gemcitabine alone.[5] Preliminary results from a study of gemcitabine and capecitabine demonstrated a 1.4 month improvement in survival when compared with gemcitabine alone;[6] however, other studies of this combination were negative.[7]
Docetaxel is a semisynthetic taxane with single-agent activity in PC. An objective response rate (RR) of 15% was observed in a phase II study of chemotherapy-naïve patients with advanced PC treated with docetaxel 100 mg/m2 every 3 weeks.[8] Response rates observed in two other phase II studies of docetaxel utilizing this dose and schedule were 5% and 6%.[9, 10] With lower doses of docetaxel (60 mg/m2 every three to four weeks), the RR was 0%.[11] Of the 118 chemotherapy-naïve patients treated with single-agent docetaxel in these four studies, one achieved a complete response (CR) and eight achieved partial responses (PR), for an overall RR of 8%.
The modest tumor responses achieved using cytotoxic chemotherapy in this disease may, in part, be due to the frequent alterations of p16INK4A, an inhibitor of the cyclin-dependent kinases (CDK) 4 and 6, observed in PC. p16INK4A dysfunction due to methylation, allelic loss, or mutation is observed in up to 85% of cases,[12-14] resulting in cell cycle dysregulation and tumor progression. The adenoviral-mediated reintroduction of p16INK4A significantly inhibits the growth of PC cell lines and established subcutaneous pancreatic tumors in nude mice.[15] Furthermore, treatment of pancreatic cell lines with chemotherapy followed by adenoviral-mediated delivery of p16INK4A results in substantial reduction of cell viability when compared with chemotherapy alone, suggesting that agents targeting the cell cycle may have efficacy in the treatment of PC.[16, 17] Flavopiridol is a CDK inhibitor that binds directly to the ATP binding pockets of CDK1, CDK2, CDK4, and CDK6 , resulting in kinase inhibition at nanomolar concentrations and cell cycle arrest at both G1/S and G2/M. The addition of flavopiridol to chemotherapy enhances the apoptotic effects of therapy in a number of preclinical models.[18-21]
In a completed phase I clinical trial of weekly docetaxel and flavopiridol, we demonstrated that the combination is tolerable and has encouraging clinical activity.[22] We thus designed this phase II study of docetaxel and flavopiridol in patients with gemcitabine-refractory, metastatic PC.
Patients and Methods
Patients
Patients ≥18 years with histologically confirmed adenocarcinoma of the pancreas, measurable metastatic disease, Karnofsky performance status (KPS) ≥80%, and acceptable end-organ function as defined by pretreatment total white blood cell count ≥2,500/mm3, absolute neutrophil count (ANC) ≥1,500/mm3, platelet count ≥100,000/mm3, normal serum creatinine or creatinine clearance ≥60 mL/min/1.73 m2 for patients with a creatinine above the upper limit of normal (ULN), total serum bilirubin ≤1.5 times the ULN, serum alkaline phosphatase ≤5 times the ULN, and serum AST and ALT levels ≤2.5 times the ULN were eligible. Patients must have disease progression on a prior gemcitabine-based regimen. Progression may have occurred while receiving therapy in the adjuvant setting, within three months of completing adjuvant therapy, while receiving therapy for locally advanced disease, or after receiving one regimen in the metastatic setting.
Patients were excluded for any of the following reasons: treatment with >1 line of prior chemotherapy, prior docetaxel or flavopiridol, ongoing toxic effects from prior therapy, grade 2 or greater peripheral neuropathy, any serious or uncontrolled infection, symptomatic cardiac or pulmonary disease, diabetes not adequately controlled, known CNS metastasis, pregnancy or lactation, or HIV infection. No chemotherapy or targeted therapy was allowed within two weeks of study entry (6 weeks for nitrosoureas and mitomycin C). No radiotherapy was allowed within 4 weeks of study entry. No concurrent chemotherapy, radiotherapy, or other investigational agents was allowed.
All patients were informed of the investigational nature of the study and provided written informed consent in accordance with institutional and federal guidelines. The institutional review board of Memorial Sloan-Kettering Cancer Center (MSKCC) oversaw this trial.
Treatment
Docetaxel 35 mg/m2 was administered intravenously over 30 minutes followed four to six hours later by flavopiridol 80 mg/m2 administered intravenously over 60 minutes on days 1, 8, and 15 of each 28 day cycle. Dexamethasone 8 mg PO BID was administered for a total of three doses, with the first dose taken approximately 12 hours prior to treatment. Antiemetic agents were administered at the discretion of the treating physician. Patients continued on therapy until the development of tumor progression or excessive adverse events (AE).
Dose Delays and Dose Adjustments
AE were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0. Therapy was held for an ANC <1000/mm3, platelet count <75,000/mm3, total serum bilirubin >1.5 times the ULN, AST and/or ALT >5 times the ULN, or alkaline phosphatase >5 times the ULN. Dose adjustments for AE are described in the Appendix (online version only).
Study Evaluations
Pretreatment evaluation included a complete medical history and physical examination, complete blood count with differential, comprehensive metabolic profile including liver function tests, LDH, prothrombin time/partial thromboplastin time, serum pregnancy test for females of child-bearing potential, and 12-lead EKG. Baseline tumor assessments included a CA 19-9, chest radiograph, and computed tomography scans and/or MRI. Tumor status was reassessed by CA 19-9 on the first day of each cycle, and by imaging studies performed every two cycles using the Response Evaluation Criteria in Solid Tumors (RECIST).[23]
Statistical Considerations
The primary endpoint of this study was tumor response. Secondary endpoints included toxicity, time to progression (TTP), and overall survival (OS). A Simon two-stage design was employed to evaluate the primary endpoint using a projected response rate of 5% for docetaxel alone in the second-line setting and 25% for docetaxel in combination with flavopiridol.[24] If no responses were observed among the first 9 patients treated, the study was to be terminated. If ≥1 responses were observed, enrollment was to be extended to 30 patients. If ≥4 responses were observed, this regimen would be considered worthy of further testing. There is a 90% probability that this design will recommend this regimen for further evaluation if the true response rate is 25%. This probability drops to 5% if the true response rate is 5%.
Results
Patient Characteristics
Ten patients with advanced, gemcitabine-refractory PC were enrolled between March 2006 and December 2006 at MSKCC. Of these 10 patients, 9 were evaluable for response. One patient unexpectedly developed an elevated alkaline phosphatase, AST, and ALT on cycle 1, day 1 of therapy not present on screening bloodwork ultimately determined to be due to biliary obstruction. He was taken off study, deemed inevaluable, and replaced as required by the study protocol.
Baseline characteristics are outlined in Table 1. The nine evaluable patients received ≥2 cycles of therapy and were accessible for all study endpoints. The median number of cycles administered was 2.0 (range, 2.0 – 2.25).
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (N = 10) | |
|---|---|---|
| Evaluable Patients | 9 (90%) | |
| Age, years | ||
| Median | 64 | |
| Range | 49 - 81 | |
| Sex | ||
| Male | 4 (40%) | |
| Female | 6 (60%) | |
| Karnofsky Performance Status | ||
| Median | 80 | |
| Range | 70 - 90 | |
| Sites of Disease | ||
| Liver | 9 (90%) | |
| Lymph nodes | 3 (30%) | |
| Lung | 4 (40%) | |
| Peritoneum | 3 (30%) | |
| CA 19-9, U/mL | ||
| Median | 178 | |
| Range | 6 - 39,966 | |
| Prior Surgery | 2 (20%) | |
| Prior Adjuvant Therapy | ||
| Gem→Concurrent 5-FU/RT→Gem | 1(10%) | |
| Gem/Erlotinib/RT→Gem/Erlotinib (ref 30) | 2 (20%) | |
| Prior Metastatic Treatment Regimens | ||
| Gem +/- Bevacizumab (CALGB 80303; ref 23) | 3 (30%) | |
| Gem | 1 (10%) | |
| Gem/Capecitabine | 1 (10%) | |
| FDR Gem/Erlotinib | 1 (10%) | |
| Capecitabine/Erlotinib | 1 (10%) |
Response to Treatment and Survival
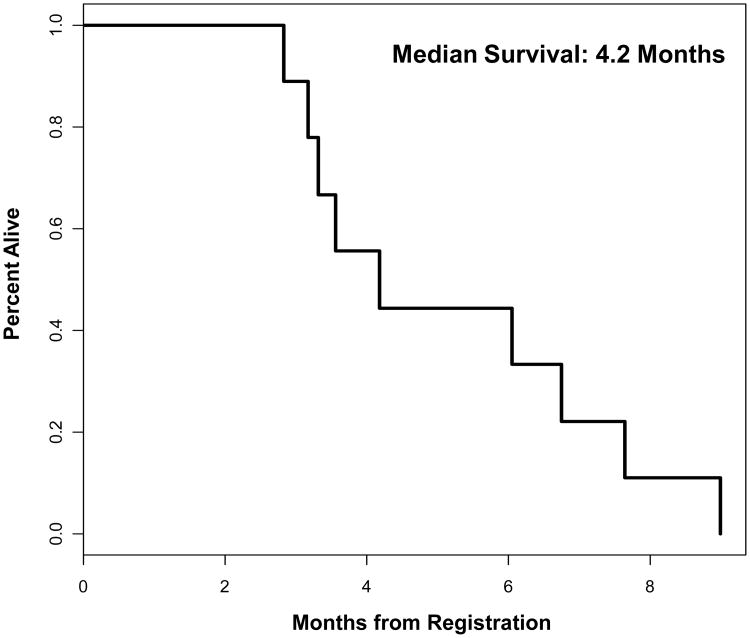
We observed no objective response as assessed by RECIST on this study. Six patients (67%) demonstrated disease progression following two cycles of therapy. Three (33%) achieved SD following two cycles of therapy, with one of these patients achieving a 20% reduction in tumor size; however, these results were not verified by confirmatory imaging. No patient completed three cycles of therapy. The median time to treatment failure (disease progression or excess AE requiring discontinuation of treatment) was 8 weeks (range, 7 – 14). The median OS from the time of initiation of therapy on protocol was 4.2 months (range, 2.8 – 6.9), with no patients alive at the time of analysis (Figure 1).
Figure 1.
Overall survival.
The three patients who achieved SD were removed from the study following cycle 3, week 1 of therapy due to AE unrelated to therapy. One patient achieved a minor response, with a 20% reduction in the size of his target lesions. Following cycle 3, week 1 of therapy, he was hospitalized for a pulmonary embolus (PE), clinically deteriorated, and was unable to receive further treatment. A second patient achieved SD following two cycles of therapy; however, following cycle 3, week 1 of therapy, she was admitted for a complete large bowel obstruction requiring jejunal sigmoid bypass. Following a prolonged hospitalization, she was discharged to hospice and received no further therapy. A third patient who was a 76 year-old male with insulin-dependent diabetes mellitus, achieved a mixed response; however, there was a questionable new hepatic lesion as well as an incidental PE observed on his restaging studies. Because of clinical benefit in terms of pain and fatigue, he was continued on therapy. Following cycle 3, week 1 of therapy, he was hospitalized for symptomatic hypoglycemia adjudicated as being unrelated to protocol therapy. He was subsequently admitted a second time for a partial small bowel obstruction, also adjudicated as unrelated to protocol therapy, which resolved with conservative management. Given these multiple complications, he ultimately opted to discontinue therapy.
Adverse Events
AE adjudicated as related to therapy are summarized in Table 2. Six (67%) patients experienced at least one grade 3 or worse AE and 3 (33%) patients experienced at least one grade 4 AE, where the attribution was possibly, probably, or definitely related to treatment. Three patients (33%) experienced one dose delay due to AE, with one (11%) experiencing two such delays. Seven patients (78%) required at least one dose reduction, with one of these seven (11%) requiring two dose reductions. One patient experienced an asymptomatic rise in ALT ≥1.5 times the ULN that mandated a protocol-specified dose-reduction; this transaminitis was attributed to disease progression and was not felt to be therapy-related. Other AE requiring dose reduction included three episodes of grade 4 neutropenia (33%), four episodes of grade 3 fatigue (44%), and two episodes of grade 3 diarrhea (22%). Two patients (22%) developed thromboembolic disease, both with a DVT and PE. These were adjudicated as being related to the underlying disease process and were not considered a toxicity from therapy.
Table 2.
Adverse Event Possibly, Probably, or Definitely Related to Treatment According to National Cancer Institute Common Toxicity Criteria for Adverse Events Version 3.0. (n = 9)
| Toxicity Grade | |||||
|---|---|---|---|---|---|
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3/4 |
| Hematologic | |||||
| Anemia | 6 (67%) | 3 (33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 2 (22%) | 1 (11%) | 1 (11%) | 0 (0%) | 1 (11%) |
| Leukopenia | 3 (33%) | 1 (11%) | 2 (22%) | 2 (22%) | 4 (44%) |
| Lymphopenia | 0 (0%)` | 1 (11%) | 5 (56%) | 1 (11%) | 6 (67%) |
| Neutropenia | 0 (0%) | 1 (11%) | 0 (0%) | 3 (33%) | 3 (33%) |
| Nonhematologic | |||||
| Fatigue | 3 (33%) | 2 (22%) | 3 (33%) | 0 (0%) | 3 (33%) |
| Nausea | 4 (44%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 5 (56%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anorexia | 5 (56%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Weight Loss | 4 (44%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diarrhea | 3 (33%) | 2 (22%) | 2 (22%) | 0 (0%) | 2 (22%) |
| Dehydration | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 1 (11%) |
| Hyperglycemia | 1 (11%) | 4 (44%) | 3 (33%) | 1 (11%) | 4 (44%) |
| Hypoglycemia | 1 (11%) | 1 (11%) | 1 (11%) | 0 (0%) | 1 (11%) |
| Alkaline Phosphatase | 6 (67%) | 2 (22%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ALT | 5 (56%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| AST | 6 (67%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperbilirubinemia | 2 (22%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Thrombosis | 0 (0%) | 0 (0%) | 0 (0%) | 2 (22%) | 2 (22%) |
Discussion
We evaluated the combination of docetaxel and flavopiridol in nine patients with metastatic, gemcitabine-refractory PC in this phase II study. Utilizing a stringent two-stage statistical design, we demonstrate that this regimen has limited clinical activity in the second-line setting. Despite our requirement of a high performance status for eligibility, we observed significant AE associated with therapy.
Given the results observed in patients with PC on the phase I study of this regimen, we were surprised by the lack of activity and marked toxicity seen on this phase II study. Whereas six of the ten patients (60%) with pancreas cancer treated on the phase I study achieved either an objective response (one PR lasting 5.8 months; one durable CR lasting > two years) or stable disease (SD; four patients with SD lasting between 3.4 and 5.8 months), only three of the nine patients (33%) achieved SD in this study, with none achieving an objective response. In the phase I study, two of the 27 patients (7%) enrolled experienced a DLT: one of six patients treated with docetaxel 35 mg/m2 and flavopiridol 70 mg/m2 developed grade 3 mucositis, and one of six patients treated with docetaxel 35 mg/m2 and flavopiridol 80 mg/m2 developed grade 4 neutropenia. On this phase II study, seven of the nine patients (78%) required at least one dose reduction for AE. Patients enrolled on the phase II study were older (median age 64 years vs. 56 years) and had a lower KPS (median KPS 80 vs. 90). As performance status is a powerful predictor of outcome in advanced PC,[25] these observations may, in part, explain the marked difference in response and toxicity demonstrated in the two studies. Unfortunately, serum and tissue samples for pharmacokinetic/pharmacodynamic studies were not collected as part of this study; thus, data comparing serum drug levels or tumor responses to therapy between trials are not available.
The treatment of PC in the second-line setting remains challenging. Indeed, only 16-57% of patients with PC receiving first-line therapy on phase III studies have been reported to receive second-line therapy, with less than 2% receiving further therapy on a clinical trial,[26] in part, because of a deterioration in performance status.[7, 26-28] Tumor response rates observed in studies evaluating various regimens in the second-line setting range from 0% to 24%, with median survival times ranging from 3.4 to 10.3 months.[29, 30] The results of our study reflect the challenges of treating patients with PC in the second-line setting, where the risk/benefit equation is tightly balanced.
Prior studies have demonstrated improved treatment outcomes in patients with good performance status.[7, 27] It is thus conceivable that if this and other regimens were tested in the first-line setting, accruing patients with less AE from both disease and prior therapy, better outcomes might be achieved. Given the negative results of studies evaluating various regimens of gemcitabine combined with chemotherapy or targeted agents such as bevacizumab[25] or cetuximab,[31] it would be reasonable to reassess our approach to the development of novel therapies for patients with PC, and consideration should be given to testing novel regimens in the upfront setting.
In summary, the combination of docetaxel and flavopiridol has limited activity and significant toxicity when administered to patients with PC in the second-line setting. Given the difficulties inherent in evaluating novel therapies for PC in the second-line setting, evaluation of novel treatment regimens for this disease in the front-line setting should be considered.
Acknowledgments
Research Support: R01 CA67819-07 (PI: Gary K. Schwartz)
Appendix (Dose Adjustments)
For grade 3 or 4 neutropenia, patients were re-treated after recovery at dose level -1 (docetaxel 25 mg/m2 and flavopiridol 60 mg/m2), with growth factor support administered at the discretion of the treating physician. For grade 2 thrombocytopenia, patients were re-treated after recovery without dose reduction. In the case of recurrent thrombocytopenia, reduction to dose level -1 was instituted at the time of platelet recovery. In the case of both elevated transaminases and alkaline phosphatase, or in the case of isolated hyperbilirubminemia, patients were re-treated after recovery at dose level -1. For an AST and/or ALT between 1.5 – 5 times the ULN and an alkaline phosphatase ≤5 times the ULN, patients were treated at dose level -1 without a delay in therapy.
Therapy was held for diarrhea until resolution of symptoms without the use of anti-diarrheal agents for ≥24 hours. For grade 3 or 4 diarrhea that persisted despite the use of anti-diarrheal medications, patients were retreated at dose level -1 upon resolution of symptoms. In the case of grade 2 neuropathy, docetaxel was reduced from 35 mg/m2 to 25 mg/m2, with no dose reduction for flavopiridol; patients were taken off study for grade 3 or greater neuropathy. For other grade 3 or 4 non-hematologic toxicities, treatment was held until resolution of symptoms to grade 1 or less, and then reinstituted at dose level -1.
Toxicities requiring a second dose level reduction resulted in reduction of the docetaxel dose to 20 mg/m2, with no further dose reduction for flavopiridol. Toxicity that necessitated a third dose reduction, or toxicity that caused two or more treatment delays within a cycle, resulted in patient removal from the study.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008;8:110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh M, Maitra A. Precursor lesions of pancreatic cancer: Molecular pathology and clinical implications. Pancreatology. 2007;7:9–19. doi: 10.1159/000101873. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase iii trial of the national cancer institute of canada clinical trials group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Chau I, Stocken D, et al. Phase iii randomised comparison of gemcitabine (gem) versus gemcitabine plus capecitabine (gem-cap) in patients with advanced pancreatic cancer. Eur J Cancer. 2005;3:4. Abst PS11. [Google Scholar]
- 7.Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Kohne CH, Mingrone W, Stemmer SM, Tamas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase iii trial of the swiss group for clinical cancer research and the central european cooperative oncology group. J Clin Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 8.Rougier P, Adenis A, Ducreux M, de Forni M, Bonneterre J, Dembak M, Clouet P, Lebecq A, Baille P, Lefresne-Soulas F, Blanc C, Armand JP. A phase ii study: Docetaxel as first-line chemotherapy for advanced pancreatic adenocarcinoma. Eur J Cancer. 2000;36:1016–1025. doi: 10.1016/s0959-8049(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 9.Lenzi R, Yalcin S, Evans DB, Abbruzzese JL. Phase ii study of docetaxel in patients with pancreatic cancer previously untreated with cytotoxic chemotherapy. Cancer Invest. 2002;20:464–472. doi: 10.1081/cnv-120002146. [DOI] [PubMed] [Google Scholar]
- 10.Androulakis N, Kourousis C, Dimopoulos MA, Samelis G, Kakolyris S, Tsavaris N, Genatas K, Aravantinos G, Papadimitriou C, Karabekios S, Stathopoulos GP, Georgoulias V. Treatment of pancreatic cancer with docetaxel and granulocyte colony-stimulating factor: A multicenter phase ii study. J Clin Oncol. 1999;17:1779–1785. doi: 10.1200/JCO.1999.17.6.1779. [DOI] [PubMed] [Google Scholar]
- 11.Okada S, Sakata Y, Matsuno S, Kurihara M, Sasaki Y, Ohashi Y, Taguchi T. Phase ii study of docetaxel in patients with metastatic pancreatic cancer: A japanese cooperative study. Cooperative group of docetaxel for pancreatic cancer in japan. Br J Cancer. 1999;80:438–443. doi: 10.1038/sj.bjc.6690375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attri J, Srinivasan R, Majumdar S, Radotra BD, Wig J. Alterations of tumor suppressor gene p16ink4a in pancreatic ductal carcinoma. BMC Gastroenterol. 2005;5:22. doi: 10.1186/1471-230X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE. Frequent somatic mutations and homozygous deletions of the p16 (mts1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- 14.Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W, Baylin SB, Kern SE, Herman JG. Abrogation of the rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–3130. [PubMed] [Google Scholar]
- 15.Ghaneh P, Greenhalf W, Humphreys M, Wilson D, Zumstein L, Lemoine NR, Neoptolemos JP. Adenovirus-mediated transfer of p53 and p16(ink4a) results in pancreatic cancer regression in vitro and in vivo. Gene Ther. 2001;8:199–208. doi: 10.1038/sj.gt.3301394. [DOI] [PubMed] [Google Scholar]
- 16.Halloran CM, Ghaneh P, Shore S, Greenhalf W, Zumstein L, Wilson D, Neoptolemos JP, Costello E. 5-fluorouracil or gemcitabine combined with adenoviral-mediated reintroduction of p16ink4a greatly enhanced cytotoxicity in panc-1 pancreatic adenocarcinoma cells. J Gene Med. 2004;6:514–525. doi: 10.1002/jgm.540. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi S, Shirasawa H, Sashiyama H, Kawahira H, Kaneko K, Asano T, Ochiai T. P16ink4a expression adenovirus vector to suppress pancreas cancer cell proliferation. Clin Cancer Res. 1999;5:4182–4185. [PubMed] [Google Scholar]
- 18.Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK. Flavopiridol enhances the effect of docetaxel in vitro and in vivo in human gastric cancer cells. Mol Cancer Ther. 2003;2:549–555. [PubMed] [Google Scholar]
- 19.Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, Schwartz GK. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of cpt-11 in hct116 colon cancer monolayers and xenografts. Clin Cancer Res. 2001;7:4209–4219. [PubMed] [Google Scholar]
- 20.Jung CP, Motwani MV, Schwartz GK. Flavopiridol increases sensitization to gemcitabine in human gastrointestinal cancer cell lines and correlates with down-regulation of ribonucleotide reductase m2 subunit. Clin Cancer Res. 2001;7:2527–2536. [PubMed] [Google Scholar]
- 21.Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999;5:1876–1883. [PubMed] [Google Scholar]
- 22.Fornier MN, Rathkopf D, Shah M, Patil S, O'Reilly E, Tse AN, Hudis C, Lefkowitz R, Kelsen DP, Schwartz GK. Phase i dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13:5841–5846. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase ii clinical trials. Controlled clinical trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Kindler HL, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, McLeod HL, Mulcahy MF, Schilsky RL, Goldberg RM B CaLG. A double-blind, placebo-controlled, randomized phase iii trial of gemcitabine (g) plus bevacizumab (b) versus gemcitabine plus placebo (p) in patients (pts) with advanced pancreatic cancer (pc): A preliminary analysis of cancer and leukemia group b (calgb) Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25 Abst 4508. [Google Scholar]
- 26.Schrag D, Archer L, Wang X, Romanus D, Mulcahy M, Goldberg R (CALGB) CaLGB. A patterns-of-care study of post-progression treatment (rx) among patients (pts) with advanced pancreas cancer (apc) after gemcitabine therapy on cancer and leukemia group b (calgb) study #80303. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25 Abst 4524. [Google Scholar]
- 27.Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, Vehling-Kaiser U, Fuchs M, Fleckenstein D, Gesierich W, Uthgenannt D, Einsele H, Holstege A, Hinke A, Schalhorn A, Wilkowski R. Randomized phase iii trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 28.Boeck S, Heinemann V. Second-line therapy in gemcitabine-pretreated patients with advanced pancreatic cancer. J Clin Oncol. 2008;26:1178–1179. doi: 10.1200/JCO.2007.15.3304. author reply 1179. [DOI] [PubMed] [Google Scholar]
- 29.Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C, Fuchs CS. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 30.Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, Hendlisz A, Van Laethem JL. Gemcitabine and oxaliplatin (gemox) in gemcitabine refractory advanced pancreatic adenocarcinoma: A phase ii study. Br J Cancer. 2006;94:481–485. doi: 10.1038/sj.bjc.6602966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip PA, Benedetti J, Fenoglio-Preiser C, Zalupski M, Lenz H, O'Reilly E, Wong R, Atkins J, Abruzzese J, Blanke C. Phase iii study of gemcitabine [g] plus cetuximab [c] versus gemcitabine in patients [pts] with locally advanced or metastatic pancreatic adenocarcinoma [pc]: Swog s0205 study. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25:LBA4509. [Google Scholar]