Abstract
The present study investigated whether pharmacological postconditoning with netrin-1 is cardioprotective against ischemia reperfusion (I/R) injury, and the underlying signaling mechanisms. Langendorff perfused hearts isolated from wild-type (WT) C57BL/6 or DCC+/− mice underwent a 20 min of ischemia, followed by a 60 min of reperfusion, in the presence or absence of netrin-1, or netrin-1 in combination with U0126 (MEK1/2 inhibitor), or PTIO (nitric oxide/NO scavenger). In WT mice, netrin-1 postconditioning dramatically reduced infarct size to 17.0±2.5%, from 40.5±4.2% in the untreated I/R group. U0126 or PTIO alone had no effect on infarct size but abolished the effects of netrin-1. The protective effect of netrin-1 was markedly diminished in DCC+/− mice (44.5±2% vs. 15±2.6 % for infract size in DCC+/− vs. DCC+/+ group). Our results indicate that netrin-1, given as a pharmacological postconditioning agent, induces cardioprotection via a DCC-dependent mechanism that involves ERK1/2 activation and NO production. Combined with our previous findings, netrin-1 treatment proves to be extremely and consistently beneficial whenever delivered to the heart, establishing its substantial promises for being developed into a robust therapeutic strategy for acute myocardial infarction.
Keywords: Netrin-1, Pharmacological postconditioning, Cardioprotection, Ischemia reperfusion (I/R) injury, deleted in colorectal cancer (DCC), ERK1/2, nitric oxide (NO)
2. INTRODUCTION
Cardiovascular diseases represent a major cause of death worldwide, with coronary artery disease being one of the most prevalent manifestations. Coronary artery disease involving myocardial ischemia and reperfusion can result in myocardial infarction. Pharmacological postconditioning has generated considerable interest for development of novel therapeutics. For example, pharmacological postconditioning with various agents such as opioid 1, bradykinin 2 and insulin 3, has been shown to be cardioprotective in animal models. However, none of these has yet been translated into clinical practice, or tested for their potential untoward complications.
Netrin-1 is a laminin-related protein initially identified as a neuronal guidance cue, functioning by directing axonal development 4. Others and we have shown that netrin-1 is also potent regulator of angiogenesis 5-7. In a recent study we demonstrated that among the netrin-1 receptors expressed in the heart, deleted in colorectal cancer (DCC) turns out to mediate netrin-1 induced cardioprotection via activation of a ERK1/2/eNOS/NO/DCC feed-forward mechanism 8. The effects of netrin-1 on NO production in the heart share similarities with our previous observations that netrin-1 promotes angiogenesis via production of NO 7.
In the present study we aimed to examine whether and how netrin-1, given specifically as a pharmacological postconditioning agent at the onset of reperfusion, also provokes cardioprotection. The underlying signaling mechanisms were also investigated. Interestingly, netrin-1 postconditoning resulted in marked reduction in infarct size, while the protective effect was reversed in DCC+/− mice. The cardioprotection was also attenuated when hearts were treated with MEK/ERK1/2 inhibitor U0126, or NO scavenger PTIO (2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), implicating intermediate roles of MEK/ERK1/2 activation and NO production. Combined with our previous findings, netrin-1 treatment proves to be extremely and consistently beneficial whenever it is delivered to the heart, establishing its substantial promises for being developed into a robust therapeutic strategy for acute myocardial infarction.
3. MATERIALS AND METHODS
3.1. Materials
Purified recombinant mouse netrin-1 was purchased from R&D Systems (Minneapolis, MN). Other chemicals and reagents, except for the antibodies, were purchased from Sigma in the highest purity (Sigma-Aldrich, St. Louis, MO, USA).
3.2. Animals
Male C57BL/6J mice (6-8 weeks old) were obtained from the Jackson Laboratories (Bar Harbor, ME). DCC+/− mice were kindly provided by Dr. Marc Tessier-Lavigne from Rockefeller University. Mice were housed under a pathogen-free condition. The use of animals and experimental procedures were approved by the Institutional Animal Care and Usage Committee at the University of California Los Angeles (UCLA).
3.3. Langendorff perfusion and experimental protocol
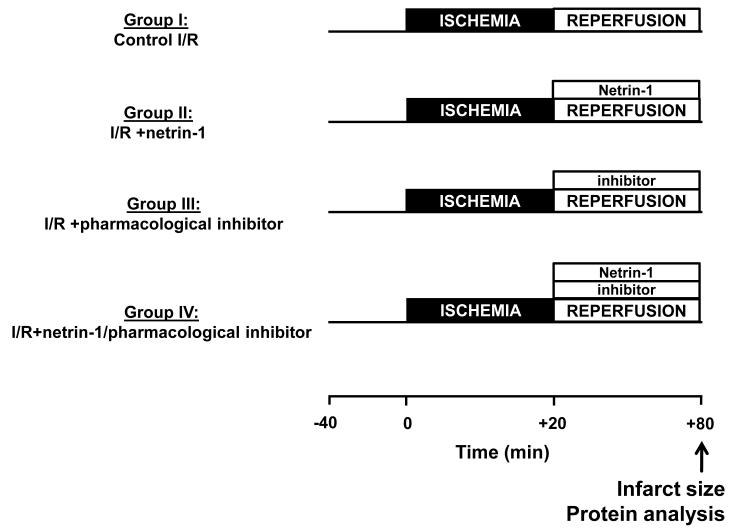
The mice were anesthetized with intraperitoneal pentobarbitone (60 mg/kg). Hearts were harvested, and the aortas were cannulated with a 20-gauge stainless steel blunt needle and transferred to the Langendorff rig and perfused retrograde instantly, with modified Krebs—Henseleit buffer (KHB), which contained (in mmol/L): NaCl 118.0, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, D-Glucose 10 at constant pressure (80±1 mm Hg). All hearts were allowed to stabilize for 40 min. After the stabilization period, hearts underwent a 20 min of global normo-thermic ischemia, followed by a 60 min of reperfusion, and then harvested for analyses of infarct size. The experimental protocol is illustrated in Figure 1, in which netrin-1 and/or pharmacological agents were started at the time of reperfusion (Fig. 1).
Figure 1.
Experimental protocol and groups. Diagrammatical description of experimental groups and the global ischemia/reperfusion (I/R) protocol using a Langendorff constant pressure perfusion system. The basal setup comprised of a 40 min of stabilization, a 20 min of global normo-thermique ischemia, and a 60 min of reperfusion. The treatment groups differed only during the 60 min of reperfusion. All of the drugs were given at the onset of the reperfusion. (I) Control I/R: No treatment (vehicle, Krebs—Henseleit buffer/KHB or DMSO<0.01%); (II) I/R+netrin-1: netrin-1 (100 ng/mL); (III) I/R+pharmacological inhibitors: U0126 (50 μmol/L, DMSO<0.01%) or PTIO (50 μmol/L); (IV) I/R+netrin-1/pharmacological inhibitors: a mix of netrin-1/U0126 or netrin-1/PTIO.
Specifically, the different treatment groups were: (I) Control I/R, KHB only or KHB with DMSO<0.01%, as a vehicle; (II) I/R+netrin-1, perfusion with 100 ng/mL of netrin-1; (III) I/R+MEK1/2 inhibitor U0126 (50 μmol/L, DMSO<0.01%) or NO scavenger PTIO (60 μmol/L); (IV) I/R+a mix of netrin-1 (100 ng/mL)/U0126 (50 μmol/L, DMSO<0.01%) or a mix of netrin-1 (100 ng/mL)/PTIO (60 μmol/L). The concentrations of netrin-1 and pharmacological inhibitors used in this study were chosen according to our published observations 8.
3.4. Infarct size analysis
Infarct size was determined by triphenyl tetrazolium chloride (TTC) staining. In brief, at the end of the experimental protocol hearts were infused with 1% TTC in phosphate-buffered solution (pH 7.4) for 10 min prior to snap freezing at −20°C. While frozen, the hearts were sliced perpendicular to the long-axis of the heart at 1 mm intervals and de-stained in 10% formaldehyde solution to increase contrast between necrotic and viable myocardium. The heart slices were then digitally photographed for planimetry using NIH Image 1.62. Infarct size is expressed as an infarct-to-risk zone ratio (the risk zone is the whole ventricular volume in this global ischemic model).
3.5. Statistical analysis
All values are expressed as Mean±SEM. Comparisons between two groups are done using a t-test. Comparisons of more than two groups were performed using a one way ANOVA analysis with Newman-Keuls test as a post-hoc test. Statistical significance was defined as a value of p<0.001.
4. RESULTS
4.1. Postconditioning with netrin-1 induces cardioprotection: Role of ERK1/2 and NO
As shown in Figure 2, netrin-1 postconditioning significantly reduced infarct size to 17.0%±2.5%, from 43.3%±4.6% in the untreated control I/R group (p<0.001, n=6). In U0126 alone or PTIO alone group, myocardial infarct size was not statistically different compared to the control I/R group (44.8%±3.0%, 39.8%±1.7%, respectively, n=6). In combination with either of these two inhibitors, the cardioprotective effect of netrin-1 was completely reversed (Figure 2). In the I/R+netrin-1/U0126 and I/R+netrin-1/PTIO groups, infarct sizes, at 44.8%±2.0% and 43.5%±2.9% respectively, were not significantly different compared to the control group (n=6).
Figure 2.
Netrin-1 postconditioning induced cardioprotection: Role of NO and ERK1/2. Myocardial infarct sizes in the different experimental groups as described in Figure 1, obtained using WT DCC+/+ mice, are presented. Infarct size is expressed as a percentage of area at risk, which is the whole transverse section of the whole ventricle. Representative transversely sectioned heart slices are displayed on the top panel. Infarcted areas are seen in white after TCC staining. *p<0.001 vs. Control I/R; †p<0.001 vs. I/R+netrin-1. The number of animal per experimental groups was six.
4.2. Postconditioning with netrin-1 induces cardioprotection: Role of DCC
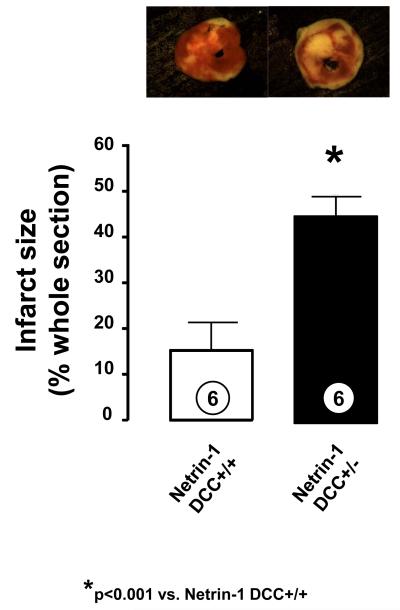
In additional experiments, hearts from DCC+/+ and DCC+/− mice were subjected to I/R and netrin-1 postconditioning. Netrin-1 induced reduction in infarct size in DCC+/+ hearts was completely attenuated in the DCC+/− mice (44.5±2.0% vs. 15.0±2.6% for DCC+/− vs. DCC+/+ respectively, p<0.001, n=6, Figure 3).
Figure 3.
Netrin-1 postconditioning induced cardioprotection: Role of DCC. Myocardial infarct sizes in netrin-1 postconditioned DCC+/− mice and DCC+/+ mice are presented. Infarct size is expressed as a percentage of area at risk, which is the whole transverse section of the whole ventricle. Representative transversely sectioned heart slices are displayed on the top panel. Infarcted areas are seen in white after TCC staining. *p < 0.001 vs. netrin-1 DCC+/+. The number of animal per experimental group was six.
5. DISCUSSION
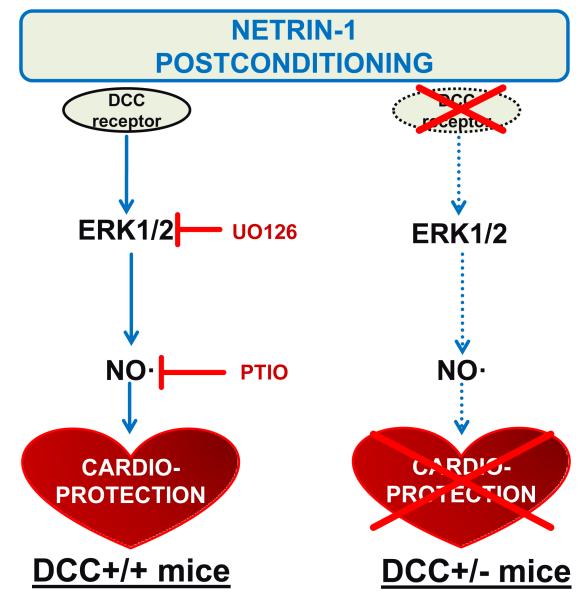
The significance of the study comes from the demonstrations of 1) high efficacy of netrin-1 postconditioning in cardioprotection; 2) roles of DCC, ERK1/2 and NO in cardioprotective effects of netrin-1 postconditioning. These findings are schematically summarized in Figure 4. Despite of growing literatures, urgent needs remain for identification of effective cardioprotective agent that can be easily translated into clinical practice. Together with our earlier report 8, we have shown that netrin-1, a previously known neuronal pathfinder and angiogenic regulator 4,7,9, serves as an extremely potent cardioprotective agent whenever delivered to the heart, either pre-, during, or at the onset of reperfusion.
Figure 4.
Signaling pathways mediating netrin-1 postconditioning induced cardioprotection. Differences in cardioprotective signaling pathways induced by netrin-1 postconditioning in DCC +/+ mice (A), compared to DCC +/− (B) mice. Our data have demonstrated roles of DCC, ERK1/2 and NO in mediating cardioprotection provoked by pharmacological postconditioning with netrin-1. Our previous findings have also established the sequence of activation for the signaling components involved, namely a DCC-ERK1/2-NO cascade8. Absence of DCC in the DCC+/− mice leads to lack of activation in ERK1/2 and NO, resulting in diminished cardioprotection in response to netrin-1.
Previous studies have implicated a role of NO in cardioprotection 10-13; although the evidences are derived mostly from usage of NO donors or genetic manipulation of NO synthase. In the present study we found that pharmacological postconditioning with netrin-1, induces NO-dependent cardioprotection, which shares similarity with our previous findings of alternative netrin-1 treatment 8. An intermediate role of ERK1/2 is also identified. This is consistent with previously established role of ERK1/2 in cardioprotection 14-16. Others and we have demonstrated an upstream role of ERK1/2 in eNOS phosphorylation and activation in vascular endothelial cells 7,17-19, and this pathway can also be turned on in the heart 8,20.
We conclude that pharmacological postconditioning with netrin-1 at a low concentration of 100 ng/mL, potently prevents myocardial injury in response to ischemia reperfusion. Our data suggest that the cardioprotective effects of netrin-1 postconditioning are specifically mediated by the DCC receptor, and DCC-dependent ERK1/2 and NO activation. Taken together with our previous findings that netrin-1 induces cardioprotection when infused into the heart before and during reperfusion, netrin-1 treatment proves to be extremely and consistently beneficial whenever it is delivered to the heart, establishing its substantial promises for being developed into a robust therapeutic strategy for acute myocardial infarction.
6. ACKNOWLEDGEMENTS
This study was supported by National Institute of Health National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL088975 (HC), HL108701 (HC, DGH), and an American Heart Association Established Investigator Award (EIA) 12EIA8990025 (HC).
7. REFERENCES
- 1.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–6. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 2.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–16. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, Wang L, Cohen MV, Downey JM. Myocardial protection by insulin is dependent on phospatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol. 1999;94:188–98. doi: 10.1007/s003950050142. [DOI] [PubMed] [Google Scholar]
- 4.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 5.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–5. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS(s1177)/NO/DCC feed-forward mechanism. J Mol Cell Cardiol. 2010;48:1060–70. doi: 10.1016/j.yjmcc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–4. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegfried MR, Carey C, Ma XL, Lefer AM. Beneficial effects of SPM-5185, a cysteine-containing NO donor in myocardial ischemia-reperfusion. Am J Physiol. 1992;263:H771–7. doi: 10.1152/ajpheart.1992.263.3.H771. [DOI] [PubMed] [Google Scholar]
- 11.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–73. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 12.Vondriska TM, Zhang J, Song C, Tang XL, Cao X, Baines CP, Pass JM, Wang S, Bolli R, Ping P. Protein kinase C epsilon-Src modules direct signal transduction in nitric oxide-induced cardioprotection: complex formation as a means for cardioprotective signaling. Circ Res. 2001;88:1306–13. doi: 10.1161/hh1201.092994. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, Janssens S, Feelisch M, Lefer DJ. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–23. doi: 10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- 14.Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H1481–8. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 17.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;275:30707–15. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63:325–31. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 19.Merla R, Ye Y, Lin Y, Manickavasagam S, Huang MH, Perez-Polo RJ, Uretsky BF, Birnbaum Y. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am J Physiol Heart Circ Physiol. 2007;293:H1918–28. doi: 10.1152/ajpheart.00416.2007. [DOI] [PubMed] [Google Scholar]
- 20.Koricanac G, Tepavcevic S, Zakula Z, Milosavljevic T, Stojiljkovic M, Isenovic ER. Interference between insulin and estradiol signaling pathways in the regulation of cardiac eNOS and Na(+)/K(+)-ATPase. Eur J Pharmacol. 2011;655:23–30. doi: 10.1016/j.ejphar.2011.01.016. [DOI] [PubMed] [Google Scholar]