Abstract
Background
Vitamin D-binding protein (DBP) and catabolism have not been examined in childhood chronic kidney disease (CKD).
Methods
Serum vitamin D [25(OH)D, 1,25(OH)2D, 24,25(OH)2D], DBP, intact parathyroid hormone (iPTH), and fibroblast growth factor-23 (FGF23) concentrations were measured in 148 participants with CKD stages 2–5D secondary to congenital anomalies of the kidney/urinary tract (CAKUT), glomerulonephritis (GN), or focal segmental glomerulosclerosis (FSGS). Free and bioavailable 25(OH)D were calculated using total 25(OH)D, albumin and DBP.
Results
All vitamin D metabolites were lower with more advanced CKD (p<0.001) and glomerular diagnoses (p≤0.002). Among non-dialysis participants, DBP was lower in FSGS vs. other diagnoses (FSGS-dialysis interaction p=0.02). Winter season, older age, FSGS and GN, and higher FGF23 were independently associated with lower free and bioavailable 25(OH)D. Black race was associated with lower total 25(OH)D and DBP, but not free or bioavailable 25(OH)D. 24,25(OH)2D was the vitamin D metabolite most strongly associated with iPTH. Lower 25(OH)D, black race, greater CKD severity, and higher iPTH were independently associated with lower 24,25(OH)2D, while higher FGF23 and GN were associated with greater 24,25(OH)2D.
Conclusions
Children with CKD exhibit altered catabolism and concentrations of DBP and free and bioavailable 25(OH)D, and there is an important impact of their underlying disease.
Keywords: Vitamin D catabolism, vitamin D-binding protein, glomerular disease, chronic kidney disease, pediatric
INTRODUCTION
Vitamin D deficiency is highly prevalent in children with chronic kidney disease (CKD).[1–6] The literature to date has focused on reduced renal production of circulating 1,25-dihydroxyvitamin [1,25(OH)2D] and deficiency of its substrate, total 25(OH)D. The impact of CKD severity and a patient's underlying renal disease on vitamin D-binding protein (DBP) and vitamin D catabolism has not been addressed in childhood CKD.
DBP transports vitamin D metabolites in blood and plays a pivotal role in renal vitamin D metabolism. 25(OH)D and 1,25(OH)2D circulate bound to DBP (85–90%) or albumin (10–15%), with < 1% in their free forms. The binding affinity of DBP for these vitamin D metabolites is considerably greater than that of albumin (7 × 108 M−1 vs. 6 × 105 M−1 for 25(OH)D and 3.7 × 107 M−1 vs. 5.4 × 104 M−1 for 1,25(OH)2D).[7–11] Vitamin D-DBP complexes filtered by the glomerulus are reabsorbed via megalin/cubilin-mediated endocytosis in the proximal tubule. The renal 1-α-hydroxylation of 25(OH)D to 1,25(OH)2D via the enzyme CYP27B1 depends on this process.[12, 13] Changes in DBP concentrations secondary to glomerular and/or tubular damage in CKD could impair vitamin D transport and metabolism, and may influence the bioavailability of 25(OH)D to target tissues. A recent study in adult hemodialysis patients demonstrated that bioavailable 25(OH)D, the fraction that is not bound to DBP, was better correlated with measures of mineral metabolism than total 25(OH)D concentrations.[7] Risk factors for low bioavailable vitamin D concentrations have not been addressed in children with CKD.
Vitamin D catabolism is an incompletely understood determinant of steady-state vitamin D metabolite concentrations in blood and tissue. CYP24A1 is the key enzyme involved in the catabolism of 25(OH)D and 1,25(OH)2D; 24,25-dihydroxyvitamin D [24,25(OH)2D] is the main product of 25(OH)D catabolism. Like CYP27B1, CYP24A1 is expressed in most tissues and regulated by 1,25(OH)2D.[14–16] In vitro and animal studies have shown that renal CYP24A1 is suppressed by parathyroid hormone (PTH)[17–19] and induced by fibroblast growth factor-23 (FGF23).[20–22] In a recent study in adults with CKD, lower estimated glomerular filtration rate (eGFR) was strongly associated with lower serum 24,25(OH)2D concentrations, and the negative correlation of PTH with 24,25(OH)2D was stronger than its correlations with 25(OH)D or 1,25(OH)2D.[23] Down regulation of CYP24A1 may represent an appropriate response to tissue-concentration 1,25(OH)2D deficiency[14] in CKD, and higher 24,25(OH)2D concentrations may provide a more accurate measure of functional vitamin D status.
We recently reported that hypoalbuminemia and glomerular disease, particularly FSGS, were independent risk factors for low 25(OH)D concentrations in pediatric CKD, adjusted for age, race, season, and CKD severity.[4] We hypothesized that the lower vitamin D concentrations in participants with glomerular disease was due to lower serum DBP concentrations. The objective of this study was to expand the assessment of vitamin D metabolism in this cohort to include measures of serum DBP, FGF-23, and 24,25(OH)2D in order to identify correlates of bioavailable and free 25(OH)D concentrations and vitamin D catabolism in childhood CKD.
METHODS
Study Participants
This cross-sectional study of children and adolescents, ages 5 to 21 years, with CKD was conducted at the Children's Hospital of Philadelphia (CHOP) and Cincinnati Children's Hospital Medical Center (CCHMC), as previously described.[4] The original study reported total vitamin D concentrations in 182 participants with CKD compared to 276 healthy controls. The current study was limited to participants with the following underlying diseases: (1) CAKUT, including aplastic/hypoplastic /dysplastic /cystic kidneys and obstructive uropathy; (2) Glomerulonephritis (GN), including membranoproliferative GN, IgA nephropathy, Alport's syndrome, systemic lupus erythematosus, and Wegener's granulomatosis; and (3) FSGS. The 21 participants with other causes of CKD were not included here. A total of 148 (92%) had sufficient stored serum to measure FGF23 and DBP and estimate free and bioavailable 25(OH)D concentrations. 140 (87%) had sufficient stored serum to measure 24,25(OH)2D. As this was an ancillary study performed on stored serum, we did not have sufficient stored serum samples for the controls from our original study to measure DBP. There were no urine samples available to measure total protein excretion or DBP.
The Institutional Review Boards at CHOP and CCHMC approved the study protocol. Informed consent was obtained from participants ≥ 18 years of age, and assent along with parental consent from subjects < 18 years, as appropriate.
Anthropometry and Race
Height was measured with a stadiometer and weight with a digital scale. Height and body mass index (BMI, kg/m2) Z-scores were calculated using national reference data.[24] Information on race was obtained by self-report and classified according to NIH categories.
Laboratory Measurements
Non-fasting blood samples were collected. Serum DBP was measured in duplicate (CV <10%) using an enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). The inter-assay CV was 1.6–3.6%, and recovery was 98–103%. Serum 25(OH)D and 1,25(OH)2D concentrations were measured by radioimmunoassay (RIA; DiaSorin Inc., Stillwater, MN).[25] Intra-assay CV for 25(OH)D was 2.2% and for 1,25(OH)2D was 7–11%.[26] Serum 24,25(OH)2D was measured using a combined tandem mass spectrometry assay for 24,25(OH)2D and 25(OH)D.[23, 27] The method has a lower limit of quantification (20% CV) of 0.5 ng/mL and inter-assay imprecision of 8.58% at 1.5 ng/mL. This assay simultaneously generated measures of 25(OH)D. The RIA and LC-MSMS measures of 25(OH)D were highly correlated among the 140 participants with both results (spearman's rho = 0.97, p <0.001). The data presented here are limited to the RIA results.
Intact PTH (iPTH) and bioactive PTH (1-84PTH) concentrations were measured by RIA with a CV of 3–5% and 3–10%, respectively (Scantibodies Clinical Laboratory, Santee, CA).[28] Intact FGF23 was measured via ELISA (Kainos Laboratories Inc., Tokyo, Japan; CV 6.7–12.4%). Serum albumin and creatinine were measured by spectrophotometric enzymatic assay (Vitros, Johnson & Johnson Co., Rochester, NY) with CV's of 1–2% and 1–5%, respectively. Serum calcium and phosphorus were measured using standard clinical methods with CV's of 1.3% and ≤ 2.1%, respectively; calcium concentration was corrected for albumin.[29]
Estimated glomerular filtration rate (eGFR, ml/min/1.73m2) was calculated based on height and serum creatinine using the estimating equation generated in the Chronic Kidney Disease in Children Study (CKiD).[30] Participants were categorized into three groups according to CKD stage[31]: stage 2–3 (eGFR 30–89 ml/min/1.73m2; n=60), stage 4–5 (eGFR < 30 ml/min/1.73m2; n=43), and stage 5D (maintenance dialysis; n=45).
Estimates of Free and Bioavailable 25(OH)D
Serum free and bioavailable 25(OH)D were calculated using total 25(OH)D, DBP, and albumin concentrations and the recently developed equations of Powe et al.[32] Powe et al. adapted validated equations for free and bioavailable testosterone,[33] by replacing testosterone, sex hormone-binding globulin, and albumin and their respective binding constants with those of 25(OH)D, DBP, and albumin. Bioavailable 25(OH)D represents the 25(OH)D that is not bound to DBP, i.e. free + albumin-bound 25(OH)D. Estimates of free 25(OH)D were also calculated according to the original equation derived by Bikle et al.[8] Estimates using this equation were highly correlated (R = 0.93) with free 25(OH)D measured by centrifugal ultrafiltration.[8] The affinity constants for the binding of vitamin D are the same for both equations: 7 × 108 M−1 for DBP versus 6 × 105 M−1 for albumin. The concentrations of free 25(OH)D derived from the two different equations were highly correlated (spearman's rho = 1, p < 0.001). The equations used to calculate free and bioavailable 25(OH)D are described in detail in the Supplementary Material of Powe et al.[32] and summarized below:
Concentrations of albumin, DBP and 25(OH)D in these equations are in mol/L, and free and bioavailable 25(OH)D were then converted to pg/ml and ng/ml respectively for ease of interpretation of results.
Disease Characteristics and Medications
Medical charts were reviewed for the date of diagnosis of CKD, underlying etiology of renal disease, dialysis history, and prior medications. Information on current medications and vitamin D intake from multivitamins and dietary supplements was obtained by questionnaire and review of the medical record.
Statistical Analyses
All analyses were performed using STATA 11.0 (Stata Corporation, College Station, TX). A two-sided p-value of <0.05 was considered statistically significant. Distributions of all variables were assessed for normality. Descriptive statistics for continuous variables were reported as mean ± standard deviation (SD) values for normally distributed data and median and inter-quartile range (IQR) for skewed data. Group differences were assessed using the Student's t, ANOVA, Wilcoxon rank sum or Kruskal-Wallis tests as appropriate, and the nptrend command in STATA as the nonparametric test for trend across ordered groups. The Chi-square test was used to assess differences in proportions. Skewed variables were natural log-transformed as previously described.[34] Serum 25(OH)D concentrations were categorized as deficient (<20 ng/ml) or not, in accordance with the 2011 Institute of Medicine report.[35] Analyses of the association between underlying renal disease and DBP concentrations were stratified by dialysis (CKD stages 2–5 vs. stage 5D), because participants on dialysis may have insufficient glomerular filtration to demonstrate disease-specific effects related to proteinuria and proximal tubular function. Correlations were assessed by Pearson product moment or Spearman's rank correlations. The corcor program was used to test the equality of two dependent correlations.
Multivariable linear regression analysis was used to evaluate potential correlates of serum free and bioavailable 25(OH)D concentrations, including age, race (black race vs. others), sex, winter season (November-April vs. remaining months), study site, BMI Z-score, vitamin D supplementation (≥400 vs. <400 IU/day), calcitriol therapy, underlying diagnosis, CKD stage, and FGF23 concentrations. Variables were retained in the final models if the p value was <0.05. Model assumptions were assessed via graphical checks, e.g. to assess linearity of relationships and the normality of residuals.
Serum 24,25(OH)2D concentration was modeled as a function of age, sex, race, 25(OH)D, 1,25(OH)2D, DBP, PTH, FGF23, CKD stage, underlying renal disease, and calcitriol therapy. Multivariate analyses of serum 1,25(OH)2D were limited to participants not on dialysis, as the vast majority of participants on dialysis (40/45) and all 16 of the FSGS participants on dialysis were treated with calcitriol at the time of the study visit.
RESULTS
Participant Characteristics (Table 1)
Table 1.
Participant Characteristics
| CKD Participants | |
|---|---|
| N | 148 (100%) |
| Study site | |
| The Children's Hospital of Philadelphia | 93 (63%) |
| Cincinnati Children's Hospital Medical Center | 55 (37%) |
| Age category (at time of study) | |
| 5 – 8 years | 19 (13%) |
| 9 – 11 years | 29 (20%) |
| 12 – 14 years | 36 (24%) |
| 15 – 21 years | 64 (43%) |
| Age at chronic kidney disease (CKD) diagnosis (years) | 4.4 (0, 10.6) |
| Male | 91 (61%) |
| Race | |
| White | 104 (70%) |
| Black | 37 (25%) |
| Other | 7 (5%) |
| Winter season | 68 (46%) |
| Height Z-score | −0.71 (−1.61, +0.26) |
| Body mass index Z-score | 0.20 (−0.62, +1.40) |
| Underlying etiology of renal disease | |
| Congenital anomalies of the kidney and urinary tract (CAKUT) | 100 (68%) |
| Glomerulonephritis (GN) | 21 (14%) |
| Focal segmental glomerulosclerosis (FSGS) | 27 (18%) |
| CKD stage | |
| 2 | 13 (9%) |
| 3 | 47 (32%) |
| 4 | 25 (17%) |
| 5 | 18 (12%) |
| 5D | 45 (30%) |
| Dialysis modality | |
| Hemodialyis | 29 (64%) |
| Peritoneal dialysis | 16 (36%) |
| Vitamin D supplement usage ≥ 400 IU/day | 25 (17%) |
| Receiving calcitriol therapy by CKD stage | |
| 2/3 | 11 (18%) |
| 4/5 | 33 (77%) |
| Dialysis | 40 (89%) |
Data are presented as n(%) or Median (inter-quartile range)
The median age of our cohort was 14.1 years (IQR 11.1, 17.5). 61% of participants were male, 25% were black, and 32% had glomerular disease. Nearly half of the study visits were conducted during the winter season. Participants were evenly distributed amongst CKD stages. While the use of calcitriol therapy was common, particularly in advanced stage CKD, only 17% of the cohort was receiving nutritional supplementation of at least 400 IU of vitamin D daily.
Serum concentrations of vitamin D metabolites and DBP
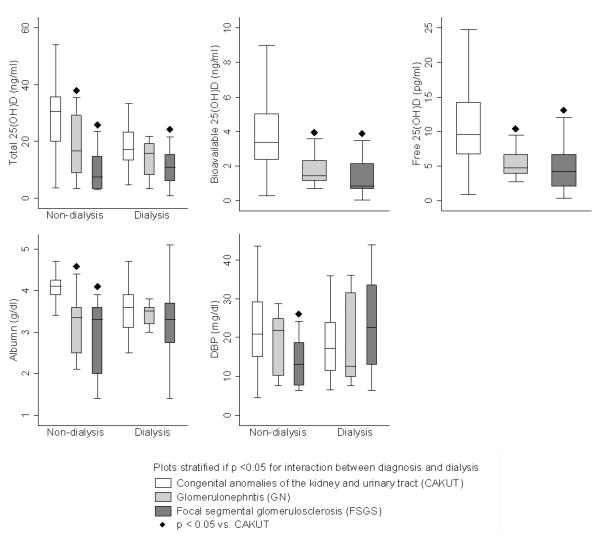
Serum concentrations of total, free, and bioavailable 25(OH)D, 24,25(OH)2D and 1,25(OH)2D were each lower with more advanced CKD stage and differed by underlying etiology of renal disease (Table 2 and Figure 1). Serum albumin differed significantly by CKD stage, but DBP did not. DBP and albumin concentrations were not significantly correlated (Spearman's rho = 0.15, p = 0.08). The prevalence of 25(OH)D deficiency (< 20 ng/mL) among participants with diagnoses of FSGS and GN was 85% and 71%, respectively, as compared to 33% among participants with CAKUT (p <0.001).
Table 2.
Serum concentrations of vitamin D metabolites, albumin, and vitamin D-binding protein (DBP) according to:
| a) chronic kidney disease (CKD) stage | ||||
|---|---|---|---|---|
| CKD 2/3 n = 60 | CKD 4/5 n = 43 | Dialysis n = 45 | p1 | |
| Total 25(OH)D (ng/ml) | 30.6 (18.9, 35.7) | 22.0 (11.4, 31.9) | 14.1 (8.3, 19.4) | <0.001 |
| Free 25(OH)D (pg/ml) | 10.3 (7.0, 14.4) | 8.2 (5.2, 11.6) | 5.2 (3.8, 8.1) | <0.001 |
| Bioavailable 25(OH)D (ng/ml) | 3.6 (2.5, 5.2) | 2.7 (1.8, 3.7) | 1.6 (0.9, 2.3) | <0.001 |
| Albumin (g/dl) | 4.1 (3.8, 4.3) | 4.0 (3.5, 4.2) | 3.4 (3.0, 3.7) | <0.001 |
| DBP (mg/dl) | 20.2 (14.0, 27.9) | 20.8 (11.2, 28.8) | 19.5 (11.1, 28.2) | >0.6 |
| 1,25(OH)2D (pg/ml) | 37.0 29.2, 45.2 | 32.9 22.4, 39.7 | 16.7 11.3, 22.3 | <0.001 |
| 24,25(OH)2D (ng/ml) | 2.5 1.7, 3.9 | 1.0 0.5, 2.2 | 0.5 0.3, 0.8 | <0.001 |
| b) underlying renal disease | ||||
|---|---|---|---|---|
| CAKUT n = 100 | GN n = 21 | FSGS n = 27 | p1 | |
| Total 25(OH)D (ng/ml) | 29.1 (17.5, 34.7) | 15.9 (8.8, 20.6) | 10.4 (4.9, 14.9) | <0.001 |
| Free 25(OH)D (pg/ml) | 9.6 (6.8, 14.3) | 4.8 (3.9, 6.7) | 4.2 (2.0, 6.7) | <0.001 |
| Bioavailable 25(OH)D (ng/ml) | 3.4 (2.4, 5.0) | 1.4 (1.1, 2.3) | 0.8 (0.7, 2.1) | <0.001 |
| Albumin (g/dl) | 4.1 (3.7, 4.2) | 3.4 (3.0, 3.6) | 3.3 (2.6, 3.6) | <0.001 |
| DBP (mg/dl) | 20.6 (13.6, 28.7) | 20.8 (10.1, 28.2) | 16.8 (9.9, 24.2) | 0.55 |
| 1,25(OH)2D (pg/ml) | 35.5 24, 43.1 | 22.7 13.2, 34.3 | 19.4 15.1, 28.1 | <0.001 |
| 24,25(OH)2D (ng/ml) | 1.8 0.7, 2.7 | 1.2 0.5, 1.9 | 0.4 0.3, 0.5 | <0.001 |
Results presented as median (IQR)
ANOVA, Kruskal-Wallis or test for trend among groups.
CAKUT = congenital anomalies of the kidney and urinary tract. GN = glomerulonephritis. FSGS = focal segmental glomerulosclerosis.
Figure 1.
Serum concentrations of total, bioavailable and free 25(OH)D, albumin and vitamin D-binding protein (DBP) by underlying renal diagnosis.
When limited to pre-dialysis participants, DBP concentrations were significantly lower in those with FSGS compared to all other diagnoses (median 13.1 vs. 21.0 mg/dl; p = 0.01), and this difference persisted after adjustment for lower DBP concentrations in black participants. However, among dialysis participants, DBP concentrations did not differ according to underlying diagnosis (p = 0.3, Figure 1). The test for interaction (p = 0.02) between FSGS and dialysis indicated that the differences observed in these stratified analyses were statistically significant. That is, the association between DBP and underlying disease was significantly different between dialysis and non-dialysis participants. For total 25(OH)D and albumin, there were similarly significant interactions between diagnosis and dialysis (all p <0.02); that is, the lower 25(OH)D and albumin concentrations in glomerular disease were more pronounced among non-dialysis participants. DBP concentrations did not differ according to dialysis modality (hemodialysis vs. peritoneal dialysis) among those on dialysis (p = 0.3). Among non-dialysis participants, FSGS was also associated with significantly lower 1,25(OH)2D concentrations (p = 0.01), adjusted for the concentration of 25(OH)D, iPTH and FGF23.
Correlates of Free and Bioavailable 25(OH)D Concentrations
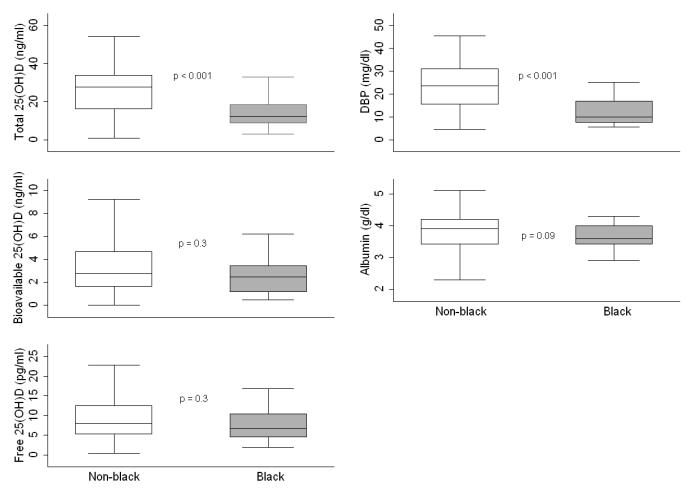
In bi-variate analyses, winter season, older age, being on dialysis, diagnoses of FSGS and GN, calcitriol therapy and higher FGF23 concentrations were associated with lower free and bioavailable 25(OH)D concentrations (all p ≤ 0.03). Serum total 25(OH)D and DBP were both significantly lower in black compared to non-black participants; however, free and bioavailable 25(OH)D and albumin concentrations did not differ (Figure 2). Table 3 summarizes the comparative multivariable linear regression analysis for correlates of the different vitamin D metabolites. For ease of clinical interpretation the untransformed outcomes are presented when transformation did not impact model findings. As we previously demonstrated, older age, winter season, black race, being on dialysis, underlying diagnosis of FSGS, and lower serum albumin were independently associated with lower total 25(OH)D. Interestingly, DBP was not associated with total 25(OH)D. Winter season, older age, and diagnoses of FSGS or GN were significant independent correlates of lower free and bioavailable 25(OH)D, but in contrast to total 25(OH)D, black race and CKD stage were not associated with free or bioavailable 25(OH)D in multivariable analyses. Since albumin and DBP are included in the formulae to calculate free and bioavailable 25(OH)D, they were excluded from these models. Using the formula for free 25(OH)D derived by Bikle et al.[8] did not change the model results. Without CKD stage in their respective models, FGF23 was inversely associated with free and bioavailable 25(OH)D in multivariable analysis (all transformed, p <0.03). As expected based on their substrate dependence, 25(OH)D was a significant determinant of 1,25(OH)2D (spearman's rho = 0.53, p < 0.001) and 24,25(OH)2D (spearman's rho = 0.81, p < 0.001). Dialysis therapy was associated with lower 1,25(OH)2D independent of 25(OH)D.
Figure 2.
Serum concentrations of total, bioavailable and free 25(OH)D, albumin, and vitamin D-binding protein (DBP) by race.
Table 3.
Comparative multivariable linear regression analysis for correlates of vitamin D metabolites
| Model R2 | Total 25(OH)D (ng/ml) 0.63 | Bioavailable 25(OH)D (ng/ml) 0.28 | Free 25(OH)D (pg/ml) 0.22 | ln[1,25(OH)2D] 0.39 | ln[24,25(OH)2D] 0.77 |
|---|---|---|---|---|---|
| Age (yrs) vs. 5–8 | |||||
| 9 – 11 | −5.2 (−9.9, −0.6) * | −0.7 (−1.9, 0.5) | −1.6 (−4.9, 1.7) | −0.1 (−0.2, 0.1) | −0.1 (−0.4, 0.2) |
| 12 – 14 | −8.5 (−13.1, −4.0) *** | −1.5 (−2.6, −0.3) * | −4.1 (−7.4, −0.9) * | −0.1 (−0.2, 0.1) | 0.03 (−0.2, 0.3) |
| 15 – 21 | −11.6 (−15.9, −7.3) *** | −1.7 (−2.8, −0.6) ** | −4.5 (−7.5, −1.4) ** | −0.1 (−0.3, 0.1) | 0.02 (−0.3, 0.3) |
| Winter season | −5.9 (−8.6, −3.1) *** | −0.9 (−1.6, −0.2) ** | −2.5 (−4.5, −0.6) * | −0.02 (−0.1, 0.1) | −0.1 (−0.3, 0.04) |
| Black race | −7.7 (−11.3, −4.2) *** | 0.2 (−0.6, 1.0) | 0.4 (−1.8, 2.6) | 0.1 (−0.01, 0.3) | −0.2 (−0.5, −0.02) * |
| CKD stage vs. 2/3 | |||||
| 4/5 | −2.3 (−5.6, 1.1) | −0.4 (−1.3, 0.4) | −0.8 (−3.2, 1.6) | 0.0004 (−0.1, 0.1) | −0.3 (−0.6, −0.1) *** |
| 5D | −4.7 (−8.1, −1.2) ** | −0.7 (−1.6, 0.2) | −1.2 (−3.6, 1.3) | −0.3 (−0.4, −0.2) *** | −0.9 (−1.1, −0.6) *** |
| Diagnosis vs. CAKUT | |||||
| GN | −2.1 (−6.4, 2.2) | −1.4 (−2.4, −0.3) ** | −3.1 (−5.9, −0.2) * | −0.1 (−0.2, 0.1) | 0.5 (0.2, 0.7) *** |
| FSGS | −7.3 (−11.4, −3.2) *** | −1.8 (−2.8, −0.9) *** | −4.5 (−7.2, −1.9) *** | −0.1 (−0.3, 0.1) | −0.05 (−0.3, 0.2) |
| Albumin (per g/dl) | 5.7 (3.3, 8.1) *** | −0.04 (−0.1, 0.1) | −0.01 (−0.2, 0.1) | ||
| DBP (per mg/dl) | 0.09 (−0.05, 0.24) | 0.002 (−0.004, 0.01) | 0.002 (−0.01, 0.01) | ||
| 25(OH)D (per ng/ml) | 0.01 (0.003, 0.02) ** | 0.04 (0.03, 0.05) *** | |||
| Intercept | 16.3 (5.7, 26.9) ** | 5.7 (4.7, 6.7) *** | 15.4 (12.6, 18.2) *** | 3.8 (3.4, 4.2) *** | −0.4 (−1.1, 0.2) |
Albumin and DBP are part of the formulae to calculate free and bioavailable 25(OH)D and were therefore, not included in their models due to collinearity.
p < 0.05
p≤ 0.01
p ≤ 0.001
DBP, vitamin D-binding protein; CKD, chronic kidney disease; CAKUT, congenital anomalies of the kidney and urinary tract
Correlates of 24,25(OH)2D
In separate analyses adjusted only for 25(OH)D, higher serum iPTH and FGF23, greater CKD severity, and calcitriol therapy were associated with lower 24,25(OH)2D concentration, and diagnosis of GN was associated with higher 24,25(OH)2D. In multivariate regression analysis (Tables 3 and 4), greater 25(OH)D and GN remained statistically significant correlates of higher 24,25(OH)2D, and black race, greater CKD severity and iPTH were associated with lower 24,25(OH)2D. The expected positive association between FGF23 and 24,25(OH)2D emerged in multivariate analysis after adjustment for confounding by CKD severity and iPTH (both of which lead to increased FGF23). The R2 of the multivariate model for correlates of 24,25(OH)2D was 0.79 indicating that it explained 79% of the variability in this measure.
Table 4.
Multivariable linear regression model for correlates of serum 24,25(OH)2D concentration
| ln[24,25(OH)2D] | β-coefficient | 95% CI | p-value |
|---|---|---|---|
| 25(OH)D | 0.04 | 0.04, 0.05 | <0.001 |
| Black race | −0.20 | −0.38, −0.01 | 0.04 |
| CKD stage (versus 2/3) | |||
| 4/5 | −0.34 | −0.56, −0.13 | 0.002 |
| 5D | −0.84 | −1.09, −0.59 | <0.001 |
| Glomerulonephritis (vs. other diagnoses) | 0.47 | 0.25, 0.68 | <0.001 |
| ln(PTH) | −0.09 | −0.16, −0.02 | 0.02 |
| ln(FGF23) | 0.05 | 0.003, 0.10 | 0.04 |
Model R2 = 0.79
CKD, chronic kidney disease; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23
Comparative associations between vitamin D metabolites, calcium and PTH
Serum calcium was positively associated with total 25(OH)D (spearman's rho = 0.19, p = 0.02) and 24,25(OH)2D (spearman's rho = 0.28, p <0.001), but not 1,25(OH)2D, free 25(OH)D or bioavailable 25(OH)D. There were no statistically significant differences between the correlation coefficients for vitamin D metabolites with calcium. iPTH was inversely associated with total, free and bioavailable 25(OH)D (spearman's rho of −0.48, −0.38 and −0.41, respectively, all p <0.001) and with 24,25(OH)2D (spearman's rho = −0.60, p <0.001) and 1,25(OH)2D (spearman's rho = −0.29, p <0.001). There was a significant difference between the correlation coefficients for 24,25(OH)2D versus 1,25(OH)2D with iPTH (p = 0.002), but otherwise there was no statistically significant difference among these correlations. Substituting PTH1-84 for iPTH did not impact findings.
DISCUSSION
To our knowledge, this is the first study to assess free and bioavailable 25(OH)D, and 24,25(OH)2D concentrations and their determinants in pediatric CKD. Prior studies of free vitamin D concentrations in patients with renal disease are limited, but raised concerns regarding the interpretation of total concentrations in the setting of urinary losses of DBP and albumin. Proteinuria frequently complicates pediatric CKD; 75% of participants in the CKiD cohort were proteinuric at baseline, 14% of whom had nephrotic-range proteinuria.[36] Given the burden of proteinuria in childhood CKD, the assessment of free and protein bound vitamin D concentrations is an important first step towards understanding the determinants and consequences of abnormal vitamin D metabolism in this population.
In our prior study of this cohort, we reported that glomerular disease, particularly FSGS, was an independent risk factor for 25(OH)D deficiency, compared with CAKUT.[4] We hypothesized that lower 25(OH)D concentrations in glomerular diseases were due to urinary losses of DBP. This subsequent study confirmed lower serum DBP concentrations in pre-dialysis participants with FSGS. However, total 25(OH)D was not associated with serum DBP, and glomerular diseases were associated with lower free and bioavailable 25(OH)D concentrations, suggesting that impaired vitamin D metabolism likely involves mechanisms extending beyond urinary losses of binding proteins. Further supporting this hypothesis is recent evidence that anti-proteinuric therapy in CKD patients reduced urinary DBP loss, but did not impact serum DBP or vitamin D concentrations.[37] Our finding that FSGS was associated with significantly lower DBP concentrations among pre-dialysis CKD, but not dialysis participants suggests that the underlying disease does not impact serum DBP in the absence of sufficient glomerular filtration and urine output. In a related study, Prytula et al. reported that serum DBP concentrations in 10 children with CKD stage 4–5 were within the normal reference range;[38] however, they did not assess the impact of the underlying disease and excluded patients with nephrotic syndrome. They also documented similar DBP concentrations in 16 children on peritoneal dialysis, despite dialysate losses of DBP. Similarly, we did not find lower serum DBP in the 16 participants on peritoneal dialysis compared to those on hemodialysis or those with CKD stage 4–5.
While circulating 1,25(OH)2D is primarily derived from the renal hydroxylation of DBP-bound 25(OH)D, multiple other cell populations, including monocytes, express CYP27B1 for localized synthesis of 1,25(OH)2D. A series of seminal experiments demonstrated that induction of the cathelicidin antimicrobial protein in human monocytes depends on serum 25(OH)D concentration and established a pivotal role for DBP in regulating availability of 25(OH)D to monocytes, suggesting that free or bioavailable, not DBP-bound, vitamin D determines this innate immune response.[39–41] Given the infectious complications of patients with CKD, particularly when complicated by nephrotic-range proteinuria, the finding of lower free and bioavailable 25(OH)D in glomerular disease may have important clinical implications.
Normally, vitamin D metabolites in serum are bound to DBP [85–88% for 25(OH)D and 1,25(OH)2D] or albumin, with < 1% of 25(OH)D and 1,25(OH)2D in their free forms.[8–11] Vitamin D-DBP complexes are freely filtered by the glomerulus and reabsorbed, along with numerous other ligands including PTH, via megalin/cubilin-mediated endocytosis in the proximal tubule. The conversion of 25(OH)D to 1,25(OH)2D by CYP27B1 depends on this process,[12, 13] and animal studies have shown that blocking this reabsorptive pathway has far greater adverse effects on mineral metabolism and skeletal health than DBP deficiency.[42, 43] The contribution of proximal tubular dysfunction to disordered mineral metabolism in CKD has not been elucidated. The fact that FSGS was associated with lower 1,25(OH)2D, independent of substrate [i.e. DBP-bound 25(OH)D], implicates impaired tubular endocytosis and 1α-hydroxylation of DBP-bound 25(OH)D in vitamin D deficiency in FSGS.
Recently, concentrations of fibroblast growth factor 23 (FGF23), a key regulator of phosphorus and vitamin D metabolism, were reported to be significantly higher in children with glomerular vs. non-glomerular disease. (Portale AA, Wolf MS, Salusky IB, et al.: Disordered mineral metabolism in the CKiD children: role of FGF23. 44th Annual Meeting of the American Society of Nephrology, Philadelphia, PA, November 2011) FGF23 inhibits CYP27B1 activity and induces the renal CYP24A1 responsible for catabolism of 25(OH)D and 1,25(OH)2D.[21, 44, 45] The lower free and bioavailable 25(OH)D and total 1,25(OH)2D concentrations in FSGS participants in our study here persisted after adjustment for, and were therefore, not explained by FGF23.
Bhan et al.[7] reported that bioavailable 25(OH)D was more tightly linked with measures of mineral metabolism (serum calcium and PTH) than total 25(OH)D in 94 incident adult dialysis patients. Calcium was not associated with total 25(OH)D (r=0.01, p=0.92), but was positively correlated with bioavailable 25(OH)D (r=0.26, p=0.01). Similarly, total 25(OH)D was not associated with PTH (β=−0.21, p=0.28) but bioavailable 25(OH)D was (β=−0.32, p=0.02). We did not corroborate these findings in our pediatric cohort. By contrast, total 25(OH)D was associated with serum calcium, while free and bioavailable 25(OH)D were not (though there was no statistically significant difference among the correlations), and iPTH was inversely and comparably associated with total, free and bioavailable 25(OH)D (spearman's rho of −0.48, −0.38 and −0.41, respectively). It must be noted that our two study populations differed considerably in terms of their distribution of age, CKD severity and underlying disease.
This study corroborates recent findings of altered vitamin D catabolism in adults with CKD.[23] As in adults, more advanced CKD was strongly associated with lower 24,25(OH)2D concentrations. Importantly, this finding persisted after adjustment for both the concentration of substrate [25(OH)D] available as well as the principal hormonal influences of PTH and FGF23 on vitamin D catabolism. Whether the lower serum 24,25(OH)2D concentrations in CKD are a function of declining renal mass, proximal tubular dysfunction, or an appropriate compensatory mechanism in the setting of tissue-level 1,25(OH)2D deficiency remains to be determined. The latter is supported by the fact that, as in adults, 24,25(OH)2D was most strongly correlated with iPTH. To our knowledge, this is also the first clinical demonstration of the independent reciprocal associations of iPTH and FGF23 with serum 24,25OH2D concentration in children with CKD.
As in the recent adult study,[23] black race was associated with lower 24,25(OH)2D concentrations, independent of 25(OH)D, CKD severity, underlying diagnosis, iPTH and FGF23. Together with the findings of lower DBP and comparable free 25(OH)D in black compared to non-black participants, this emphasizes racial differences in vitamin D handling and catabolism that must be considered in the assessment of vitamin D status and which may render total 25(OH)D an incomplete index of sufficiency. The novel observation of higher 24,25(OH)2D in participants with glomerulonephritis, almost half of whom had systemic inflammatory disease, raises questions regarding the effect of inflammation on CYP24A1 activity. [46]
This study has several limitations. First, the cross-sectional design limits our ability to make causal inferences. Second, as discussed above, the under-representation of FSGS in the CKD stage 2/3 category limits the generalizability of our findings to patients with less advanced CKD or preserved renal function. Third, direct measures of free 25(OH)D are currently not commercially available; therefore, concentrations were estimated based on total 25(OH)D, DBP and albumin concentrations. However, a recent study using these equations reported that bone density was associated with free (r = 0.41, p = 0.003) and bioavailable (r = 0.44, p = 0.002), but not total 25(OH)D (r = 0.18, p = 0.24) in healthy young adults, supporting the validity of these equations.[32] Furthermore, use of the equation developed by Bikle et al. yielded comparable results in this study, and were highly correlated (r = 0.93) with free 25(OH)D measured by centrifugal ultrafiltration in prior studies.[8] Black patients may have a different distribution of DBP genotypes relative to non-black patients which may impact the affinity of DBP and hence the actual amount of bioavailable and free 25(OH)D. We do not have genotype data on this cohort, but future studies incorporating such genotype data and novel mathematical modeling of free 25(OH)D are needed.[47] Finally, as this analysis was an ancillary to an earlier bone study and urine collection was not a focus of the original study, we were limited by lack of urine specimens, and consequent lack of data on the magnitude of proteinuria and urinary DBP loss.
This study has important strengths. It is one of the largest studies of mineral metabolism in childhood CKD and the first to examine concentrations of vitamin D-binding protein, free and bioavailable 25(OH)D, and 24,25(OH)2D. This study is unique in its comprehensive assessment of mineral metabolism with simultaneous measures of vitamin D metabolites, iPTH, FGF23, calcium and phosphorus. We used a novel and accurate mass-spectrometry assay for 24,25(OH)2D[23] as compared to earlier, smaller studies in adults which used a competitive binding assay with potential for cross-reactivity with 25(OH)D and 1,25(OH)2D.[48, 49] The heterogeneity of our study population enabled us to examine the impact of underlying disease on concentrations of vitamin D and its binding protein. Our pre-dialysis population was representative based on comparison to CKiD.[36]
In summary, we observed that among children and adolescents with CKD, glomerular disease, especially FSGS, was independently associated with lower free and bioavailable 25(OH)D concentrations. Pre-dialysis participants with FSGS also had lower 1,25(OH)2D concentrations for a given concentration of 25(OH)D substrate. Together these findings support the hypothesis that disturbances in vitamin D metabolism in these patients extend beyond increased glomerular filtration and urinary loss of its binding proteins to include aberrant tubular handling of 25(OH)D downstream from the glomerulus. We also demonstrated that greater CKD severity is associated with lower 24,25(OH)2D concentrations, even after adjustment for the significant and reciprocal hormonal influences of iPTH and FGF23, and that glomerulonephritis was independently associated with increased 24,25(OH)2D. Thus, children with CKD not only have nutritional deficiency and decreased 1-α hydroxylation of 25(OH)D; they exhibit altered catabolism and concentrations of DBP and free and bioavailable 25(OH)D, and there is an important impact of their underlying disease. Future longitudinal studies are needed to examine the associations of free and bioavailable vitamin D concentrations with clinical outcomes such as infection, cardiovascular disease and bone disease.
ACKNOWLEDGEMENTS
We would like to thank the study participants and their families for their time and dedication. We greatly appreciate the assistance of the Clinical Research Coordinators and the staff at the Clinical Translational Research Centers (CTRC) at CCHMC and CHOP in the conduct of this study, particularly Samir Sayed, B.S. in the CTRC Translational Core Laboratory at CHOP for his work on the DBP assay.
Funding sources: This project was supported by NIH grants R01-DK060030 (MBL), R01-HD040714 (MBL), K24-DK076808 (MBL) and by the National Center for Research Resources, Grants UL1RR024134 and M01-RR-08084, which are now at the National Center for Advancing Translational Sciences, Grants UL1TR000003 and UL1TR000077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Laskin was supported by a Career Development Award in Comparative Effectiveness Research (KM1CA156715-01)
Dr. Denburg was funded by a National Kidney Foundation / Amgen KDOQI Research Fellowship, The Nephcure Foundation-American Society of Nephrology Research Grant and K23DK093556.
Footnotes
Disclosure statement: Dr. de Boer receives research funding from Abbott Laboratories. The remaining authors have nothing to disclose.
REFERENCES
- 1.Ali FN, Arguelles LM, Langman CB, Price HE. Vitamin D deficiency in children with chronic kidney disease: uncovering an epidemic. Pediatrics. 2009;123:791–796. doi: 10.1542/peds.2008-0634. [DOI] [PubMed] [Google Scholar]
- 2.Belostotsky V, Mughal MZ, Berry JL, Webb NJ. Vitamin D deficiency in children with renal disease. Arch Dis Child. 2008;93:959–962. doi: 10.1136/adc.2007.134866. [DOI] [PubMed] [Google Scholar]
- 3.Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A. Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol. 2010;25:2483–2488. doi: 10.1007/s00467-010-1639-2. [DOI] [PubMed] [Google Scholar]
- 4.Kalkwarf HJ, Denburg MR, Strife CF, Zemel BS, Foerster DL, Wetzsteon RJ, Leonard MB. Vitamin D deficiency is common in children and adolescents with chronic kidney disease. Kidney Int. 2012;81:690–697. doi: 10.1038/ki.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK. Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol. 2008;23:1831–1836. doi: 10.1007/s00467-008-0842-x. [DOI] [PubMed] [Google Scholar]
- 6.Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr. 2009;154:906–911. e901. doi: 10.1016/j.jpeds.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 9.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 10.Feldman D, Malloy PJ, Gross C. Vitamin D: Biology, action, and clinical implications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. Academic Press; 2001. pp. 257–303. [Google Scholar]
- 11.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 12.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 13.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci U S A. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology. 1997;138:2233–2240. doi: 10.1210/endo.138.6.5170. [DOI] [PubMed] [Google Scholar]
- 15.Ohyama Y, Noshiro M, Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991;278:195–198. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- 16.Petkovich M, Jones G. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens. 2011;20:337–344. doi: 10.1097/MNH.0b013e3283477a7b. [DOI] [PubMed] [Google Scholar]
- 17.Shinki T, Jin CH, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, Okuda K, Suda T. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992;267:13757–13762. [PubMed] [Google Scholar]
- 18.Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci U S A. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zierold C, Reinholz GG, Mings JA, Prahl JM, DeLuca HF. Regulation of the procine 1,25-dihydroxyvitamin D3-24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 and parathyroid hormone in AOK-B50 cells. Arch Biochem Biophys. 2000;381:323–327. doi: 10.1006/abbi.2000.1964. [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J. 2005;390:325–331. doi: 10.1042/BJ20041799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 22.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 23.Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B, Schwartz SM, Himmelfarb J, Kestenbaum B, de Boer IH. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82:693–700. doi: 10.1038/ki.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 26.Clive DR, Sudhaker D, Giacherio D, Gupta M, Schreiber MJ, Sackrison JL, MacFarlane GD. Analytical and clinical validation of a radioimmunoassay for the measurement of 1,25 dihydroxy vitamin D. Clin Biochem. 2002;35:517–521. doi: 10.1016/s0009-9120(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 27.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1639–1642. doi: 10.1016/j.jchromb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao P, Scheibel S, D'Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 29.Serum-Calcium. Lancet. 1979;1:858–859. [PubMed] [Google Scholar]
- 30.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 32.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 34.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24:503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine 2011 Dietary reference intakes for calcium and vitamin D. Washington, DC: 2011. http://www.ncbi.nlm.nih.gov/books/NBK56070/ [Google Scholar]
- 36.Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doorenbos CR, de Cuba MM, Vogt L, Kema IP, van den Born J, Gans RO, Navis G, de Borst MH. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol. 2012;128:56–61. doi: 10.1016/j.jsbmb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Prytula A, Wells D, McLean T, Balona F, Gullett A, Knott C, Cantwell M, Hassen K, Ledermann S, Rees L, Shroff R. Urinary and dialysate losses of vitamin D-binding protein in children on chronic peritoneal dialysis. Pediatr Nephrol. 2011;27:643–649. doi: 10.1007/s00467-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 39.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 42.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- 43.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 45.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 46.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–4808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7:e30773. doi: 10.1371/journal.pone.0030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishimura E, Nishizawa Y, Inaba M, Matsumoto N, Emoto M, Kawagishi T, Shoji S, Okuno S, Kim M, Miki T, Morii H. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55:1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 49.Koenig KG, Lindberg JS, Zerwekh JE, Padalino PK, Cushner HM, Copley JB. Free and total 1,25-dihydroxyvitamin D levels in subjects with renal disease. Kidney Int. 1992;41:161–165. doi: 10.1038/ki.1992.22. [DOI] [PubMed] [Google Scholar]