Abstract
Several recent studies have found a conserved microRNA (miRNA) family, the miR-34s, to be direct transcriptional targets of p53. miR-34 activation can recapitulate elements of p53 activity, including induction of cell-cycle arrest and promotion of apoptosis, and loss of miR-34 can impair p53-mediated cell death. These data reinforce the growing awareness that non-coding RNAs are key players in tumour development by placing miRNAs in a central role in a well-known tumour-suppressor network.
Three decades of intensive investigation have placed p53 at the center of a complex molecular network regulating diverse physiological responses to cancer-related stresses1,2. Until recently, the components of the p53 network consisted solely of protein-coding genes, including those acting upstream to regulate p53 activity, those functioning downstream to mediate p53 effects, and those forming regulatory feedback loops.
Recent studies have identified miR-34 as a microRNA (miRNA) component of the p53 network, for the first time revealing an interplay between proteins and non-coding RNAs in this crucial tumour-suppressor pathway3–7. miRNAs are a class of small regulatory RNAs that mediate post-transcriptional silencing of specific target mRNAs8,9. The miR-34 family of evolutionarily conserved miRNAs are directly induced by p53 in response to DNA damage and oncogenic stress3–7. Ectopic expression of miR-34 recapitulates the biological effects of p53, inducing growth arrest and apoptosis, through its ability to dampen the expression of pro-proliferation and anti-apoptotic genes3–7. These findings suggest that miRNAs, and in a broader sense non-coding RNAs, may be previously unrecognized but integral components of established oncogene and tumour-suppressor networks.
miRNAs in cancer
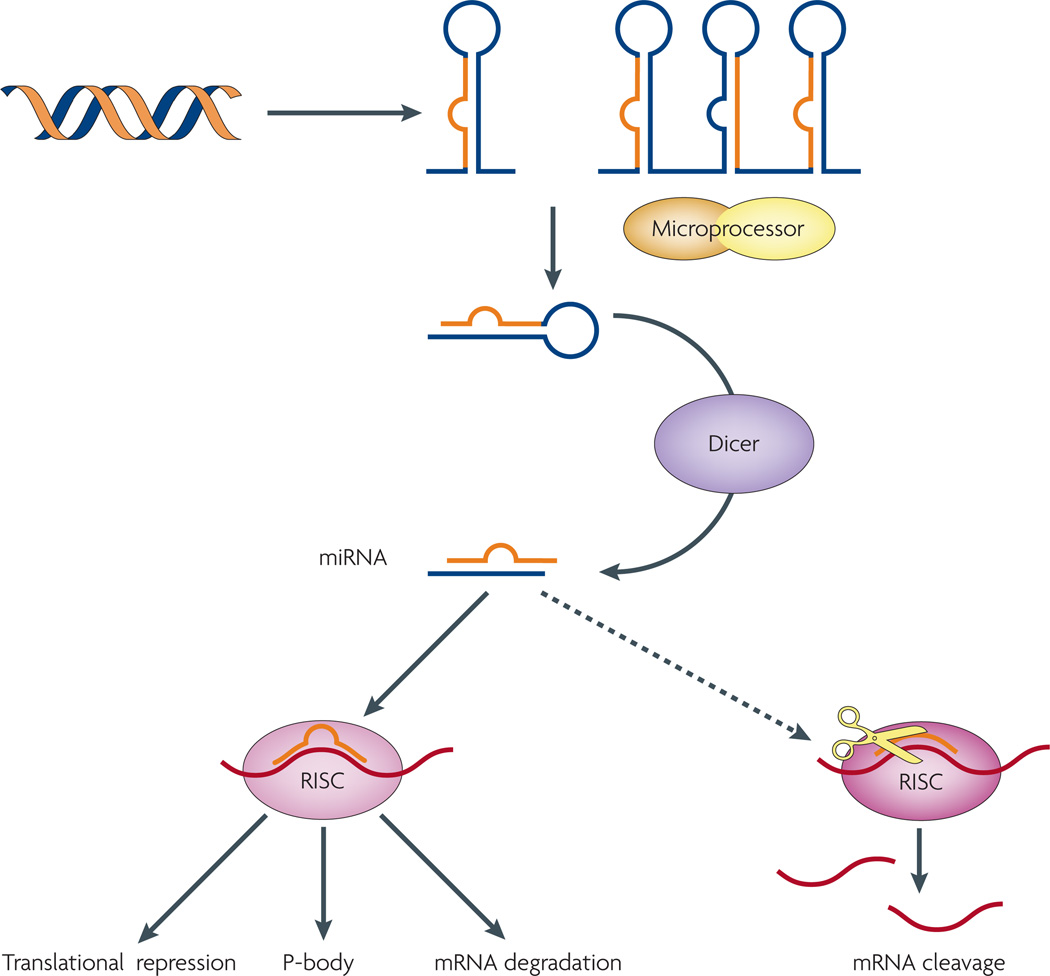
First identified as small, non-coding RNAs essential for the timing of larval development in worms, a large number of miRNAs have been and are still being discovered in nearly all metazoans. Most animal miRNAs share common biogenesis and effector machineries (FIG. 1). Mature miRNAs often range from 20–22 nucleotides in length as a result of two sequential processing reactions, which liberate mature species from their stem-loop precursors8,9. After maturation, these small RNAs are incorporated into the RNA-induced silencing complex (RISC), through which they mediate post-transcriptional gene silencing of specific mRNA targets8,9. Identification of targets is achieved through imperfect base pairing to the miRNA as a whole, with recognition relying mainly on sequences at the miRNA 5′ end, known as the seed10. This flexibility affords miRNAs the ability to regulate large programmes of gene expression. As the sequences that miRNA seeds recognize are found in many genes, their flexibility makes it difficult to identify physiologically relevant miRNA–target relationships from sequence analysis alone.
Figure 1. Current model of miRNA biogenesis and post-transcriptional silencing.
Nascent transcripts of microRNA (miRNA) genes are processed by microprocessor into a stem-loop precursor, which is further processed by Dicer into a mature miRNA duplex, which often displays imperfect base-pairing. One strand of the miRNA duplex gets incorporated into the effector complex RISC (RNA-induced silencing complex), which recognizes specific targets through imperfect base-pairing and induces post-transcriptional gene silencing. Several mechanisms have been proposed for this mode of regulation: miRNAs can induce the repression of translation initiation, mark target mRNAs for degradation by deadenylation, or sequester targets into the cytoplasmic P-body.
The precursors of miRNAs are generally conventional RNA polymerase II products. Like protein-coding genes, miRNAs show complex patterns of tissue-specific expression that combine with their capacity to regulate many targets to permit control of many crucial developmental and physiological decisions8,9. As such, miRNAs have a profound impact on many processes that are frequently disrupted during malignant transformation, including cell proliferation11, apoptosis, stress responses, maintenance of stem cell potency and metabolism. The relevance of miRNAs to cancer was suggested by changes in their expression patterns12,13 and recurrent amplification and deletion of miRNA genes in tumours14,15. This has been translated in several cases into functional validation of roles in tumour initiation and progression using mouse models and cultured tumour cells. Several miRNAs have emerged as candidate components of oncogene and tumour-suppressor networks. The miR-17-92 cluster16–18, miR-372-373 (REF. 19) and miR-155/BIC (REF. 20) have been implicated as proto-oncogenes in B-cell lymphomas and testicular cancers. On the other hand, miR-15-16 is frequently deleted in patients with chronic lymphocytic leukaemia (CLL)21, and more importantly, evidence from expression studies and functional studies has revealed the potential tumour-suppressive roles of let-7 in various cancers22,23, possibly owing to its ability to repress key oncogenic components, including Ras and HMGA2.
In addition to roles for several individual miRNAs in tumour initiation and progression, global alterations in miRNA expression patterns are a common feature of tumour cells13,24. Generally, miRNAs that characterize differentiated cell types show reduced expression in tumours, whereas expression of those that are expressed during early development and in stem cells is retained24,25. Consistent with this, the suppression of key components of the miRNA biogenesis machinery in cancer cells has been reported to promote transformation both in vitro and in vivo26.
The true extent to which the disruption of miRNA pathways has a role in tumorigenesis remains to be determined. However, early indications are that this family of genes is intimately integrated into the regulatory processes that are normally disrupted during transformation. Moreover, the placement of several miRNAs into known oncogenic and tumour-suppressor networks is beginning to solve longstanding mysteries of how the circuitry of these pathways is wired.
Identification of p53-regulated miRNAs
An illustration of the role of a miRNA in a well-established tumour-suppressor network was provided recently by five independent studies that linked members of the miR-34 family with p53 (REFS 3–7). The p53 network acts as a sensor for many cancer-associated stress signals, including DNA damage, telomere depletion, oncogene activation, hyperactive cytokine signalling and hypoxia2. These signals are translated into effects on cell proliferation, cell death, DNA repair and angiogenesis2 through the function of p53 as a sequence-specific transcriptional regulator. Despite extensive efforts over the past three decades to link downstream targets of p53 to specific biological effects, many puzzles remain. For example, several studies indicated that p53 could also repress the expression of target genes through a mechanism that had not been fully elucidated27,28. Moreover, genetic studies of p53-regulated protein-coding genes had not yet provided a complete picture of how regulation of these targets might lead to commonly observed effects of p53 activation, such as G1 cell-cycle arrest or apoptosis, in all tissue settings29. Given the potentially broad consequences of activating miRNA expression, it seemed possible that non-coding RNAs might contribute to p53 function. This prompted several efforts to search for links between p53 and miRNAs. These converged into one exciting finding — the identification of miR-34s as key p53 transcriptional targets capable of regulating cell proliferation and cell death3–7.
Most studies focused on examining global miRNA expression profiles and correlating expression patterns with p53 status. He and colleagues profiled miRNAs in wild-type and p53-null mouse embryonic fibroblasts (MEFs) carrying various additional oncogenic lesions5. Raver-Shapira et al. studied a lung cancer cell line harbouring a temperature-sensitive TP53 allele6. Chang and co-workers set out to identify miRNAs whose expression increased after genotoxic stress in a p53-dependent manner4, and Tarasov et al. launched their screen for p53-regulated miRNAs using a tetracycline-inducible TP53 allele7. Using a complementary, bioinformatic approach, Bommer and colleagues revisited a previous study of genome-wide p53 chromatin immunoprecipitation (ChIP)30, in which all putative p53 binding sites had been attributed to their nearest protein-coding genes3,30. In all of these studies, miR-34 family members emerged as prime candidates for p53-regulated miRNAs.
First identified in Caenorhabditis elegans, mir-34 encodes an evolutionarily conserved miRNA, with single orthologues in several invertebrate species. In vertebrates, mir-34 diverged into a family of three homologous miRNAs — mir-34a, mir-34b and mir-34c. The mature mir-34a sequence is located within the second exon of its non-coding host gene, nearly 30 kb downstream of its first exon, which contains a predicted p53 binding site3–7. Both mir-34b and mir-34c are located within a single non-coding precursor (mir-34b/c), whose transcriptional start site is adjacent to a predicted p53 binding site3,5. Both of these p53-binding sites are evolutionarily conserved and match the consensus derived from p53-regulated protein-coding targets30.
Extensive studies were carried out, both in vitro and in vivo, to validate the regulation of miR-34 family miRNAs by p53. Both exogenous and physiological stresses are able to induce miR-34 expression in multiple cell culture systems and animal tissues in a p53-dependent manner. The induction of miR-34s by p53 does not rely on de novo protein synthesis6, but does depend on having intact p53 binding sites within their putative promoter regions3,5,6. The kinetics and magnitude of miR-34 induction is comparable to that observed for the canonical p53 target, the cyclin-dependent kinase (CDK) inhibitor p21 (REFS 5,7), as is the approximate binding affinity of p53 for mir-34 promoters as measured by ChIP3,5,6. Together, these studies provide compelling evidence that miR-34 miRNAs are bona fide p53 targets. Thus, p53 acts as a tumour suppressor by both positively and negatively regulating gene expression; the negative regulation occurs, at least in part, through the positive effects of p53 on the expression of noncoding RNAs.
miR-34 mimics the effects of p53
Given a solid connection between miR-34s and p53, studies quickly shifted focus to answering one key biological question — is miR-34 sufficient and/or necessary for any of the biological outcomes elicited by p53? So far, these have mainly probed two of the best-studied p53 outputs, growth arrest and apoptosis.
The effects of simulating miR-34a activation, either through delivery of synthetic mature miRNAs or through ectopic expression of miRNA precursors, were examined in various biological systems. In most cases, key p53 effects were recapitulated in a context-dependent manner. Overexpression of miR-34a in primary fibroblasts and in certain tumour cell lines produced a significant cell-cycle arrest, evident by an increase in the G1 population at the expense of the S-phase population3,5. It is worth noting that when miR-34 is ectopically overexpressed in IMR90 human lung fibroblast cells, ~60% of the infected cells exhibit morphological and molecular alterations characteristic of cellular senescence. In a different set of cell types, mostly tumour cell lines, the observed effect of miR-34a overexpression was an increased apoptotic response, although the degree of cell death varied. Interestingly, in the case of HCT116 colon cancer cell lines, the predominant effect of miR-34a overexpression was growth arrest at 48 hours after transfection5, but apoptosis at 72 hours after transfection4,5. Mature miR-34b and miR-34c are nearly identical to miR-34a and have similar biological activities in several proliferation assays3,5. Given the potential of miRNAs to recognize many targets through imperfect base pairing, the pleiotropic effects of miR-34 may simply reflect the different spectrum of target mRNAs available in a given system, and this may represent a recurring theme within miRNA-mediated regulatory pathways.
Although the pro-apoptotic effects of miR-34a are modest, miR-34a is essential for p53-mediated apoptosis in some settings. Direct support for a crucial role of miR-34a in p53-induced cell death came from the study by Raver-Shapira et al., in which inhibition of miR-34a by LNA (locked nucleic acid) oligos strongly attenuated p53-dependent apoptosis in U2OS cells in response to genotoxic stress6. LNA oligos are locked in the 3′ endo conformation, thus increasing their hybridization energy. This property is exploited in the use of these agents as competitive inhibitors of miRNA activity. It is worth noting that in that study, the pro-apoptotic effect of miR-34a overexpression, albeit in MCF7 and H1299 rather than U2OS cells, was relatively mild. In addition, loss of mir-34a in mouse embryonic stem cells dampened the apoptotic effects of differentiation stimuli such as addition of retinoic acid and withdrawal of leukaemia inhibitory factor (LIF)3. These studies did not resolve the question of whether miR-34 is essential for p53-mediated G1 arrest. Given the redundancy in both cell-cycle regulatory pathways and in the miR-34 family, addressing this issue will probably have to wait until genetic lesions in all three mir-34 homologues are created.
Mechanisms of miR-34 action
miRNAs act by inhibiting gene expression. Thus the precise mechanisms by which miR-34 contributes to p53 activity can be revealed through identification of its regulatory targets. Both bioinformatic and experimental approaches have been used to address this issue31, and consistent with the predicted p53–miR-34 circuit, some miR-34-regulated genes are repressed after p53 activation32. Microarray analysis showed that the induction of miR-34s led to the downregulation of hundreds of mRNAs, which were enriched for cell cycle regulators. Collectively, these were also enriched for mRNAs that could bind miR-34 seed regions, and several individual genes, including CDK4, CDK6, cyclin E2 and E2F3 have been experimentally validated as miR-34 targets by western blotting. In most cases, a direct regulatory relationship was established by fusing the 3′ untranslated region (UTR) of each candidate target (containing the miR-34 seed complementary region) to luciferase and demonstrating miR-34-induced repression3–7. Unlike proliferation genes, antiapoptotic genes as a whole are not enriched in the miR-34-repressed set or in bioinformatics predictions of miR-34 targets. However, the anti-apoptotic protein BCL2 is downregulated by miR-34 in several cell types, consistent with a role for miR-34 in p53-mediated apoptosis3.
In classic studies of miRNA function in worms and flies, it was often true that one or few miRNA targets could account for the regulation of a specific biological process33,34. Although the suppression of any of several miR-34 targets can mimic its biological effects, it seems most likely that collective regulation of multiple genes by miR-34 is responsible for the full range of its physiological effects (FIG. 2).
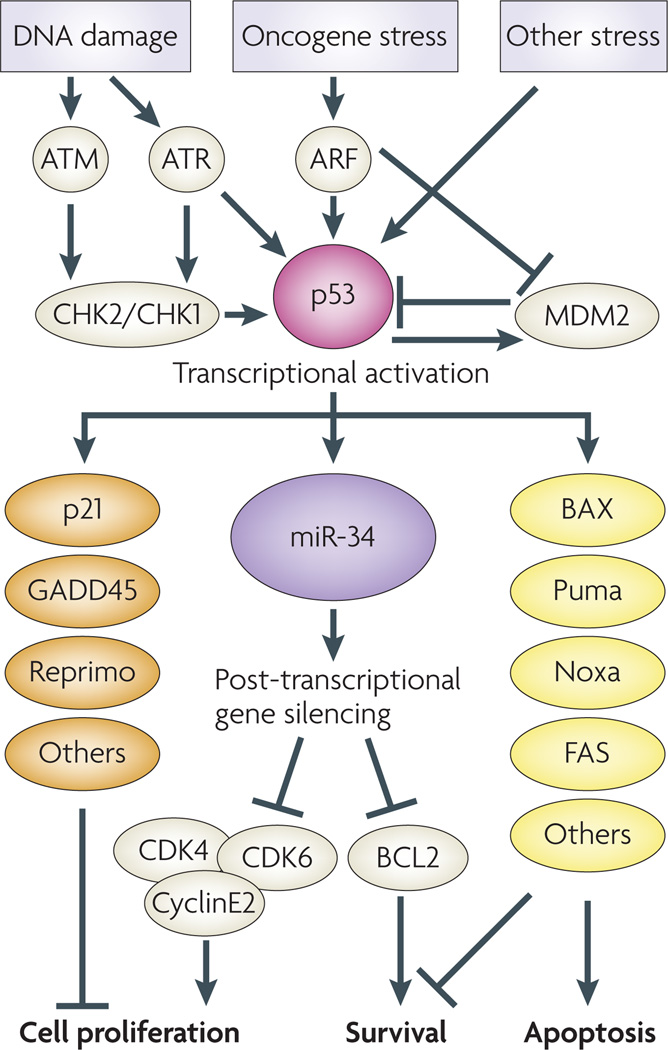
Figure 2. A model of the p53–miR- 34 network in regulating cell proliferation and cell death.
miR-34 is a direct transcriptional target of p53, which in turn downregulates genes required for proliferation and survival. Along with other p53 targets, such as p21 and BAX, miR-34-family miRNAs promote growth arrest and cell death in response to cancer related stress. ATM, ataxia talangiectasia mutated; ATR, ataxia telengiectasia and RAD3-related; CDK, cyclin-dependent kinase; CHK, checkpoint kinase
miR-34 in cancer
Deletions of mir-34 family miRNAs have been reported in human cancers. mir-34a is located within 1p36, a region of frequent heterozygous deletion in many tumour types35. The chromodomain protein CHD5, another candidate tumour suppressor located within 1p36, is capable of activating p53 through ARF (encoded by CDKN2A)36. Thus, the loss of 1p36 may affect the integrity of the p53 pathway both upstream and downstream of p53. Minimal deletions containing mir-34b and mir-34c have also been found in breast and lung cancer15, which is consistent with significant reduction of miR-34b/c expression in non-small-cell lung cancer cell lines3. In human tumours, the selective pressure to lose miR-34s may be relieved by frequent mutation of p53. Thus, genetic alterations in mir-34s are more likely to occur in tumour types that characteristically contain wild-type p53. For example, Welch and colleagues reported that mir-34a is frequently deleted or downregulated in neuroblastoma cell lines37,38.
miR-34 in the midst of the p53 pathway
The CDK inhibitor p21 was recognized more than a decade ago as a key mediator of p53-induced growth arrest. However, disruption of p21 in mice failed to completely negate this output of the p53 pathway29. This predicted the existence of other essential players in this process, which had not been discovered after more than a decade of searching. A possible explanation for this failure is the exclusive focus on protein-coding genes, as these new results have raised the possibility that noncoding RNAs cooperate with protein-coding genes in various p53 effector pathways. The placement of a miRNA in the p53 pathway may also help to explain another longstanding mystery. An examination of p53-responsive transcriptional programmes revealed a large number of genes that are quickly repressed upon p53 activation27,28. It now seems likely that at least some of these observations can be explained as secondary effects of the induction of repressive small RNAs (FIG. 2).
These recent reports raise the question of whether there might be other miRNAs that connect to p53 — either as downstream effectors or as regulators of p53 or its modifiers. Searches for p53-regulated miRNAs predated the recent studies of miR-34 (REF. 39). As many as a dozen miRNAs exhibit expression patterns indicative of p53-dependent regulation. Several different studies have generated largely non-overlapping sets of miRNA as candidate p53 targets, possibly owing to the differences in the biological systems studied and the detection methods used. A small number of miRNAs, including miR-26a and miR-182, were identified in multiple independent studies and are interesting candidates for further investigation4,6,7. Strikingly, the interplay between p53 and miRNAs may not be limited to a purely linear relationship. Overexpression of miR-372 and miR-373 can bypass senescence induced by oncogenic Ras, which depends on p53 (REF. 19). This effect is achieved by suppression of a p53 target, LATS2 (REF. 19), which not only inhibits proliferation, but also forms a positive-feedback loop with p53 (REF. 40).
Conclusions
We are only beginning to understand the realm of non-coding RNAs, whose representation in the genome, according to comparative analyses, increases as a function of genome complexity. The discovery that a conserved family of miRNAs is central to a crucial tumour-suppressor pathway may reflect ancient connections between noncoding RNAs and the regulation of developmental and physiological decisions, whose disruption can lead to tumour development. A single miRNA can affect many downstream targets. This raises an alternative possibility that the adoption of non-coding RNAs permits pathways to simultaneously regulate many aspects of a particular cellular process. It remains to be seen whether prevalent links between small RNAs and tumorigenesis indicate any special biology of these species or whether such observations simply reflect the function of miRNAs as flexible regulators of gene-expression programmes.
Acknowledgements
I.H. is supported by a K99 grant from the US National Institutes of Health (NIH). X.H. is supported by a predoctoral fellowship from the DOB Breast Cancer Research Program. This work was supported in part by grants from the NIH (S.W.L. and G.J.H.) and a kind gift from Kathryn W. Davis (G.J.H.).
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
BCL2 | CDK4 | CDK6 | CDKN2A | CHD5 | cyclin E2 | E2F3 | HMGA2 | LATS2 | LIF | p21 | p53
FURTHER INFORMATION
Scott Lowe’s website: http://www.cshl.edu/public/SCIENCE/lowe.html
Greg Hannon’s website: http://www.cshl.edu/public/SCIENCE/hannon.html
All links are active in the online pdf
References
- 1.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein BB, Lane DD, Levine AAJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Bommer GT, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Chang TC, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raver-Shapira N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Tarasov V, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 10.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Stress-induced reversal of microRNA repression and mRNA P-body localization in human cells. Cold Spring Harb. Symp. Quant. Biol. 2006;71:513–521. doi: 10.1101/sqb.2006.71.038. [DOI] [PubMed] [Google Scholar]
- 12.Iorio MV, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 13.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol. Rep. 2006;16:845–850. [PubMed] [Google Scholar]
- 15.Calin GA, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dews M, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nature Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 19.Voorhoeve PM, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Tam W, Dahlberg JE. miR-155/BIC as an oncogenic microRNA. Genes Chromosomes Cancer. 2006;45:211–212. doi: 10.1002/gcc.20282. [DOI] [PubMed] [Google Scholar]
- 21.Calin GA, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SM, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 25.Thomson JM, et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nature Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 27.Yu JJ, et al. Identification and classification of p53-regulated genes. Proc. Natl Acad. Sci. USA. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao RR, et al. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–993. [PMC free article] [PubMed] [Google Scholar]
- 29.Brugarolas J, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 30.Wei CL, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 31.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Spurgers KB, et al. Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J. Biol. Chem. 2006;281:25134–25142. doi: 10.1074/jbc.M513901200. [DOI] [PubMed] [Google Scholar]
- 33.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 34.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 35.Versteeg R, et al. 1p36: every subband a suppressor? Eur. J. Cancer. 1995;31A:538–541. doi: 10.1016/0959-8049(95)00037-j. [DOI] [PubMed] [Google Scholar]
- 36.Bagchi A, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 37.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 38.Gaur A, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 39.Xi Y, Shalgi R, Fodstad O, Pilpel Y, Ju J. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin. Cancer Res. 2006;12:2014–2024. doi: 10.1158/1078-0432.CCR-05-1853. [DOI] [PubMed] [Google Scholar]
- 40.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]