Abstract
Green tea and hibiscus are widely consumed as traditional beverages in Yemen and some regional countries. They are relatively cheap and the belief is that they improve health state and cure many diseases. The aim of this study was to evaluate the potential protective and antibacterial activity of these two famous plants in vitro through measuring their antibacterial activity and in vivo through measuring nonenzymatic kidney markers dysfunction after induction of nephrotoxicity by gentamicin. Gram positive bacteria like MRSA (methicillin resistant Staphylococcus aureus) were isolated from hospitalized patients' different sources (pus and wound) and Gram negative bacteria including E. coli and P. aeruginosa were used in vitro study. In addition, the efficacy of these plants was assessed in vivo through measuring nonenzymatic kidney markers including S. creatinine and S. urea. Green tea was shown antimicrobial activity against MRSA with inhibition zone 19.67 ± 0.33 mm and MIC 1.25 ± 0.00 mg/mL compared with standard reference (vancomycin) 18.00 ± 0.00 mg/mL. Hibiscus did not exhibit a similar effect. Both Hibiscus- and green tea-treated groups had nephroprotective effects as they reduced the elevation in nonenzymatic kidney markers. We conclude that green tea has dual effects: antimicrobial and nephroprotective.
1. Introduction
In China, the usage of green tea was started for more than 4,000 years. The major species is originated from leaves of Camellia sinensis. Currently, this herb's use is widespread in Asia and West countries [1]. Many polyphenols are present in green tea leaves like catechins and epigallocatechin gallate. In addition, it contains tocopherols, carotenoids, ascorbic acid (vitamin C), minerals such as chromium, manganese, selenium, or zinc, and other minor phytochemical compounds [1, 2]. Consequently, animal and human studies showed that regular intake of green tea may have health benefits against cardiovascular diseases, cancer, and many types of infectious pathogens [3]. Green tea can support the immune system as well as improve the cognitive function [4]. Hibiscus sabdariffa calyces are a famous plant cultivated and used widely in Africa, especially Egypt and Sudan as this region has a long history of the use of this drink. Hibiscus tea is known to be effective in lowering blood pressure [5] and cholesterol [6]. In addition, researchers propose that consuming hibiscus could aid in the prevention of human cancer [7]. Hibiscus sabdariffa calyces contain different components including mucilage, polysaccharides, pectins, polyphenols, organic acids, ascorbic acid, citric, malic and tartaric acids [8].
This study aimed firstly to assess and evaluate the antibacterial activity of two plants Camellia sinensis leaves and Hibiscus sabdariffa calyces through measuring the inhibition zone of tested plants compared with antibiotic references in vitro study. Secondly, the study aimed to compare the potential nephroprotective effect of two tested plants in in vivo study.
2. Materials and Methods
2.1. Materials
2.1.1. Drugs and Natural Products
Gentamicin sulfate was purchased from Alpha Aleppo pharmaceutical Ind.
Fresh green tea leaves and Hibiscus sabdariffa calyces were bought from special herbal stores in Sana'a city. The two plants were identified and authenticated at Pharmacognosy department, University of Science and Technology (UST).
2.1.2. Animals and Bacteria
Different bacteria species Gram positive including methicillin resistant Staphylococcus aureus (MRSA) and Gram negative bacteria including Escherichia coli and Pseudomonas aeruginosa were supplied by the UST hospital laboratory where these were isolated freshly from infected patients.
Experimental animals being adult male New Zealand White Rabbits with weight range from 1–1.5 kg and age 7 months ±1 week were supplied by the Faculty animal house. They were fed with fresh green grass and housed in stainless steel cages (2500 cm2 with a height of 35 cm) away from direct sunlight.
2.2. Preparation of Extracts
The dried plants subsequently were ground separately using a blender to fine powder.
Two kilograms (2 kg) of each dried plant material (green tea and hibiscus) were introduced separately into a Whatman paper thimble and then extracted by refluxing with water as solvent system in a Soxhlet apparatus for six hours. A second extraction was made using 80% methanol and concentrated using a rotary evaporator at 40°C and finally dried in a vacuum dissector at 40°C. The resulting residue which weighed 32.52 g (recovery 10.33%) was later stored under 4°C until required. A 10 mg/mL solution of the extract was prepared in distilled water before administration to the rabbits [9].
2.3. Determination of Antibacterial Activity
Disc diffusion assay [10] was used to determine the antimicrobial activity of the investigated extracts. Sterile filter paper discs of 6 mm diameter were impregnated with 35 μL of the extracted solution (equivalent to 7 mg of dried extract). The paper's discs were allowed to evaporate and then placed on the surface of agar plates that were previously inoculated with microbial cell. Plates were kept for 2 hours in refrigerator to enable prediffusion of extract into the agar. The plates then were incubated overnight (18 hours) at 37°C. Vancomycin, co-trimoxazole, and piperacillin were used as positive control for methicillin resistant Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, respectively. At the end of incubation period, the antibacterial activity was evaluated by measuring the inhibition zones (diameter of inhibition zone plus diameter of the disc). An inhibition zone of 14 mm or greater is considered as high antibacterial activity [10].
2.4. Minimum Inhibitory Concentration
According the method of Olaleye and Mary Tolulope, 2007, serial dilution of plant extract was used: 20, 10, 5, 2.5, 1.25, 0.625, and 0.313 mg/mL. Each mL (innocula) was poured into petri dish and allowed to set after the agar was also poured. A 3 mm sterile cork borer was used to make wells. The serial freshly prepared dilutions were poured into these wells. The plates were incubated at 37°C for 24 h. Finally, the growth of tested microorganisms (m.o) was observed and compared with clear zone. The least concentration of plant extract that inhibits growth of tested m.o is considered as minimum inhibitory concentration (MIC) [11].
2.5. Animal Study Design
Twenty-four rabbits were divided into four groups randomly. Each group contained six animals. Before starting the experiment, all animals were kept for five days to be acclimatized.
Nephrotoxicity was induced in groups II, III, and IV intraperitoneally by gentamicin (80 mg/kg body weight) for seven consecutive days [12, 13]. Dose of 250 mg/kg body weight, of the Hibiscus sabdariffa calyx extract was administered to rabbits in group III, and 300 mg/kg body weight, of Camellia sinensis was given to rabbits in group IV, respectively. Rabbits in group I were given distilled water and kept as a control. All groups received tested agents via an oral route using a gavage needle once daily for seven days.
At the end of experiment, light anesthesia with halothane was used, and the animals were dissected. Blood samples were collected directly through the cardiac puncture and kept in container free from anticoagulant and allowed to clot for 20 minutes and centrifuged at 4000 rpm for 15 minutes.
Sera were collected using micropipettes and analyzed. Nonenzymatic markers of kidney dysfunction were measured including serum creatinine [14] and urea [15]. All the experimental procedures were in accordance with the guidelines for the care and use of laboratory animals, and approval from the Institutional Research and Ethics Committee, UST, was received prior to the experiments.
2.6. Statistical Analysis
Results of this work were expressed as mean ± standard error of the mean (S.E.M) by using the Statistical Analysis (SPSS) software package version 18.0. ANVOA was used to compare between groups. P < 0.05 was considered as significant.
3. Results
In in vitro study, water and methanolic extracts of both Camellia sinensis and Hibiscus sabdariffa were tested against MRSA (pus), MRSA (wound), E. coli, and P. aeruginosa and compared with the documented references. Only water and methanolic extracts of Camellia sinensis were shown 21, 22, 18, and 19 mm inhibiting zones, respectively. Extracts of Hibiscus sabdariffa did not produce antibacterial activity against tested bacterial species. This experiment was repeated three times to get the average inhibition zone of the tested extracts as shown in (Tables 1 and 2).
Table 1.
Antibacterial activity of water and methanolic extracts of Camellia sinensis and Hibiscus sabdariffa on tested Gram positive and Gram negative bacteria.
| Bacteria species | Reference | T.w | T.m | H.w | H.m |
|---|---|---|---|---|---|
| MRSA (pus) | Van. [19] | 21 | 22 | R | 10 |
| MRSA (wound) | Van. [17] | 18 | 19 | R | R |
| E. coli | GT. [33] | R | R | R | R |
| P. aeruginosa | Pi. [30] | R | R | R | 11 |
T.w: water extract of green tea; T.m: methanolic extract of green tea; H.w: water extract of hibiscus; H.m: methanolic extract of hibiscus; Van.: vancomycin; GT: gentamicin; Pi: piperacillin; R: resistance.
Table 2.
Detection of MIC (mg/mL) of methanolic extract of Camellia sinensis by using MRSA (wound) species (n = 3).
| Bacteria | Inhibition zone (mm) | MIC (mg/mL) | |
|---|---|---|---|
| 10 mg/L | Van. 1 mg/L | G.T | |
| Staph. aureus | 19.67 ± 0.33 | 18.00 ± 0.00 | 1.25 ± 0.00 |
| E. coli | 0 | 0 | 0 |
| P. aeruginosa | 0 | 0 | 0 |
Results are expressed as mean ± SE; n = 3; MIC: minimum inhibitory concentration; G.T: green tea; Van.: vancomycin.
Regarding the in vivo study, levels of creatinine and urea in sera, gentamicin group displayed significant increase in urea (110.5 ± 17.52 mg/dL) and creatinine (1.62 ± 0.72 mg/dL) compared with control group. However, the green tea-treated group demonstrated significant reduction in urea (43.83 ± 3.45 mg/dL) and creatinine (0.617 ± 0.167 mg/dL) compared with gentamicin group. The hibiscus-treated group also revealed significant reduction in both nonenzymatic markers of kidney dysfunctions urea (43.30 ± 6.47 mg/dL) and creatinine (0.733 ± 0.114 mg/dL) compared with gentamicin group. There was insignificant difference between two tested plants as shown in (Figures 1(a) and 1(b)).
Figure 1.
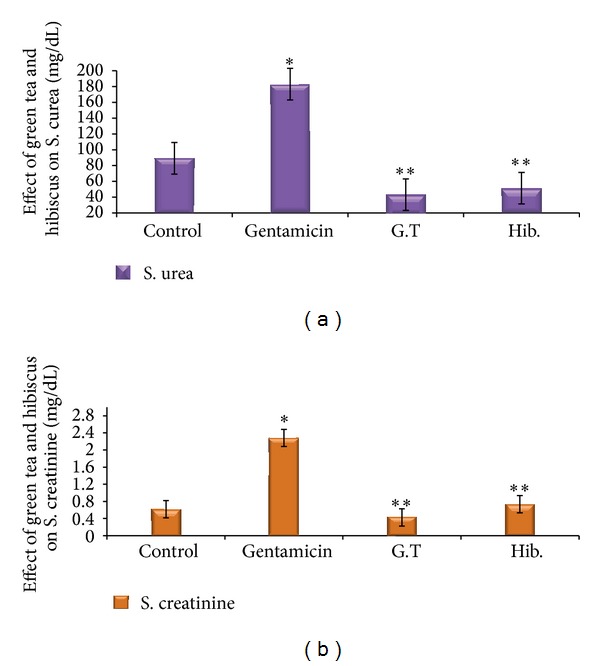
(a) Effect of green tea (300 mg/d) and hibiscus (250 mg/d) on the (mean ± SE) nonenzymatic markers of kidney dysfunction (serum urea mg/dL) for 7 days in adult rabbits (n = 6). *Significant as compared with control at P value < 0.05. **Significant as compared with gentamicin-induced nephrotoxicity at P value < 0.05. G.T: green tea, Hib: hibiscus. (b) Effect of green tea (300 mg/d) and hibiscus (250 mg/d) on the (mean ± SE) nonenzymatic markers of kidney dysfunction (serum creatinine mg/dL) for 7 days in adult rabbits (n = 6). *Significant as compared with control at P value < 0.05. **Significant as compared with gentamicin-induced nephrotoxicity at P value < 0.05. G.T: green tea, Hib: hibiscus.
4. Discussion
Staphylococcus aureus is a contagious type of bacteria that can cause serious infection, especially nosocomial infections [16]. In the present study, both methanolic and water extracts of plant Camellia sinensis have been tested. The extracts displayed antimicrobial effect especially against Gram-positive bacteria as they inhibited the growth of methicillin resistant Staph. aureus. The antimicrobial effect of green tea extract is similar to that of the reference used (vancomycin), although it had no activity against gram negative bacteria like E. coli and P. aeruginosa. Hamilton-Miller, 1995, and Yamada et al., 2006, supported our findings. They found that green tea catechins possess strong antibacterial activity [17, 18].
The antimicrobial activity of green tea (Camellia sinensis) against MRSA may be related to the presence of epicatechin gallate (ECG) and epigallocatechin gallate (EGCG). These two polyphenols that are abundant in green tea (Camellia sinensis) were identified as candidates for treating S. aureus. Chromosomal factors, known as “fem factors”, are links between branched-chain cell wall peptide formation and β-lactam rings that are necessary for S. aureus, to show resistance to methicillin. Scientists have identified ECG as a potential inhibitor of these fem factors. In addition, ECG also has been recognized as a selective growth inhibitor of MRSA because it damages the cell wall of the bacteria. It is only effective when ECG is present in high concentrations [19–22].
However, this study showed that methanolic and water extracts of hibiscus flowers were free from antimicrobial activity being neither against Gram-positive nor against Gram-negative bacteria. Our result disagreed with Mahadevan et al., 2009 and VimalinHena, 2010, who found that the organism Staphylococcus aureus is sensitive towards both the leaves and flowers hot aqueous extract of hibiscus. This effect is due to the polyphenolic nature of the flavonoid gossypetin [23, 24].
Antioxidant compounds are very important for inhibition of oxidative damage to biological target molecules. They are used for prevention and treatment of many diseases such as cancer and cardiovascular, autoimmune, and neurodegenerative diseases as well as inflammatory effect [25]. Gentamicin was used in this study to induce kidney dysfunction as a model of nephrotoxicity. Silan et al. 2007 and Soliman et al. 2007 supported our findings. They showed that gentamicin produced an elevation in the concentrations of biochemical indicators of kidney function such as blood urea nitrogen (BUN) and serum creatinine [26]. Both green tea- and hibiscus-treated group had shown significant nephroprotective effects. They reduced biochemical indicators or nonenzymatic markers of the kidney dysfunction compared with gentamicin-induced nephrotoxicity. Okoko and Oruambo 2008 agree with our outcomes. They found that Hibiscus sabdariffa extract reduced the levels of serum creatinine, urea, and the elevation of the levels of kidney GSH and catalase in rats [27].
The exact mechanism of action is not clear. There are many suggestions; the extract of this plant may lower the level of lipid peroxidation that is elevated in response to some toxic materials like gentamicin. Another suggestion points toward the interaction between the phytochemicals and the toxic materials. In addition, calyces of Hibiscus sabdariffa contain potent antioxidant components including vitamin C and tocopherol. This explains the protective effect of this plant as it functions in the conversion of α-tocopheroxy radical to α-tocopherol or reduction of Ca+2 dependent permeabilization of renal cortex mitochondria [27–29]. On the other hand, antioxidant and radical scavenger of these calyces are related to the presence of flavonoids known as anthocyanins [27, 30, 31].
Koyner et al., 2008 and Ali et al., 2011, showed that several extracts of medicinal plants including green tea have been tested against gentamicin-induced nephrotoxicity in rats. The basis of the protective action of those plant extracts is not known with certainty, but it was thought to be directly related to their antioxidant properties [32, 33]. In addition, Kadkhodaee et al., 2005 and Kadkhodaee et al., 2007, found that the nephroprotective effect of green tea may be due to the presence of vitamins C and E in its composition [34, 35]. As previously noted, green catechins can act as scavengers of free radicals caused by reactive oxygen species and prevent free radical damage [36].
5. Conclusion
From this study, we conclude that green tea is a good and potent antimicrobial agent, mainly against MRSA, since it has a superior effect than hibiscus in this manner. According to their potential nephroprotective effect, both plants showed very close efficacy without any significant difference between them in their ability to ameliorate nephrotoxicity induced by gentamicin. This may due to the potent antioxidant activity of these two plants. Further studies are needed to elucidate the presence of other components in green tea and hibiscus that may have other health benefits such as anticancer or neuroprotective effects.
Acknowledgments
This study was funded by University of Science and Technology. The senior author would like to thank Faculty of Pharmacy and UST hospital lab team as well as the Aulaqi Specialized Med. Lab. that supported this work. Special thanks to Professor Lenny Rhine for his hard work to revise and edit this paper.
Conflict of Interests
There is no conflict of interests regarding the publication of this paper.
References
- 1.Reich E, Schibli A, Widmer V, Jorns R, Wolfram E, DeBatt A. HPTLC methods for identification of green tea and green tea extract. Journal of Liquid Chromatography and Related Technologies. 2006;29(14):2141–2151. [Google Scholar]
- 2.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea—a review. Journal of the American College of Nutrition. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 3.Gradišar H, Pristovšek P, Plaper A, Jerala R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. Journal of Medicinal Chemistry. 2007;50(2):264–271. doi: 10.1021/jm060817o. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Li W, Wang H. More tea for septic patients?—green tea may reduce endotoxin-induced release of high mobility group box 1 and other pro-inflammatory cytokines. Medical Hypotheses. 2006;66(3):660–663. doi: 10.1016/j.mehy.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seca AML, Silva AMS, Silvestre AJD, Cavaleiro JAS, Domingues FMJ, Neto CP. Phenolic constituents from the core of kenaf (Hibiscus cannabinus) Phytochemistry. 2001;56(7):759–767. doi: 10.1016/s0031-9422(00)00473-8. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffari-Khosravi H, Jalali-Khanabadi B-A, Afkhami-Ardekani M, Fatehi F. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. Journal of Alternative and Complementary Medicine. 2009;15(8):899–903. doi: 10.1089/acm.2008.0540. [DOI] [PubMed] [Google Scholar]
- 7.Yajloo MM, et al. Inhibition of human breast cancer cells by aqueous extractof Hibiscus gossypifolius Mill (Sour tea) Journal of the Iranian Chemical Society. 2009;6:S115–S143. [Google Scholar]
- 8.Wang C-J, Wang J-M, Lin W-L, Chu C-Y, Chou F-P, Tseng T-H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food and Chemical Toxicology. 2000;38(5):411–416. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 9.Dahiru D, Obi OJ, Umaru H. Effect of Hibiscus sabdariffacalyx extract on carbon tetrachloride induce liver damage. Biokemistri. 2003;15(1):27–33. [Google Scholar]
- 10.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 11.Olaleye MT. Cytotoxicity and antibacterial activity of Methanolic extract of Hibiscus sabdariffa . Journal of Medicinal Plants Research August. 2007;1(1):9–13. [Google Scholar]
- 12.Babu PVA, Sabitha KE, Shyamaladevi CS. Green tea impedes dyslipidemia, lipid peroxidation, protein glycation and ameliorates Ca2+-ATPase and Na+/K+-ATPase activity in the heart of streptozotocin-diabetic rats. Chemico-Biological Interactions. 2006;162(2):157–164. doi: 10.1016/j.cbi.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Silan C, Uzun O, Comunoglu NU, Gokcen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicityin rats ameliorated and healing effects of reveratrol. Biological & Pharmaceutical Bulletin. 2007;30:79–83. doi: 10.1248/bpb.30.79. [DOI] [PubMed] [Google Scholar]
- 14.Rock RC, Walker WG, Jennings CD. Nitrogen metabolites and renalfunction. In: Tietz NW, editor. Fundamentals of Clinical Chemistry. 3rd edition. Philadelphia, Pa, USA: WB Saunders; 1987. pp. 669–704. [Google Scholar]
- 15.Burtis CA, Edward ER, editors. Tietz Fundamentals of Clinical Chemistry. 5th edition. W.B. Saunders Company; 2001. [Google Scholar]
- 16.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton-Miller JMT. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrobial Agents and Chemotherapy. 1995;39(11):2375–2377. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada H, Tateishi M, Harada K, et al. A randomized clinical study of tea catechin inhalation effects on methicillin-resistant staphylococcus aureus in disabled elderly patients. Journal of the American Medical Directors Association. 2006;7(2):79–83. doi: 10.1016/j.jamda.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Yam TS, Hamilton-Miller JMT, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2’ synthesis, and β-lactamase production in Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 1998;42(2):211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Roccaro AS, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrobial Agents and Chemotherapy. 2004;48(6):1968–1973. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.boulmagd EA, Al-Mohammed HI, Al-Badry S. Synergism and postantibiotic effect of green tea extract and imipenem against methicillin-resistant staphylococcus aureus. Microbiology Journal. 2011;1(3):89–96. [Google Scholar]
- 22.Rohrer S, Berger-Bächi B. FemABX peptidyl transferases: a link between branched-chain cell wall peptide formation and β-lactam resistance in gram-positive cocci. Antimicrobial Agents and Chemotherapy. 2003;47(3):837–846. doi: 10.1128/AAC.47.3.837-846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VimalinHena J. Antibacterial potentiality of Hibiscus rosa-sinensis solvent extract and aqueous extracts against some pathogenic bacteria. Herbal Tech Industry. 2010;6(11):21–23. [Google Scholar]
- 24.Mahadevan N, Shivali S, Kamboj P. Hibiscus sabdariffa linn.—an overview. Natural Product Radiance. 2009;8(1):77–83. [Google Scholar]
- 25.Zamani N, Mianabadi M, Younesabadi M. Evaluation of anti-oxidant properties and phenolic content of Thymus transcaspicus. Journal of the Iranian Chemical Society. 2009;6:S115–S143. [Google Scholar]
- 26.Soliman KM, Abdul-Hamid M, Othman AI. Effect of carnosine on gentamicin-induced nephrotoxicity. Medical Science Monitor. 2007;13(3):BR73–BR83. [PubMed] [Google Scholar]
- 27.Okoko T, Oruambo IF. The effect of Hibiscus sabdariffa calyx extract on cisplatin-induced tissue damage in rats. Biokemistri. 2008;20(2):47–52. [Google Scholar]
- 28.Packer L, Kagan VE. Vitamin E: the Antioxidant Harvesting Centre of Membranes and Lipoproteins. New York, NY, USA: Marcel Dekker; 1993. [Google Scholar]
- 29.Campbell PI, Al-Nasser IA. Renal insufficiency induced by cisplatin in rats is ameliorated by cyclosporine-A. Toxicology. 1996;114(1):11–17. doi: 10.1016/s0300-483x(96)03411-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Xie H, Zhang L, Stewart JK, Gu X-X, Ryan JJ. cis-terpenones as an effective chemopreventive agent against aflatoxin B1-induced cytotoxicity and TCDD-induced P450 1A/B activity in HepG2 cells. Chemical Research in Toxicology. 2006;19(11):1415–1419. doi: 10.1021/tx0601307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sini JM, Umar IA, Inuwa HM. The beneficial effects of extracts of Hibiscus sabdariffa calyces in alloxan-diabetic rats: reduction of free-radical load and enhancement of antioxidant status. Journal of Pharmacognosy and Phytotherapy. 2011;3(10):141–149. [Google Scholar]
- 32.Ali BH, Al Za’abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: a mini-review of recent research. Basic and Clinical Pharmacology and Toxicology. 2011;109(4):225–232. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 33.Koyner JL, Sher Ali R, Murray PT. Antioxidants: do they have a place in the prevention or therapy of acute kidney injury? Nephron Experimental Nephrology. 2008;109(4):e109–e117. doi: 10.1159/000142935. [DOI] [PubMed] [Google Scholar]
- 34.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Experimental Physiology. 2005;90(4):571–576. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- 35.Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplantation Proceedings. 2007;39(4):864–865. doi: 10.1016/j.transproceed.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Liao S, Kao Y-H, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitamins and Hormones. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]


