Abstract
Childhood cancer survivors are at high risk of developing congestive heart failure (CHF) compared to the general population, and there is a dose-dependent increase in CHF risk by anthracycline dose. The mechanism by which this occurs has not been fully elucidated. Metabolomics, the comprehensive profile of small-molecule metabolites, has the potential to provide insight into the pathogenesis of disease states and discover diagnostic markers for therapeutic targets. We performed echocardiographic testing and blood plasma metabolomic analyses (8 pathways; 354 metabolites) in 150 asymptomatic childhood cancer survivors previously treated with anthracyclines. Median time from cancer diagnosis to study participation was 12.4 years (2.6–37.9 years); 64% were treated for a hematologic malignancy; median anthracycline dose was 350 mg/m2 (25–642 mg/m2). Thirty-five (23%) participants had cardiac dysfunction – defined as left ventricular end-systolic wall stress >2SD by echocardiogram. Plasma levels of 15 compounds in 3 metabolic pathways (carbohydrate, amino acid, and lipid metabolism) were significantly different between individuals with cardiac dysfunction and those with normal systolic function. After adjusting for multiple comparisons, individuals with cardiac dysfunction had significantly lower plasma carnitine levels (relative ratio [RR]: 0.89, p<0.01) in relation to those with normal systolic function. These findings may facilitate the development of primary prevention (treatment of carnitine deficiency prior to/ during anthracycline administration) and secondary prevention strategies (screening and treatment in long-term survivors) in patients at highest risk for CHF.
INTRODUCTION
Anthracyclines are widely used in the treatment of childhood cancer.(1) However, the use of anthracyclines is limited by their strong dose-dependent association with late-onset congestive heart failure (CHF).(1) Outcome of patients with anthracycline-related CHF is poor with 5-year mortality rates exceeding 50%.(1, 2) Anthracycline-related cardiotoxicity is mediated in part by direct myocardial injury due to formation of free radicals or toxic alcohol metabolites, disruption of myocyte fatty acid oxidation, or by topoisomerase-II-directed disruption of the myocyte mitochondrial genome.(1, 3) However, the exact mechanism of anthracycline-induced cardiotoxicity at the cellular level has not been completely elucidated.
Metabolomics refers to the comprehensive profiling of small-molecule metabolites found in biological specimens. The metabolome could represent the cumulative end product of gene expression, environmental factors or complex multi-faceted interactions between them. Importantly, metabolomic analysis has the potential to elucidate molecular pathways involved in the pathogenesis of disease states such as anthracycline-related CHF and help identify druggable targets. In a recent study(4) of patients with primary dilated cardiomyopathy (DCM), decreased levels of plasma steroid metabolites, glutamine, histidine, threonine, and increased levels of lipid β-oxidation products were associated with disease severity. Importantly, clinical response to anti-congestive treatment such as furosemide and angiotensin-1 converting enzyme inhibitors corresponded with improvement in plasma metabolites such as glutamine, threonine, and steroid metabolites. These findings informed subsequent studies evaluating response-based pharmacologic therapy to prevent progression of cardiac dysfunction in DCM.(5) Similar studies are lacking in childhood cancer survivors at high risk for anthracycline-related CHF. The current report describes how metabolomic profiling of anthracycline-exposed survivors may provide new information for the development of targeted primary or secondary prevention strategies.
MATERIALS AND METHODS
Patient eligibility and recruitment
Childhood cancer survivors were recruited between October 2010 and September 2012 from the City of Hope or the Children’s Hospital Los Angeles Childhood Cancer Survivorship Clinics. Eligibility criteria included: 1) cancer diagnosis prior to 22 years of age, irrespective of current age; 2) two or more years after completion of cancer treatment; and 3) receipt of anthracycline chemotherapy. In order to ensure heterogeneity of anthracycline exposure, survivors at high risk (HR: cumulative anthracycline dose ≥300mg/m²) and low risk (LR: 1–299mg/m²) of developing CHF per the Children’s Oncology Group Long Term Follow-up Guidelines(6) were targeted for recruitment. Furthermore, in order to enrich the cohort for those with cardiac dysfunction, patient recruitment disproportionately (2:1) targeted HR survivors over those who were LR. We excluded survivors who were actively being treated for cardiomyopathy, as pharmacologic therapy could possibly alter metabolomic profile. The study was approved by the respective institutional review boards. All study participants or their parents/ legal guardians provided informed consent.
Cardiac evaluation
Study participants underwent a detailed physical examination by their healthcare provider within the respective Survivorship Clinics, with special attention to signs and symptoms of CHF. Echocardiograms were performed on the day of clinical evaluation by a designated study technician, and consisted of complete two-dimensional (2D), M-mode, and Doppler evaluations, per the American Heart Association/ American College of Cardiology (AHA/ACC) task force practice guidelines for the clinical application of echocardiography.(7) Identical ultrasound machines (General Electric Vivid-7 echocardiography machine; General Electric) were used for all study-related echocardiographic evaluations at the two institutions. Left ventricular (LV) ejection fraction (EF) was calculated from the apical 4- and 2-chamber views using a modified Simpson biplane method.(7) LV end-systolic wall stress (ESWS) was calculated using the formula (1.35 × MAP × LVESD)/ ((4 × LVPWS) (1+LVPWS/LVESD)),(8, 9) where MAP is the mean arterial pressure as obtained by Dinamap blood pressure machine, LVESD is the LV chamber diameter in systole, and LVPWS is LV wall thickness in systole. A digitized and anonymized copy of each echocardiogram was sent to the study core cardiology laboratory (University of Michigan, Ann Arbor, MI) for a reading by the study cardiologist (SG) who was blinded to the risk status of the study participant.
Cardiac dysfunction was defined as having ESWS ≥2 standard deviations of normal (≥70 g/cm2) on the echocardiogram.(9, 10) The rationale for choosing ESWS over more conventional indices of LV systolic function such as EF was that change in EF typically occurs in the later stages of chronic pathology, signaling an extensive loss of function beyond the compensatory capacity of the myocardium. ESWS is the best-studied echocardiographic index in childhood cancer survivors aside from EF, and is a well-recognized precursor to anthracycline-related CHF.(10–12) Importantly, increase in ESWS typically precedes clinically meaningful changes in EF (<50%),(10–12) providing a reliable and prognostic echocardiographic index to study asymptomatic individuals at risk for CHF.
Metabolomic Profile
Blood samples were collected on the day of the echocardiographic assessment, and plasma was extracted within 1 hour of sample collection. Plasma samples were stored at −80°C and shipped to Metabolon, Inc. (Research Triangle, NC) for batched analytic studies. Detailed procedure for sample extraction and metabolomic analysis is described in a recent technical manuscript(13) and detailed in the appendix. Briefly, approximately 200 uL of plasma were split across three analytical platforms: gas chromatography/ mass spectroscopy (GC/MS), ultra high-performance liquid chromatography (UHPLC)/ MS-Positive and UHPLC/MS-Negative. Following peak identification and quality control (QC) filtering, integrated peak ion counts for each compound in each sample were used for statistical analysis (appendix). The dataset used for the current study included all detectable compounds of known identity (named biochemicals) that met established acceptance criteria for instrument and process variability; the final dataset included a total of 354 named biochemicals (appendix).
Clinical data collection
Self-reported questionnaires were used to obtain baseline demographics data, and medical record review allowed abstraction of the following information: date of diagnosis, type of cancer, stage of disease (if applicable), cumulative dose of anthracycline exposure and receipt of chest-directed radiation therapy. Lifetime cumulative anthracycline dose was calculated by multiplying the total dose of each anthracycline (doxorubicin, daunorubicin, epirubicin, idarubicin, and mitoxantrone) by a factor that reflects the cardiotoxic potential of each drug and then summing the products of the converted anthracyclines.(14)
Statistical analysis
Clinical characteristics of individuals with and without cardiac dysfunction
categorical variables were compared using Χ2 tests, and continuous variables were compared using independent two-sample t-tests. Data were analyzed using SPSS Version 18.0 (IBM, Armonk, NY). All statistical tests were 2-sided, and P<0.05 were considered statistically significant.
Metabolomic analyses
Data for each compound were normalized using the median values for each run (appendix). This minimizes inter-day instrument gain drift, but does not interfere with intra-day sample variability. Welch’s two-sample t-test was performed on the log-transformed data. To account for multiple comparisons, FDR method was used;(15) q-value was calculated using the method of Storey and Tibshirani,(16) a cut-off for statistical significance of <0.05 (i.e. FDR<5%) was used. The statistical analysis program ‘R’ was used for the analysis of metabolites (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria URL: http://www.R-project.org).
RESULTS
One hundred and fifty-two individuals were approached for participation in the study and 150 participated (HR: n=100; LR: n=50). One individual refused participation, and 1 did not complete the full battery of echocardiographic and blood testing (participation rate: 98.6%).
The salient characteristics of cancer survivors are presented in Table 1. Median time from cancer diagnosis to study participation was 12.4 years (range 2.6 to 37.9 years), and 63% were treated for a hematologic malignancy at a median age of 10.6 years (range 0.4 to 21.9 years). Median EF of study participants was 60% (range 50–85%), and none had clinical symptoms or signs of CHF. Thirty-five (23%) participants (29 HR, 6 LR) were found to have cardiac dysfunction at the time of evaluation; there was a dose-dependent increase in the prevalence of cardiac dysfunction by anthracycline dose (1–99 mg/m2: 5.7%, 100–299 mg/m2: 11.4%, 300–399 mg/m2: 31.4%, ≥400 mg/m2: 48.6%). The correlation between ESWS and EF was R=−0.48, p<0.01. Characteristics of patients with and without cardiac dysfunction are presented in Table 2. For patients with cardiac dysfunction, median ESWS and EF were 85 g/cm2 (70–106 g/cm2) and 58% (50–67%), respectively. In patients with normal cardiac function, they were 55 g/cm2 (30–69 g/cm2) and 62% (50–85%), respectively. Patients with cardiac dysfunction had significantly higher lifetime anthracycline exposure (400 mg/m2 vs. 300 mg/m2, p<0.01) when compared to those with normal function. The two groups were comparable with respect to sex, race/ethnicity, age at evaluation, body mass index (BMI), chest radiation exposure, mean arterial pressure, and reported use of anti-hypertensive medications at the time of evaluation
Table 1.
Patient and treatment characteristics
| Characteristics | Cancer survivors (N=150) |
|---|---|
| Sex, No. (%)* | |
| Male | 85 (56.7) |
| Female | 65 (43.3) |
| Race/Ethnicity, No. (%) | |
| Non-Hispanic white | 54 (36.0) |
| Hispanic | 73 (48.7) |
| Other | 23 (15.3) |
| Age at examination, Years* | |
| Median, range | 26.6 (6.7–55.8) |
| BMI at examination | |
| Overweight or obese** | 66 (444.0) |
| Diagnosis, No. (%) | |
| Hematologic malignancy | 95 (63.3) |
| Acute Lymphoblastic Leukemia | 53 (35.3) |
| Acute Myeloid Leukemia | 18 (12.0) |
| Lymphoma | 24 (16.0) |
| Solid tumor | 55 (36.7) |
| Ewing Sarcoma | 19 (12.7) |
| Osteosarcoma | 14 (9.3) |
| Soft tissue sarcoma | 16 (10.7) |
| Other | 6 (4.0) |
| Age at diagnosis, Years | |
| Median, range | 10.6 (0.4–21.9) |
| Lifetime Anthracycline, mg/m2 | |
| Median, range | 350 (25–642) |
| Chest radiation, No. (%) | |
| Any | 16 (10.6) |
| Time since cancer diagnosis, Years | |
| Median, range | 12.4 (2.6–37.9) |
Represents matching criteria.
Defined as ≥25 Kg/m2 if ≥18 years old, or ≥85% for age if <18 years old.
Table 2.
Patient characteristics comparing participants with cardiac dysfunction and normal function
| Characteristics | Cardiac dysfunction (N=35) |
Normal function (N=115) |
P-Value |
|---|---|---|---|
| Sex, No. (%) | |||
| Male | 16 (45.7) | 69 (60.0) | |
| Female | 19 (54.3) | 46 (40.0) | 0.19 |
| Race/Ethnicity, No. (%) | |||
| Non-Hispanic white | 12 (34.3) | 42 (36.5) | |
| Hispanic | 16 (45.7) | 57 (49.6) | |
| Other | 7 (20.0) | 16 (13.9) | 0.56 |
| Age at examination, Years (%) | |||
| ≤18 | 6 (17.1) | 28 (24.3) | |
| 19–29 | 6 (17.1) | 28 (24.3) | |
| ≥30 | 23 (65.7) | 59 (51.3) | 0.33 |
| BMI at examination | |||
| Overweight or obese** | 15 (42.8) | 51 (45.6) | 0.88 |
| Lifetime Anthracycline, mg/m2 (%) | |||
| Median, range | 400 (75–541) | 300 (25–759) | <0.01 |
| Chest radiation, No. (%) | |||
| None | 29 (82.9) | 105 (91.3) | |
| Any | 6 (17.1) | 10 (8.7) | 0.12 |
| Ejection fraction (%) | |||
| Median (Range) | 58 (50–67) | 62 (50–85) | <0.01 |
| Mean arterial pressure (MAP), mmHg | |||
| Median MAP, range | 82.7 (66–115) | 81.2 (58–135) | 0.23 |
| On anti-hypertensive medications at the time of assessment, No. (%)* | |||
| Any | 4 (11.4) | 9 (7.8) | 0.51 |
Defined as ≥25 Kg/m2 if ≥18 years old, or ≥85% for age if <18 years old.
Angiotensin converting enzyme inhibitor or beta-blocker
Plasma levels of 15 compounds in 3 metabolic pathways (carbohydrate, amino acid, and lipid metabolism) were significantly different between the two groups (Table 3). After adjusting for multiple comparisons, individuals with cardiac dysfunction had significantly lower plasma carnitine levels (relative ratio [RR]: 0.89, p<0.01) when compared to those with normal function.
Table 3.
Plasma metabolites altered in individuals with LV dysfunction
| HMDBID | KEGG | Super Pathway | Sub Pathway | Biochemical Name | Relative Ratio* (Abn : Normal) |
p-Value | q-Value |
|---|---|---|---|---|---|---|---|
| HMDB04136 | C16884 | Carbohydrate | Nucleotide sugars, pentose metabolism | Threitol | 1.29 | 0.002 | 0.075 |
| HMDB00169 | C00159 | Carbohydrate | Mannose metabolism | Mannose | 1.17 | 0.015 | 0.220 |
| N/A | N/A | Amino Acid | Glutamate metabolism | Pyroglutamine | 1.13 | 0.003 | 0.117 |
| HMDB00766 | C02847 | Amino Acid | Alanine metabolism | N-acetylalanine | 1.09 | 0.002 | 0.081 |
| HMDB00064 | C00300 | Amino Acid | Creatine metabolism | Creatine | 0.95 | 0.025 | 0.251 |
| HMDB00736 | N/A | Amino Acid | Valine, leucine, isoleucine metabolism | Isobuylcarnitine | 1.07 | 0.046 | 0.328 |
| N/A | C00318 | Lipid | Carnitine metabolism | Carnitine | 0.89 | <0.001 | 0.009 |
| HMDB02231 | N/A | Lipid | Long-chain fatty acid | Eicosenoate | 1.26 | 0.044 | 0.282 |
| HMDB06547 | C16300 | Lipid | Long-chain fatty acid | Stearidonate | 1.62 | 0.004 | 0.176 |
| HMDB01043 | C00219 | Lipid | Long-chain fatty acid | Arachidonate | 1.15 | 0.019 | 0.324 |
| N/A | C16525 | Lipid | Long-chain fatty acid | Dihomo-linoleate | 1.34 | 0.030 | 0.229 |
| N/A | N/A | Lipid | Lysolipid | 1-stearoylglycerophosphoinositol | 1.44 | 0.012 | 0.365 |
| HMDB01032 | C04555 | Lipid | Sterol/Steroid | Dehydroisoandrosterone sulfate | 0.61 | 0.003 | 0.283 |
| N/A | N/A | Lipid | Sterol/Steroid | Pregnen-diol disulfate | 0.76 | 0.011 | 0.366 |
| N/A | N/A | Lipid | Sterol/Steroid | Pregn steroid monosulfate | 0.66 | 0.035 | 0.599 |
Abbreviations: HMDIB, Human Metabolome Database ID; KEGG, Kyoto Encyclopedia of Genes and Genomes Compound Database; N/A, not available.
Ratio of the mean value of each metabolite in individuals with abnormal cardiac function divided by that in the normal group.
DISCUSSION
Anthracycline-related cardiac toxicity has emerged as one of the leading causes of morbidity and mortality in long-term survivors of childhood cancer,(1) emphasizing the need for studies that examine the pathophysiology of cardiac injury. In the current study, metabolomic profiling of asymptomatic childhood cancer survivors revealed significantly lower plasma carnitine levels in individuals with cardiac dysfunction when compared to those with normal function.
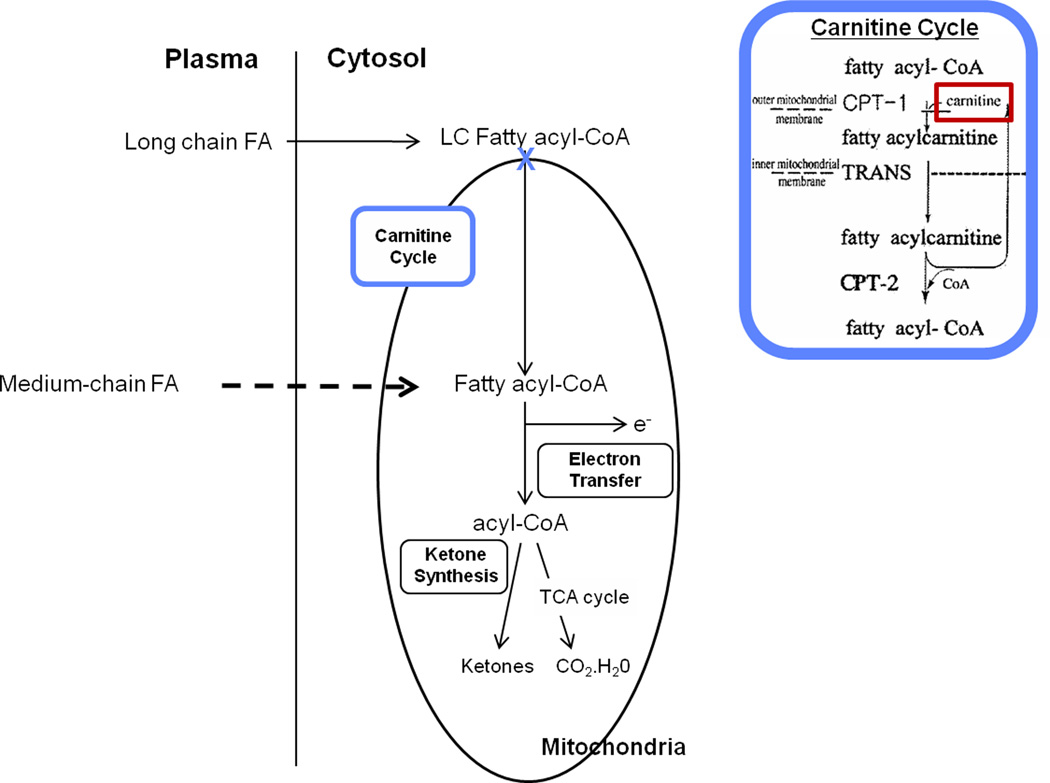
L-carnitine (the biologically active stereoisomer of carnitine) is a quaternary amine essential for oxidation of long chain fatty acids (LCFAs), which are a major substrate for energy production in the myocardium (Figure 1).(17) Carnitine homeostasis is maintained through endogenous biosynthesis of L-carnitine, absorption of carnitine from dietary sources, and elimination and reabsorption by the kidneys.(17) Cardiac myocytes contain relatively high concentrations of carnitine. Carnitine is actively transported into the cell, since myocytes are incapable of carnitine biosynthesis.(17, 18) Clinically, both primary and secondary carnitine deficiency can result in cardiomyopathy and cardiac arrhythmias, due in part to the accumulation of LCFAs and acylcarnitines that are unable to be oxidized in the mitochondria and are unavailable for energy production.(17, 19) In patients with a past history of myocardial infarction, administration of L-carnitine has been shown to lead to attenuation of left ventricular dilation, prevent LV remodeling, and is associated with a lower incidence of chronic heart failure and cardiac death.(20) These beneficial effects are thought to be due to the resumption of normal oxidative metabolism and restoration of myocardial energy reserves.16,17
Figure 1.
Role of carnitine in mitochondrial long-chain fatty acid oxidation.
Previous studies suggest that anthracyclines may exert at least part of their cardiotoxicity by inhibiting LCFA oxidation in the heart.(21) In animal models, chronic anthracycline administration results in dose-dependent decrease in expression of heart fatty acid binding protein (H-FABP) and organic cation/carnitine transporter (OCTN2) mRNA in cardiac tissues, corresponding to an increase in serum enzymes (lactate dehydrogenase [LDH], creatine phsophokinase isoenzyme [CK-MB]) of acute cardiac injury.(22, 23) OCTN2 is a high-affinity carnitine transporter which mediates carnitine transport across the plasma membrane into the cells, while H-FABP plays an important role in eliminating toxic LCFA metabolites from the cytosol.(23) Carnitine supplementation has been shown to restore H-FABP and OCTN2 gene expression to baseline, and decrease serum LDH and CK-MB levels to control values.(22, 23)
Small studies in cancer patients receiving anthracyclines have found a dose-dependent decrease in plasma carnitine levels during treatment,(24, 25) corresponding to acute impairment of LV systolic and diastolic function.(25) To date, there have been no studies that have evaluated the role of carnitine in long-term cardiac health of cancer survivors treated with anthracyclines. The findings from the current study, when confirmed in an independent cohort of anthracycline-exposed childhood cancer survivors, could facilitate the development of novel strategies for primary prevention (identification and treatment of carnitine deficiency around the time of anthracycline administration) and secondary prevention in cancer survivors at highest risk of CHF.
It is important to note that the current study utilized a cross-sectional design. As a result, we are not able to state whether the cardiac dysfunction was due to pre-existing anthracycline-mediated carnitine depletion. In addition, we cannot exclude unrelated causes of carnitine deficiency such as differences in dietary intake or gastrointestinal absorption that may account for our findings. These limitations notwithstanding, the current study provides data to support a biologically plausible mechanism for cardiac dysfunction occurring years following completion of cardiotoxic therapy. Future longitudinal studies will need to evaluate whether carnitine depletion occurs during anthracycline administration, is sustained after completion of cancer therapy, and whether these alterations in carnitine levels precede echocardiographic changes of cardiac remodeling.
Acknowledgments
Funding source: NIH/NCI: 2 K12 CA001727-14 (SHA), STOP Cancer Foundation (SHA).
Footnotes
Presented, in part, at the International Conference on Long Term Complications of Treatment of Children and Adolescents for Cancer and the American Society of Clinical Oncology, 2013.
FINANCIAL DISCLORURES
Financial support. All authors: no financial disclosures.
Financial disclosures. All authors: no financial disclosures.
References
- 1.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement From the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England journal of medicine. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological reviews. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 4.Alexander D, Lombardi R, Rodriguez G, Mitchell MM, Marian AJ. Metabolomic distinction and insights into the pathogenesis of human primary dilated cardiomyopathy. European journal of clinical investigation. 2010;41:527–538. doi: 10.1111/j.1365-2362.2010.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin JL, Atherton H, Shockcor J, Atzori L. Metabolomics as a tool for cardiac research. Nature reviews. 8:630–643. doi: 10.1038/nrcardio.2011.138. [DOI] [PubMed] [Google Scholar]
- 6.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers. 3.0 ed. Arcadia, CA: Children's Oncology Group; 2008. [Google Scholar]
- 7.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Journal of the American College of Cardiology. 2003;42:954–870. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 8.Ganame J, Claus P, Uyttebroeck A, Renard M, D'Hooge J, Bijnens B, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20:1351–1358. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, Pettersen MD, Gurckzynski J, L'Ecuyer T. Measuring stress velocity index using mean blood pressure: simple yet accurate? Pediatric cardiology. 2008;29:108–112. doi: 10.1007/s00246-007-9101-3. [DOI] [PubMed] [Google Scholar]
- 10.Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 11.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatric blood & cancer. 2005;44:600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 13.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 14.Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Yekutieli D. Controlling false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;75:289–300. [Google Scholar]
- 16.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutrition & metabolism. 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendler BS. Carnitine: an overview of its role in preventive medicine. Preventive medicine. 1986;15:373–390. doi: 10.1016/0091-7435(86)90005-8. [DOI] [PubMed] [Google Scholar]
- 19.Arsenian MA. Carnitine and its derivatives in cardiovascular disease. Progress in cardiovascular diseases. 1997;40:265–286. doi: 10.1016/s0033-0620(97)80037-0. [DOI] [PubMed] [Google Scholar]
- 20.Iliceto S, Scrutinio D, Bruzzi P, D'Ambrosio G, Boni L, Di Biase M, et al. Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. Journal of the American College of Cardiology. 1995;26:380–387. doi: 10.1016/0735-1097(95)80010-e. [DOI] [PubMed] [Google Scholar]
- 21.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26:3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong YM, Kim HS, Yoon HR. Serum lipid and fatty acid profiles in adriamycin-treated rats after administration of L-carnitine. Pediatric research. 2002;51:249–255. doi: 10.1203/00006450-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Sayed-Ahmed MM, Al-Shabanah OA, Hafez MM, Aleisa AM, Al-Rejaie SS. Inhibition of gene expression of heart fatty acid binding protein and organic cation/carnitine transporter in doxorubicin cardiomyopathic rat model. European journal of pharmacology. 2010;640:143–149. doi: 10.1016/j.ejphar.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Yaris N, Ceviz N, Coskun T, Akytuz C, Buyukpamukcu M. Serum carnitine levels during the doxorubicin therapy. Its role in cardiotoxicity. Journal of experimental & clinical cancer research : CR. 2002;21:165–170. [PubMed] [Google Scholar]
- 25.Khositseth A, Jirasakpisarn S, Pakakasama S, Choubtuym L, Wattanasirichaigoon D. Carnitine levels and cardiac functions in children with solid malignancies receiving doxorubicin therapy. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2011;32:38–42. doi: 10.4103/0971-5851.81889. [DOI] [PMC free article] [PubMed] [Google Scholar]