Abstract
Objective
Review murine xenotransplantation models for myelodysplastic syndromes (MDS).
Materials and Methods
Literature review, experimental strategies
Results
The difficulties in achieving sustained engraftment of MDS cells in immunodeficient mice may lie in innate characteristics of the MDS clone(s) and microenvironmental factors. Engraftment of very low numbers of CD45+ clonal MDS cells has been achieved with intravenous (IV) injection; higher rates of engraftment are obtained via the intramedullary route. Co-injection of certain stroma components with hematopoietic cells overcomes limitations of IV administration, allowing for engraftment of high proportions of human CD45+ cells in mouse spleen and marrow. Expression of CD146 on stroma cells conveys an engraftment-facilitating effect. Clonal MDS cells have been propagated for periods beyond 6 months and have been transplanted successfully into secondary recipients.
Conclusions
Engraftment of human clonal MDS cells with stem cell characteristics in immunodeficient mice is greatly facilitated by co-injection of stroma/mesenchymal cells, particularly with IV administration; CD146 expression on stroma is an essential factor. However, no model develops the laboratory and clinical features of human MDS. Additional work is needed to determine cellular and non-cellular factors required for the full evolution of MDS.
Keywords: Myelodysplastic syndrome, murine xenotransplantation models, stroma cells, marrow microenvironment
Introduction
Myelodysplastic syndromes (MDS) are clonal diseases of hematopoietic stem/precursor cells. The incidence in the United States has been estimated at 3.5–12.6/100,000 per year [1–3], but the incidence increases with age, reaching 30–50/100,000 per year in persons older than 70 years, a population in whom it may be the most frequent hematologic malignancy [2,3]. The average age at diagnosis in North-American and European patients is in the eighth decade of life but is lower in Asian patients [4–9]. MDS is characterized by ineffective hematopoiesis, and patients generally present with single or multilineage blood cytopenias. The prognosis varies greatly. In approximately one-third of patients, MDS will evolve to acute myeloid leukemia (AML) – hence the historical terms pre-leukemia or smoldering leukemia – while in the remaining patients declining marrow function and failure, resulting in severe anemia, infections and hemorrhagic complications, are the most frequent scenarios [10,11]. The cellular and molecular pathophysiology of MDS has been investigated extensively over the past decade, and important insights have been gained into disease mechanisms, leading to functional sub-classifications of this heterogeneous group of disorders. The identification of various somatic mutations in humans and the respective genetic modification of murine hematopoietic stem/precursor cells (HSC) has led to the development of murine MDS models that mimic many aspects of human MDS [12–14]. Additional strategies for model development include the treatment of mice with known mutagenic or carcinogenic agents [15,16] to induce a murine disease that may mimic the human disorder or to utilize immunodeficient mice that accept the implantation/injection of human tissue/cells and allow for in vivo propagation, i.e. xenotransplantation [17–19].
In vivo investigations of human MDS, however, have remained challenging. While several murine xenotransplantation models of MDS have been developed, the propagation of CD34+ cells derived from the marrows of patients with MDS, in contrast to cells from patients with AML [20,21] has proven difficult [17,18,22,23]. Consistent with those observations is the fact that very few, if any, MDS-derived myeloid cell lines that do not require growth factor support have been established [24–27]. The reasons are not entirely clear but may be related to the prominent tendency of CD34+ MDS cells to undergo “spontaneous” apoptosis that is modified by signals from the microenvironment, which profoundly affects regulation of hematopoiesis [28–35]. Thus, if components of the microenvironment support hematopoiesis and interfere with apoptosis, one approach to enhance the success of xenogeneic transplantation would be to incorporate those elements into the transplant approach. We will review currently described murine xenotransplantation models of MDS, assessing the role of growth factors and stroma or mesenchymal cells (MSC) in maintaining the human hematologic malignancy in murine hosts.
Murine xenotransplantation models of MDS
In vivo models of human diseases offer many advantages over in vitro studies by allowing longitudinal observations and possible treatment interventions in an environment closer to the human in vivo situation than in vitro experiments. However, as indicated already, propagation of clonal CD34+ cells derived from the marrow of patients with MDS has proven difficult [17,18,22,23]. Table 1 summarizes several published murine xenotransplantation models of MDS.
Table 1.
Murine xenotransplantation models of MDS
| Model | Mouse strain | Transplanted MDS cells | Added Stroma support | TBI Dose (cGy) | Clonal marker | Injection route | Proportion of mice with engraftment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | NOD/SCID | CD34+/CD38− | None | 350–375 | del(5q) | IV | 1/7 | [21] |
| 2 | NOD/SCIDβ2mnull | CD34+/CD38− | None | 350–375 | +8 | IV | 0/7 | [16] |
| 3 | NOD/SCID | BMMC | None | 300,350,375 | multiple clones | IV or IP | 34/48 | [20] |
| 4 | NOD/SCIDβ2mnull | BMMC | None | 350 | del(5q); +8 | IV | 31/43 | [22] |
| 5 | NOD/SCIDβ2mnull | BMMC | HS5 +HS27a stroma cells | 325 | del(5q); del(7q); -Y | IM | 11/15 | [15] |
| 6 | NOD/SCID IL2Rγnull (NOD)# |
WBM CD34+/CD33+ hCD45+ |
Mesenchymal stem cells | 250 | multiple clones | IM | 20/31 | [43] |
| 7 | Nod.cg-Prkdcscid IL2rgtm1wjll (NSG)# |
BMMC | HS27a stroma cells | 275 | multiple clones | IV | 44/46 | [17] |
= nomenclature as used by the authors; BMMC= bone marrow mononuclear cells; IM = intramedullary; IV = intravenously; WBM=Whole bone marrow cells.
1. Xenotransplantation without stroma support
Nilsson et al. reported on transplantation of human CD34+ cells from 7 patients with MDS whose karyotypes all contained deletion of the long arm of chromosome 5 (5q-) into NOD/SCID mice irradiated with 350–375 cGy. Only mice receiving CD34+ cells (7 × 105) from one individual patient showed engraftment of intravenously (IV) injected cells, showing up to 12% CD45+ human cells in bone marrow [23]. The same investigators then reported transplantation of CD34+ CD38− cells from patients with early stage MDS, all with karyotypes containing trisomy 8 (+ 8), and none showed engraftment [18]. Our own earlier studies showed that non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice irradiated with 350–375 cGy and transplanted with IV injected MDS marrow allowed for long-term propagation of normal but not clonal MDS cells, suggesting that the NOD/SCID environment was not conducive to the expansion of clonal MDS precursors [22]. Thanopoulou et al. reported engraftment of neoplastic cells with multi-lineage potential from patients with MDS in NOD/SCID mice, which also had β2 microglobulin deleted (NOD/SCID-β2−/− mice), and in four cases the regenerating cells in recipient mice showed the same clonal markers as the original MDS samples [24]. Importantly, these immunodeficient mice were, in addition, transgenic for the human hematopoietic growth factors interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF) and steel factor (SF), the relevance of which was stressed more recently in a report by Takagi et al. [25]. The authors suggested that the genetic alteration of MDS clonal cells may affect patterns of differentiation and responsiveness to hematopoietic growth factors, and that NOD/SCID-β2m−/− mice would be superior to NOD/SCID mice for xenotransplant experiments [24].
2. Xenotransplantation with stroma or mesenchymal stem cell support
Hematopoietic stem cells are maintained by biochemical and physical contextual signals from the microenvironment consisting of osteoblasts, mesenchymal/stroma cells, endothelial cells, pericytes, and macrophages, in addition to matrix structures and soluble factors [29,30,30,36–38]. Specifically, two distinct microenvironmental structures have been described, the subendosteal and the vascular niches [30,38]. Disruption of components of the niches will alter hematopoiesis. For example, Raaijmakers et al. showed that deletion of Dicer 1 in murine osteoblast progenitors resulted in the development of dysplastic murine hematopoiesis [29]. Others showed that in the presence of clonal MDS cells marrow stroma may exhibit abnormal gene expression and function [39,40]. Those data support the concept that the microenvironment is essential both in normal and in pathological hematopoiesis and show that bi-directional signals between hematopoietic cells and the microenvironment affect hematopoiesis.
Working with two established stroma cell lines, HS5 and HS27a, derived from a healthy marrow donor [41], we showed in an in vitro co-culture system that apoptosis-resistant clonal MDS progenitors from patients with advanced MDS acquired sensitivity to apoptosis induced by TNFα following stroma contact [42–44]. However, hematopoietic precursors that remained adherent to stroma remained viable [43,45]. Strikingly, normal hematopoietic precursors did not become sensitive to apoptosis upon stroma contact [43,44]. Based in part on these in vitro observations, Kerbauy et al. used NOD/SCID-β2m−/− mice conditioned with total body irradiation of 325 cGy, and showed engraftment of distinct clonal MDS-derived hematopoietic precursors when stroma cells (HS5 and HS27a cells combined) were co-injected via the intramedullary (IM) route; the proportion of human cells in peripheral blood, determined at 4 to 17 weeks was 0.7%–58.4% (median 8.9%) [17].
More recently, Muguruma et al. injected bone marrow CD34+ cells from patients with MDS (or AML), together with or without human mesenchymal stem cells, into the medullary space of NOD/SCID mice with deletion of the T cell receptor λ chain (NOD/SCID/IL2Rγ−/− [NOG]) mice irradiated with 250 cGy [46]. The CD34+ cells were obtained from six patients with MDS and eight patients with AML with various cytogenetic abnormalities, including −7, +8 and complex abnormalities [46]. Cells from 3 of 6 MDS patients engrafted in the bone marrow of NOG mice that received co-injections of mesenchymal stem cells. The proportion of CD45+ human cells observed in murine marrow ranged from 0.15% to 88.92% [46]. Co-injection of stroma cells derived from sites other than marrow or non-stromal cells failed to facilitate engraftment of MDS-derived cells. Human cells harvested from successfully engrafted primary murine recipients did not require the intramedullary route of injection for engraftment in secondary and subsequent transplant recipients [46], consistent with reports by others that cells from patients with AML also exhibit great heterogeneity, and some clones will engraft readily in immunodeficient mice [20,21]. Presumably, engraftment in the primary recipient selected for those clones (sub-clones) that did not require additional signals for propagation.
HS5 and HS27a, two marrow stroma cell lines derived from the same healthy donor that were used in our experiments, had been shown in previous studies to exhibit strikingly different gene expression profiles and functions [41,47]. Specifically, HS5, a rich source of cytokines, supports the growth of more mature colony-forming cells, while HS27a, which expresses various adhesion molecules, interacts directly with very primitive hematopoietic cells and favors the out-growth of cobblestone areas, a model as close to stem cell assessment as we can assay in vitro [41]. We hypothesized, therefore, that HS27a cells also would be more potent in supporting primitive clonal MDS precursors [19] and speculated that the close adherence between HS27a cells and hematopoietic cells might allow for successful engraftment even with IV injection. Thus, either HS5 or HS27a cells were mixed and co-injected intravenously with MDS marrow-derived hematopoietic cells into Nod.cg-Prkdcscid Il2rgtm1wjll (NSG) mice irradiated with 275 cGy. In clear distinction, HS27a, but not HS5 cells, facilitated engraftment of clonal MDS cells [19]. The proportion of CD45+ human cells in mice followed for up to 4 months ranged from 0.1% to 30.3% in bone marrow, and from 0.1% to 73.2% in the spleen. The multipotency of the transplanted cells was illustrated by the differentiation into CD33+, CD19+, CD14+ and CD3+ lineages. Cells harvested from marrows and spleens of the primary recipients were transplanted successfully (together with HS27a cells) into secondary recipients and continued to show the clonal cytogenetic characteristics of the patient after an overall propagation time in murine recipients extending beyond 6 months, in strong support of the stem cell-like self-renewing capacity of the transplanted clonal cells.
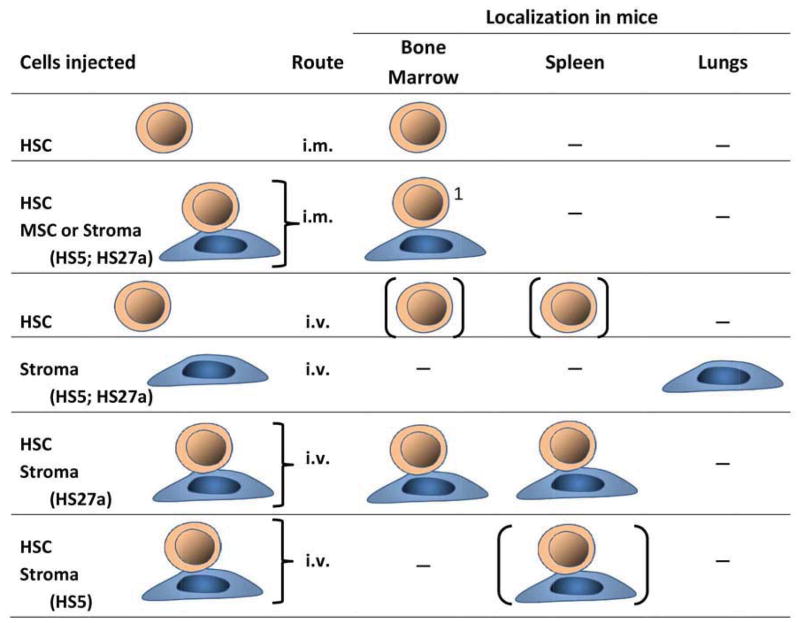
Additional data show that the glycoprotein termed “melanoma cell adhesion molecule” (MCAM/CD146), previously identified as a marrow niche marker [48–50], is highly expressed on HS27a but not HS5 cells, suggesting a direct link of CD146 to engraftment and survival of the clone(s). CD146 was originally identified as a tumor marker for melanoma, but recent work shows that CD146 is involved in various physiologic processes, such as development, signal transduction, cell migration, mesenchymal cell differentiation and angiogenesis [51]. MSCs with greater differentiation potential express higher levels of CD146 on the cell surface [52]. Corselli and colleagues showed that sorted CD146+ (but not CD146 negative) perivascular cells supported propagation of human hematopoietic stem cells with long-term reconstituting potential that engrafted in immunodeficient mice and could be serially transplanted [53]. Data from our own studies show that over-expression of CD146 in HS5 cells conveys engraftment-facilitating functions to HS5 cells similar to those of HS27a, apparently by providing a favorable microenvironment to support the survival of CD34+ clonal MDS cells in NSG mice [19]. Conversely, knock-down of CD146 in HS27a cells reduced their ability to support MDS cell propagation. Crisan et al., who had shown previously that in many organs perivascular cells, principally pericytes, expressed CD146, then demonstrated that CD146+ cells supported the long-term persistence of hematopoietic cells through cell-to-cell contact, and at least partly via Notch activation [54]. This is of note, as Pinnix et al. showed in primary melanocytes that CD146 was a direct target of Notch signaling by identifying two high-affinity binding sites within the CD146 promoter [55]. Both anti-Notch1 antibodies and inhibition of γ-secretase, required for Notch signaling, inhibited the CD146-mediated hematopoietic stem cell support by CD146+ cells [56]. A role of CD146+ stromal progenitors is further suggested by the fact that they physically associate with reticular cells, and express CXCL12 and multiple other gene products implicated in hematopoietic stem cell regulation [50]. It was of note in our experiments that CD146+ HS27a cells injected by themselves (without hematopoietic cells) were trapped in the lungs and failed to reach either marrow or spleen. While CD146+ perivascular cells have been shown to have potential for pulmonary repair [57], similar to stroma cells they are thought to have no or only limited potential to migrate. The present observations indicate, however, that stroma cells are able to travel in the company of hematopoietic cells, presumably due to tight adhesion with those cells, similar to the recent documentation of fibroblasts traveling with metastatic solid tumor cells [58]. Thus, this study demonstrates that human clonal MDS cells are able to engraft in immunodeficient mice following IV injection if concurrently specific stroma support is provided. The principle strategies underlying the various models that have been described are illustrated in Figure 1.
Figure 1.
Strategies for MDS xenotransplantation models.
Abbreviations: i.m. = intramedullary; i.v. = intravenous; HSC = hematopoietic stem/precursor cells from MDS marrow; MSC = mesenchymal stem cells; HS5 = human stroma, CD146 negative; HS27a = human stroma, CD146 positive; [] = very few human cells identified in recipient mice. The mouse strains used are described in the text.
z1No stroma cells identified. HSC only in the bone into which they were injected.
3. MDS and marrow stroma
MDS can be cured by allogeneic hematopoietic stem cell transplantation [59], suggesting that, generally, the microenvironment, including stroma, is structurally and functionally intact- stroma cells remain of patient origin even after allogeneic hematopoietic cell transplantation [60]. However, Elstner et al. showed that marrow from MDS patients formed poor adherent stromal layers [61], which may affect proliferation of MDS precursor cells [62,63]. Also, long-term bone marrow cultures from patients with MDS revealed impaired production of cytokines, such as IL-3 or hepatocyte growth factor [61,62,64–66]. The fact that healthy donor HSC, nevertheless, engraft in the marrow of MDS patients provides support for the concept that alterations in MDS stroma are dependent upon the presence of clonal MDS cells and are reversible upon their elimination. While several signals provided by stroma, including TWIST1-dependent down-regulation of p53 in clonal hematopoietic cells and and interactiosn of CD54 and CD11b/CD18, have been identified [44], signals in the reverse direction, from clonal hematopoietic cells to stroma, remain to be characterized.
In addition to CD146, the expression of several adhesion molecules, including VCAM-1, CD166, and CD29, has been shown to be altered in MDS-derived mesenchemal/stroma cells; how these abnormalities influence the pathogenesis of MDS is not clear at present [39,67]. Sacchetti et al. defined CD146/MCAM-positive cells as an important subset of stromal fibroblasts that contributes to the stem cell niche [50]. CD146/MCAM is expressed at high levels in human bone marrow stroma cells that can be assayed as CFU-Fs [50]. Using HS5 and HS27a stroma cells, Pillai et al. showed that MCAM/CD146hi HS27a cells expressed significantly higher stroma-derived factor 1-alpha (SDF-1α/CXCL12) than MCAM/CD146lo cells (e.g. HS5 cells) [49]. Levels of SDF-1α, and the CXC chemokine receptor 4 (CXCR4), which control homing, self-renewal and proliferation capacities of hematopoietic cells [68–71], are decreased in MDS cell cultures, features that are associated with reduced induction of migration of CD34+ hematopoietic cells [39]. Co-cultures of mesenchymal stromal cells from MDS patients with CD34+ cells from healthy donors resulted in reduced numbers of cobblestone area forming-cells and fewer colony forming units compared to co-cultures with mesenchymal cells from healthy donors [39]. Further, mesenchymal stem cells from MDS patients (across the entire MDS spectrum; n=106) exhibit significantly reduced growth and proliferative capacities and show premature replicative senescence, leading to a diminished ability to support CD34+ hematopoietic precursors in long-term culture-initiating cell assays. To the best of our knowledge, so far no xenotransplant data have been reported that compared stroma/mesenchymal cells from MDS patients to stroma/mesenchymal cells obtained from healthy donors for their capacity to support clonal MDS cells in murine transplant recipients.
Concluding Remarks
Several murine xenotransplantation models of MDS have been developed. Currently, the best suited recipients appear to be Nod.cg-Prkdcscid Il2rgtm1wjll (NSG) mice. Intramedullary injection of MDS cells results in engraftment of clonal MDS cells to various extents and is enhanced by co-injection of mesenchymal/stroma cells, although engraftment appears to remain confined to the bone into which the transplant occurs. Almost uniform success of engraftment is achievable by the simpler intravenous route if hematopoietic cells are co-injected with admixed stroma cells. One important characteristic of effective stroma cells was the expression of CD146. Based on data on the relevance of CD146 presented by others [50,53,54] this observation is in support of a central role of the vascular niche. The available experiments do not allow conclusions specifically in regards to osteoprogenitors and the subendosteal niche, although osteoblast progenitors have been shown in a model of murine MDS to be involved in the disease process [29]. However, none of the mice in the models described here have developed clinical features of human MDS. Therefore, if these models are to be further exploited, particularly in regards to therapeutic manipulations, additional modifications of the mice, possibly in the form of a “humanized” murine recipient, will be necessary.
Acknowledgments
This work was supported in part by National institutes of Health, grants HL095999, HL036444, CA015704, CA018029. We thank Helen Crawford and Bonnie Larson for help with manuscript preparation.
Footnotes
Conflict of Interest Disclosure
There are no financial interests/relationships with financial interest to declare relating to the topic of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1538–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 3.Polednak AP. Trend (1999–2009) in U.S. death rates from myelodysplastic syndromes: Utility of multiple causes of death in surveillance. Cancer Epidemiology. 2013 doi: 10.1016/j.canep.2013.05.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Intragumtornchai T, Prayoonwiwat W, Swasdikul D, et al. Myelodysplastic syndromes in Thailand: a retrospective pathologic and clinical analysis of 117 cases. Leuk Res. 1998;22:453–460. doi: 10.1016/s0145-2126(98)00022-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Lee JH, Shin YR, et al. Application of different prognostic scoring systems and comparison of the FAB and WHO classifications in Korean patients with myelodysplastic syndrome. Leukemia. 2003;17:305–313. doi: 10.1038/sj.leu.2402798. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes [erratum appears in Blood 1998 Feb 1;91(3):1100] Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 7.Aul C, Gattermann N, Heyll A, Germing U, Derigs G, Schneider W. Primary myelodysplastic syndromes: analysis of prognostic factors in 235 patients and proposals for an improved scoring system. Leukemia. 1992;6:52–59. [PubMed] [Google Scholar]
- 8.Mufti GJ, Stevens JR, Oscier DG, Hamblin TJ, Machin D. Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol. 1985;59:425–433. doi: 10.1111/j.1365-2141.1985.tb07329.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanz GF, Sanz MA, Vallespì T, et al. Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: A multivariate analysis of prognostic factors in 370 patients. Blood. 1989;74:395–408. [PubMed] [Google Scholar]
- 10.Greenberg PL, Baer M, Bennett JM, et al. Myelodysplastic Syndromes. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2004;1.2005 < http://www.nccn.org/professionals/physician_gls/PDF/mds.pdf>. [PubMed] [Google Scholar]
- 11.Greenberg PL. Myelodysplastic syndromes: dissecting the heterogeneity. J Clin Oncol. 2011;29:1937–1938. doi: 10.1200/JCO.2011.35.2211. [DOI] [PubMed] [Google Scholar]
- 12.Helgason CD, Damen JE, Rosten P, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louz D, van den Broek M, Verbakel S, et al. Erythroid defects and increased retrovirally-induced tumor formation in Evi1 transgenic mice. Leukemia. 2000;14:1876–1884. doi: 10.1038/sj.leu.2401887. [DOI] [PubMed] [Google Scholar]
- 14.Grisendi S, Bernardi R, Rossi M, et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437:147–153. doi: 10.1038/nature03915. [DOI] [PubMed] [Google Scholar]
- 15.Funk RK, Maxwell TJ, Izumi M, et al. Quantitative trait loci associated with susceptibility to therapy-related acute murine promyelocytic leukemia in hCG-PML/RARA transgenic mice. Blood. 2008;112:1434–1442. doi: 10.1182/blood-2008-01-132084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenske TS, McMahon C, Edwin D, et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 2006;66:5029–5038. doi: 10.1158/0008-5472.CAN-05-3404. [DOI] [PubMed] [Google Scholar]
- 17.Kerbauy DMB, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-β2microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells (Letter to the Editor) Blood. 2004;104:2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson L, Åstrand-Grundström I, Anderson K, et al. Involvement and intrinsic deficiencies of hematopoietic stem cells in MDS patients with trisomy 8 [abstract] Blood. 2001;98 (Part 1):354a#1491. [Google Scholar]
- 19.Li X, Marcondes AM, Ragoczy T, Telling A, Deeg HJ. Effect of intravenous coadministration of human stroma cell lines on engraftment of long-term repopulating clonal myelodysplastic syndrome cells in immunodeficient mice. Blood Cancer Journal. 2013;3:e113. doi: 10.1038/bcj.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez PV, Perry RL, Sarry JE, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009;23:2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agliano A, Martin-Padura I, Mancuso P, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 22.Benito AI, Bryant E, Loken MR, et al. NOD/SCID mice transplanted with marrow from patients with myelodysplastic syndrome (MDS) show long-term propagation of normal but not clonal human precursors. Leuk Res. 2003;27:425–436. doi: 10.1016/s0145-2126(02)00221-7. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson L, Astrand-Grundstrom I, Arvidsson I, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96:2012–2021. [PubMed] [Google Scholar]
- 24.Thanopoulou E, Cashman J, Kakagianne T, Eaves A, Zoumbos N, Eaves C. Engraftment of NOD/SCID-β2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood. 2004;103:4285–4293. doi: 10.1182/blood-2003-09-3192. [DOI] [PubMed] [Google Scholar]
- 25.Takagi S, Saito Y, Hijikata A, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujioka T, Matsuoka A, Tohyama Y, Tohyama K. Approach to new therapeutics: investigation by the use of MDS-derived cell lines (Review) Current Pharmaceutical Design. 2012;18:3204–3214. doi: 10.2174/1381612811209023204. [DOI] [PubMed] [Google Scholar]
- 27.Drexler HG, Dirks WG, Macleod RA. Many are called MDS cell lines: one is chosen (Review) Leuk Res. 2009;33:1011–1016. doi: 10.1016/j.leukres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 29.Raaijmakers MHGP, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2012;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells (Review) Curr Opin Hematol. 2008;15:301–306. doi: 10.1097/MOH.0b013e328303e14c. [DOI] [PubMed] [Google Scholar]
- 31.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 32.Hale MD, Hayden JD, Grabsch HI. Tumour-microenvironment interactions: role of tumour stroma and proteins produced by cancer-associated fibroblasts in chemotherapy response. Cellular Oncology. 2013;36:95–112. doi: 10.1007/s13402-013-0127-7. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. The hallmarks of cancer (Review) Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation (Review) Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Allen M, Jones JL. Jekyll and Hyde: the role of the microenvironment on the progression of cancer (Review) J Pathol. 2011;223:162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 36.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins MK, Khoruts A, Ingulli E, et al. In vivo activation of antigen-specific CD4 T cells (Review) Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 38.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013 doi: 10.3324/haematol.2013.083972. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geyh S, Oz S, Cadeddu RP, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013 doi: 10.1038/leu.2013.193. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 42.Bhagat TD, Spaulding E, Sohal D, et al. MDS marrow stroma is characterized by epigenetic alterations [abstract] Blood. 2008;112:1243–3635. [Google Scholar]
- 43.Mhyre A, Marcondes AM, Spaulding EY, Deeg HJ. Stroma-dependent apoptosis in clonal hematopoietic precursors correlates with expression of PYCARD. Blood. 2009;113:649–658. doi: 10.1182/blood-2008-04-152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Marcondes AM, Gooley TA, Deeg HJ. The helix-loop-helix transcription factor TWIST is dysregulated in myelodysplastic syndromes. Blood. 2010;116:2304–2314. doi: 10.1182/blood-2009-09-242313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerbauy DMB, Mhyre A, Bryant E, Deeg HJ. Do MDS-derived clonal hematopoietic precursors require human stroma support for survival. Current Research in Hematology. 2007;1:1–13. [Google Scholar]
- 46.Muguruma Y, Matsushita H, Yahata T, et al. Establishment of a xenograft model of human myelodysplastic syndromes. Haematologica. 2011;96:543–551. doi: 10.3324/haematol.2010.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graf L, Iwata M, Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a (Letter to Editor) Blood. 2002;100:1509–1511. doi: 10.1182/blood-2002-03-0844. [DOI] [PubMed] [Google Scholar]
- 48.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillai MM, Yang X, Balakrishnan I, Bemis L, Torok-Storb B. MiR-886-3p down regulates CXCL12 (SDF1) expression in human marrow stromal cells. PLoS ONE. 2010;5:e14304. doi: 10.1371/journal.pone.0014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion (Review) Cancer Lett. 2013;330:150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 52.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 53.Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121:2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Pinnix CC, Lee JT, Liu ZJ, et al. Active Notch1 confers a transformed phenotype to primary human melanocytes. Cancer Res. 2009;69:5312–5320. doi: 10.1158/0008-5472.CAN-08-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levesque JP. A niche in a dish: pericytes support HSC. Blood. 2013;121:2816–2818. doi: 10.1182/blood-2013-02-485144. [DOI] [PubMed] [Google Scholar]
- 57.Montemurro T, Andriolo G, Montelatici E, et al. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. Journal of Cellular & Molecular Medicine. 2011;15:796–808. doi: 10.1111/j.1582-4934.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. PNAS. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deeg HJ. Marrow stroma in MDS: culprit or bystander? Leuk Res. 2002;26:687–688. doi: 10.1016/s0145-2126(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 60.Soenen V, Kotb R, Bonnet ML, et al. Mesenchymal cells (MC) generated from patients with myelodysplastic syndromes (MDS) are devoid of cytogenetic abnormalities and support short and long-term hematopoiesis [abstract] Blood. 2001;98 (Part 1):729a–3041. [Google Scholar]
- 61.Elstner E, Wachter M, Ihle R. Bone marrow stromal culture from patients with myelodysplastic syndromes and patients at different stages of acute nonlymphocytic leukaemia. Folia Haematologica - Internationales Magazin fur Klinische und Morphologische Blutforschung. 1989;116:167–174. [PubMed] [Google Scholar]
- 62.Weimar IS, Voermans C, Bourhis JH, et al. Hepatocyte growth factor/scatter factor (HGF/SF) affects proliferation and migration of myeloid leukemic cells. Leukemia. 1998;12:1195–1203. doi: 10.1038/sj.leu.2401080. [DOI] [PubMed] [Google Scholar]
- 63.Aizawa S, Nakano M, Iwase O, et al. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro. Leuk Res. 1999;23:239–246. doi: 10.1016/s0145-2126(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 64.Mangi MH, Newland AC. Interleukin-3 in hematology and oncology: current state of knowledge and future directions (Review) Cytokines, Cellular & Molecular Therapy. 1999;5:87–95. [PubMed] [Google Scholar]
- 65.Tormo M, Marugan I, Calabuig M. Myelodysplastic syndromes: an update on molecular pathology (Review) Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societes & of the National Cancer Institute of Mexico. 2010;12:652–661. doi: 10.1007/s12094-010-0574-9. [DOI] [PubMed] [Google Scholar]
- 66.Manakova TE, Tsvetaeva NV, Levina AA, et al. In vivo production of cytokines by bone marrow stromal cells and macrophages from patients with myelodysplastic syndrome. Bulletin of Experimental Biology & Medicine. 2001;132:633–636. doi: 10.1023/a:1012559724283. [DOI] [PubMed] [Google Scholar]
- 67.Lubkova ON, Tzvetaeva NV, Momotyuk KS, Belkin VM, Manakova TE. VCAM-1 expression on bone marrow stromal cells from patients with myelodysplastic syndromes. Bulletin of Experimental Biology & Medicine. 2011;151:13–15. doi: 10.1007/s10517-011-1248-5. [DOI] [PubMed] [Google Scholar]
- 68.Crump MP, Gong JH, Loetscher P, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 70.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 71.Franz WM, Zaruba M, Theiss H, David R. Stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:675–676. doi: 10.1016/S0140-6736(03)14240-7. [DOI] [PubMed] [Google Scholar]