Abstract
Dysregulation of microRNA (miRNA) expression contributes to the pathogenesis of several clinical conditions. The aim of this study is to evaluate the associations between miRNAs and childhood acute lymphoblastic leukemia (ALL) to discover their role in the course of the disease. Forty-three children with ALL and 14 age-matched healthy controls were included in the study. MicroRNA microarray expression profiling was used for peripheral blood and bone marrow samples. Aberrant miRNA expressions associated with the diagnosis and outcome were prospectively evaluated. Confirmation analysis was performed by real time RT-PCR. miR-128, miR-146a, miR-155, miR-181a, and miR-195 were significantly dysregulated in ALL patients at day 0. Following a six-month treatment period, the change in miRNA levels was determined by real time RT-PCR and expression of miR-146a, miR-155, miR-181a, and miR-195 significantly decreased. To conclude, these miRNAs not only may be used as biomarkers in diagnosis of ALL and monitoring the disease but also provide new insights into the potential roles of them in leukemogenesis.
1. Introduction
The prevalence of cancer is 11–15/100.000 among children and leukemia is the most common malignancy with an incidence of 30.2%, hence a major cause of mortality and morbidity [1, 2]. Acute lymphoblastic leukemia (ALL) accounts for 75% of childhood leukemia [2]. Event-free-survival (EFS) rates of childhood ALL exceeded 80% with current treatment protocols and relapse as the main reason for therapy failure [3]. Pediatric leukemia has distinct features when compared to adult leukemia, especially in prognosis and treatment options [4]. There are also individual differences among patients of the same age and risk group in response to treatment and in prognosis. Genetic factors are seen as one of the likely culprits for these discrepancies. Recently, microRNAs have been shown to be important molecules in developmental and oncogenic processes. They are 19–25 nt long posttranscriptional regulator ribonucleic acid (RNA) molecules [5]. They target mRNAs, inhibit the translation into proteins, and silence specific gene expression [6]. Aberrant expression of miRNAs has been observed in many types of cancer, showing either tumor suppressive or oncogenic activity [7, 8]. The number of studies regarding the relationship between adult cancers and miRNAs is gradually increasing; however, there are still a relatively low number of studies regarding childhood malignancies. Moreover, there have been even fewer studies focusing specifically on the association of miRNAs with childhood ALL [9–15]. The aim of the present study is to identify the relevant miRNAs in the diagnosis of childhood ALL and evaluate their effect in the course of the disease.
2. Materials and Methods
2.1. Patients
A total of 45 children with newly diagnosed and untreated childhood ALL and 15 age-matched control subjects (normal peripheral blood (PB) and bone marrow (BM) smears) were enrolled in the study. Patients were consecutively included from the inpatient oncology and hematology departments of 3 hospitals. Previously diagnosed leukemia cases were excluded from the study as the miRNA expression levels might have been altered as a result of previous treatment. ALL diagnoses were confirmed via a BM aspirate showing at least 30% blast cells, in accordance with the FAB classification. All patients were diagnosed according to standard morphological, cytochemical, and immunophenotypic criteria. Patients were treated primarily with Berlin-Frankfurt-Munster- (BFM-) based national ALL protocol. This protocol was modified slightly with regard to methotrexate dosing and cranial irradiation. Following the BFM-ALL 1995 protocol, risk groups were categorized as standard (SRG), intermediate (IRG), and high (HRG) risk groups based on their age, leukocyte count, immunophenotyping, cytogenetic changes, early response to prednisone therapy, and BM remission. Patients received four drug induction regimens consisting of prednisone, asparaginase, vincristine, and daunorubicin. Complete remission (CR) was defined as a normocellular marrow with less than 5% blast cells. Patients' characteristics, such as age, sex, white blood cell (WBC) count, FAB classification, and treatment response, are available in Table 1.
Table 1.
Demographics, laboratory results, and response to the treatment in ALL patients.
| Sex | Female n (%) | 21 (48.8) |
| Male n (%) | 22 (51.2) | |
| Mean age (year old) | 6.8 ± 4.5 | |
| White blood cells | Mean (mm3) | 47.930 |
| Range (mm3) | 487–474.000 | |
| Subtype | T-lineage: 9 | |
| B-lineage: 34 | ||
| Blast BM | Mean (%) | 85 |
| Range (%) | 52–100 | |
| Blast PB | Mean (%) | 51 |
| Range (%) | 2–100 | |
|
| ||
| Characteristic | Status | n (%) |
|
| ||
| Risk group | Standard risk | 13 (30) |
| Intermediate risk | 20 (47) | |
| High risk | 10 (23) | |
| Steroid response | Good | 36 |
| Poor | 7 | |
| Response at 33 day | Remission | 43 |
| Not remission | 0 | |
| Survival | Alive | 39 |
| Dead | 4 | |
| Relapse | Yes | 0 |
| No | 39 | |
| Dead | 4 | |
ALL: acute lymphoblastic leukemia, BM: bone marrow, PB: peripheral blood.
2.2. Study Design
The cases were evaluated prospectively. The enrollment period for each patient in the study was 18 months, plus 2 years of followup. MicroRNA microarray profiling was performed on all patients at day 0 and in control cases. The significant miRNAs were confirmed by real time RT-PCR. Six months after treatment, significantly dysregulated and validated miRNAs at day 0 were again analyzed by real time RT-PCR. The change in miRNA levels over time was determined by comparing the ratio at day 0 with the ratio of those measured at 6 months. During this period, 4 patients died. Among our study population, 3 cases (2 from patient group and 1 from control group) were also excluded following RNA isolation process, due to insufficient signalization during microarray work. The significance of miRNAs that were obtained from PB samples was compared with BM samples. Aberrant miRNA expressions associated with the diagnosis, differential diagnosis, and outcome of ALL were evaluated.
2.3. RNA Extraction and Microarray Study
RNA isolation was performed on each BM and peripheral blood (PB) samples obtained from both patient and control groups. Total RNA was isolated by using Qiazol, which was then followed by miRNeasy Mini Kit (Qiagen, Valencia, USA) as per the manufacturer's instructions. Genome wide microRNA microarray profiling was performed by using a microRNA biochip platform (Febit, Heidelberg, Germany). The platform consisted of 1136 microRNA probes (Sanger, miRBase 12.0). In short, 0.7 micrograms of microRNA was labelled using miRVANA labeling kit (Ambion, USA) and then dried via SpeedVac (Thermo, Germany). Dried samples were then treated with 18 microL of hybridization buffer (Febit Biomed GmbH, Heidelberg, Germany) and placed into the biochip platform overnight. Following hybridization and washing, signals were measured. The signal enhancement procedure was processed with Geniom Real Time Analyzer (GRTA) and detection pictures were evaluated by using Geniom Wizard software. The signal intensities for all miRNAs were extracted from the raw data for each array. After background correction, the median signal intensity of the seven replicate intensity values of each miRNA was obtained. Normalization was conducted by using the freely available R software (http://www.R-project.org/).
The RNA quality control was determined by using the NanoDrop ND-1000 in which the ratios of 230/260 and 260/280 were 2. The RNA integrity number was determined by using Agilent 2100 Bioanalyzer (Agilent Technologies) and was ≥7.
The array data including raw data has been deposited in Gene Expression Omnibus (GEO) with the accession number GSE56489.
2.4. Quantitative Real Time Polymerase Chain Reaction and Validation
Significantly dysregulated miRNAs were validated by Quantitative PCR (LightCycler 480, Roche Applied Science, Mannheim, Germany) after comparing the BM microarray miRNA expressions of patients with the controls. RNA was reverse-transcribed to cDNA by using a cDNA synthesis kit (Exiqon). For miRNA quantification, the miRCURY LNA Universal RT microRNA PCR system (Exiqon) was used in combination with the predesigned primers (Exiqon). A master mix was designed for each primer set in accordance with the recommendations of the real time RT-PCR setup for “individual assays,” suggested in the kit. The reaction conditions consisted of polymerase activation/denaturation at 95°C for 10 min. For miRNA quantification, 40 amplification cycles at 95°C for 10 sec and 60°C for 1 min were performed; this was then followed by signal detection. The Delta-Delta-Ct algorithm was used to determine relative gene expression, and SNORD48 and U6 were used for housekeeping genes.
2.5. Statistical Analysis
According to mean values, only those miRNAs whose “fold change” value demonstrated ±2-fold or more expression difference were included in the study. miRNAs whose “False Discovery Rate” (FDR) corrected P value <0.05 were considered significant. FDR was determined by CLC Main Workbench 5 (CLC Bio, Denmark). Heat map and cluster analysis were performed for grouping of the miRNA data. Student's t-test was used to make comparisons in different groups by CLC Main Workbench.
2.6. Consent
This study was approved by the Local Ethics Committee, and written consent was taken from all parents of children that participated in the study.
3. Results
The patient group consisted of 43 cases with ALL in which 34 cases (79%) were B-lineage, and 9 cases (21%) were T-lineage ALL. The mean age in the ALL group was 6.8 ± 4.5. Fourteen cases constituted the control group and the mean age was 6.6 ± 5.1. The ratio of female/male was calculated 0.9 in ALL and 0.8 in the control group (Table 1). The distribution of ALL cases, according to the risk groups, was as follows: 13 cases (30%) were in the SRG, 20 cases (47%) were in the IRG, and 10 cases (23%) were in the HRG. Upon followup, 4 ALL patients (9%) (2 cases with SRG B-lineage ALL, 2 cases with HRG B-lineage ALL) died due to infectious complications (Table 1). Risk groups and response to the treatment of ALL cases are summarized in Table 1.
3.1. miRNA Analysis
miRNAs from PB samples were not correlated with BM samples. Significant miRNAs in PB of patients when compared to control cases were totally different to miRNAs in BM. Therefore, all analysis and considerations in different categories were made by using BM samples. We propose using BM samples for the miRNA analysis in hematological malignancies because they reflect the leukemic process more efficiently, when compared to PB. With reference to the controls' (n = 14), significantly dysregulated miRNAs in BM samples according to the microarray study (n = 43) are shown in Table 2. A total of 13 miRNAs (miR-548i, miR-708, miR-181b, miR-449a, miR-146a, miR-155, miR-181a, miR-3121, miR-181a, miR-128, miR-1323, miR-195, and miR-587) showed upregulation and 2 miRNAs (miR-640, miR-145) showed downregulation. Heat map and cluster analysis in ALL patients and control cases (FDR P value <0.05) were given in Figure 1. Confirmation analysis of significantly dysregulated miRNAs in the patient group by real time RT-PCR revealed 5 upregulated miRNAs (miR-128, miR-146a, miR-155, miR-181a, and miR-195) in ALL (Table 3). Following 6 months of treatment, the level of miR-146a, miR-155, miR-181a, and miR-195 which were found to be upregulated at diagnosis and validated by real time RT-PCR decreased significantly (Table 3). The change in expression level of miR-128, though decreased by 50%, was not significant throughout this period. The expression change of these relevant miRNAs was also presented in Figure 2.
Table 2.
Significant miRNA profile compared to control cases in the microarray study and validation results in real time RT-PCR. According to mean values, only those miRNAs whose “fold change” value demonstrated ± 2-fold or more expression difference were included in the study. miRNAs whose “False Discovery Rate” (FDR) corrected P value <0.05 were considered significant. FDR is calculated by using CLC Main Workbench 5 (CLC Bio, Denmark). A total of 13 miRNAs showed upregulation (shown in bold) and 2 miRNAs showed downregulation.
| miRNA | Microarray | High/Low Expression (n)* | RT-PCR | High/Low Expression (n)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ALL | Ratio | P value | FDR P | Control | ALL | log2RR | P | |||
| hsa-miR-548i | 1.84 | 22.12 | 12.50 | 3.90E − 03 | 0.03 | 23/0 | 0.000112 | 0.007942 | 6.15 | 0.590 | 21/0 |
| hsa-miR-708 | 46.55 | 488.77 | 10.00 | 6.55E − 04 | 0.01 | 21/7 | 0.002949 | 19.01167 | 12.65 | 0.291 | 28/3 |
| hsa-miR-181b | 72.46 | 467.47 | 6.25 | 7.09E − 06 | 3.83E − 04 | 36/3 | 0.0468 | 48.42431 | 10.02 | 0.251 | 33/2 |
| hsa-miR-449a | 6.66 | 23.84 | 3.57 | 2.69E − 03 | 0.03 | 14/0 | 0.003653 | 0.013143 | 1.85 | 0.163 | 7/0 |
| hsa-miR-146a | 326.65 | 1135.73 | 3.45 | 8.31E − 05 | 1.79E − 03 | 35/2 | 0.068189 | 24.37251 | 8.48 | 0.008 | 35/1 |
| hsa-miR-155 | 170.68 | 515.25 | 3.03 | 7.93E − 05 | 1.79E − 03 | 32/2 | 0.063027 | 31.66987 | 8.97 | 0.009 | 34/0 |
| hsa-miR-181a* | 104.93 | 298.17 | 2.86 | 6.54E − 03 | 0.04 | 25/4 | 0.012341 | 0.766604 | 5.96 | 0.123 | 35/2 |
| hsa-miR-3121 | 12.04 | 33.64 | 2.78 | 6.95E − 03 | 0.04 | 21/0 | 0.023803 | 0.001278 | −4.22 | 0.209 | 0/0 |
| hsa-miR-181a | 661.35 | 1810.68 | 2.70 | 3.66E − 03 | 0.03 | 29/7 | 0.770556 | 80.63069 | 6.71 | 0.002 | 34/2 |
| hsa-miR-128 | 720.72 | 1713.49 | 2.38 | 2.67E − 06 | 2.88E − 04 | 30/0 | 0.245556 | 11.3962 | 5.54 | 0.009 | 33/4 |
| hsa-miR-1323 | 34.21 | 81.96 | 2.38 | 1.05E − 03 | 0.01 | 25/2 | 0.000686 | 0.000024 | −4.84 | 0.199 | 0/0 |
| hsa-miR-195 | 962.76 | 2251.43 | 2.33 | 9.67E − 04 | 0.01 | 28/4 | 0.012433 | 1.250504 | 6.65 | <0.001 | 32/3 |
| hsa-miR-587 | 33.83 | 69.93 | 2.08 | 5.67E − 03 | 0.04 | 17/4 | 0.000523 | 0.000146 | −1.84 | 0.326 | 1/0 |
| hsa-miR-640 | 91.53 | 45.07 | −2.03 | 7.18E − 03 | 0.04 | 3/30 | 0.050227 | 0.000498 | −6.66 | 0.191 | 0/0 |
| hsa-miR-145 | 498.27 | 197.73 | −2.52 | 8.09E − 05 | 1.79E − 03 | 2/35 | 1.201444 | 14.55961 | 3.60 | 0.440 | 26/10 |
FDR: false discovery rate, ALL: acute lymphoblastic leukemia.
*“High/Low Expression (n)” means the number of cases in which particular miRNA expression is over the normal range. Normal range was accepted for the 95% CI of control cases.
Figure 1.
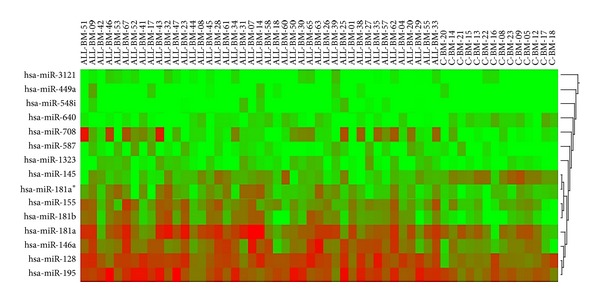
Heat map and cluster analysis in ALL patients and control cases (FDR P value <0.05). The figure shows the relative expression of bone marrow (ALL-BM) miRNAs in ALL patients and control cases (c-BM). miRNAs are in columns representing the 15 miRNAs, samples in rows representing 43 ALL patients and 14 control cases. The color scale shown on the top illustrates the expression level of the indicated miRNA across all samples: red means that a miRNA expression value is higher than its average expression across all samples (upregulated), and green means a lower expression value (downregulated).
Table 3.
Significant miRNAs after validation by real time RT-PCR at day 0 were given. Those miRNAs were reevaluated after 6 months of treatment and the expression change of all miRNAs except miR-128 was significant.
| miRNA | RR | |
|---|---|---|
| Validated by RT-PCR (at diagnosis) | hsa-miR-128 | 11.396200 |
| hsa-miR-146a | 24.372519 | |
| hsa-miR-155 | 31.669875 | |
| hsa-miR-181a | 80.630694 | |
| hsa-miR-195 | 1.250504 | |
|
| ||
| Real time RT-PCR (after 6 months) | hsa-miR-128 | 6.073660 |
| hsa-miR-146a | 0.334798 | |
| hsa-miR-155 | 1.201844 | |
| hsa-miR-181a | 4.987600 | |
| hsa-miR-195 | 0.206393 | |
RR: relative ratio.
Figure 2.
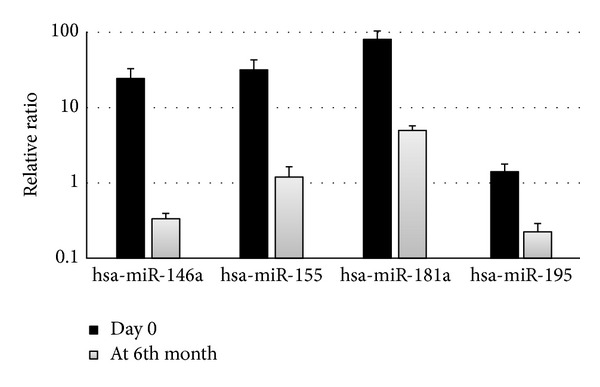
Diagram of significantly changed miRNAs in ALL patients after 6 months of treatment. The figure shows the change in expression levels of 4 significantly dysregulated miRNAs after 6 months of treatment. The change in miRNA levels over time was determined by comparing the ratio at day 0 with the ratio of those measured at 6 months by using quantitative real time RT-PCR.
The most significantly upregulated miRNAs and their fold changes in T-lineage ALL and B-lineage ALL are summarized in supplemental Table 1 (see Supplementary Table 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2014/967585). miR-548i, miR-3140, and miR-181b in T-lineage ALL and miR-708, miR-181b, and miR-369-3p in B-lineage ALL were the most distinctive miRNAs among all.
4. Discussion
In this study a total of 1136 miRNAs were studied in children with ALL using microarray platform to reveal their contribution with regard to diagnosis, classification, and treatment period. Microarray study revealed 15 miRNAs dysregulated, when compared to the control cases. The results of real time RT-PCR for 5 miRNAs (miR-128, miR-146a, miR-155, miR-181a, and miR-195) were consistent with our microarray results; however, the other 10 miRNAs (miR-548i, miR-708, miR-181b, miR-369-3p, miR-449a, miR-3121, miR-181a, miR-1323, miR-587, and miR-181a-2) contradicted the results of our microarray study. This discrepancy may be due to the small sample size (n = 43) used for microarray assay [16]. Array platforms are specifically used for high throughput studies to define significantly dysregulated miRNAs and involve background correction, normalization, and summarization, from which it is recommended that the results be confirmed via other methods. Real time RT-PCR is accepted as a “gold standard” for the confirmation of array platforms [17]; therefore, our data is based on RT-PCR results. After 6 months of follow-up period following chemotherapy, miRNA expression profiles were reevaluated, and their potential involvement in cancer biology was assessed. As presented in Table 3 and shown in Figure 2, the level of all oncogenic miRNAs except miR-128 which were confirmed by real time RT-PCR significantly decreased. This data has not been reported in the literature.
miR-155 is an oncogenic miRNA which has been shown to be dysregulated in many studies and is suggested as a putative prognostic factor [18, 19]. After 6 months of followup including chemotherapy, it was found to be downregulated in our study. It can be speculated that this result is the reflection of the fact that after 6 months, blasts which overexpress miR-155 have disappeared. Although miR-155 is a potent inhibitor of myeloid differentiation and was found to be upregulated particularly in AML patients [20, 21], it has been reported that this miRNA is also an important factor in lymphopoiesis and immune response [22–24]. The study by Wang et al. has shown that miR-155 was expressed at a significantly higher level in ALL than in AML [25]; therefore, it can be speculated that, in view of our data combined with the literature, miR-155 could be used as a valuable biomarker in the diagnosis and maintenance of ALL.
miR-146 has been reported to have a role in innate immunity by downregulating TRAF6 and IRAK1 genes, and miR-146a is regulated by NF-kappa [26, 27]. Combined with miR-155 and miR-181a, which were found to be overexpressed in our study, those 3 miRNAs are associated with genes involved in innate immunity and inflammation, including TLR4, TLR8, IRF8, and IL6R [28]. In the light of the information reported in the literature, altered expression of miR-146a, miR-155, and miR-181a could be suggested as additional factors which may lead to leukemia development by causing deleterious effect on normal immune function [29, 30]. miR-181a has been reported to have a role in the development and in the differentiation of both B cells and cytotoxic T cells [31, 32]. It has also been shown that miR-181a repressed the expression of genes which play a role in thymocyte maturation, such as Bcl-2, CD69, and the T-cell receptor, and the members of the miR-181 family were found to be significantly overexpressed in T-cell leukemia in our study. These findings support the suggestion that future studies should focus on miR-181 family in the management of ALL.
Studies have shown that miR-195 has a regulatory function in the cell cycle and cell proliferation [33, 34]. It is expressed in many cancers including chronic lymphocytic leukemia which was one of the 5 most expressed in our patients [35]. However, there is no study in the literature regarding the impact of this miRNA on acute leukemia patients.
miR-128 together with let-7b and miR-223 was found to be the most discriminatory miRNAs involving ALL and AML [36, 37]. miR-128 was found to be expressed at a significantly higher level in ALL than in AML; therefore, in the light of our results, miR-128 can be used to diagnose and discriminate ALL and AML cases accurately.
Some miRNAs are already known to be related to specific subtypes of pediatric ALL. As reported by Schotte et al., genetic subtypes such as MLL-rearranged, TEL-AML1 positive, hyperdiploid, and drug-resistant leukemic cells display characteristic miRNA signatures in pediatric ALL [38]. Fulci et al. have studied 470 miRNAs in adult ALL patients and identified miRNAs discriminative of the ALL subsets, namely, T-cell and B-cell ALL [39]. miRNAs which were highly discriminative of the different subgroups in this study were not consistent with the results of the present study. The inconsistency between these two studies may be caused by the different age group characteristics. Wang et al. studied 23 miRNAs, which were reported to be associated with hematopoiesis and/or leukemogenesis, to show differentially expressed miRNAs in ALL and AML [25]. Among those identified as differentially expressed miRNAs, miR-128 is remarkable because it also showed significant overexpression in our study.
The limitation of our study lies in the studying of expression levels in specimens containing both normal and blast cells where the results may be affected by the contribution of normal cells. The mean blast cell level in our study, however, was found to be 85% (range: 52–100) meaning this issue may be avoided.
When the age group and its characteristics of childhood leukemia are considered, our data could add important contributions to the literature. The first is the studying of the largest miRNA profile in ALL and the presentation of novel miRNAs associated with leukemogenesis; the second contribution is identifying miRNAs as discriminative of T-lineage versus B-lineage ALL. Moreover, our results confirmed the importance of certain miRNAs such as miR-128, miR-146a, and miR-181a in childhood ALL. The final and possibly the most important contribution is the prospective design of our study that we were able to evaluate miRNAs throughout a treatment period.
In conclusion, the discovery of miRNAs and their association with disease have provided valuable information on potential diagnostic and/or prognostic biomarkers, as well as monitoring the disease progression. In our study, miR-128, miR-146a, miR-155, miR-181a, and miR-195 were found to be significantly dysregulated which may help provide new insights into the diagnosis and prognosis of childhood ALL. Further studies, with larger subject numbers, are needed to clearly demonstrate the effect of miRNAs in leukemogenesis and its practical implications.
Supplementary Material
“Significant miRNA profile compared to control cases by microarray study in T-lineage ALL and B-lineage ALL and their fold changes are given. Upregulated miRNAs are shown in bold. No significant miRNA was detected after FDR correction in died ALL patients”
Acknowledgment
The authors would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK) for financial support under Grant 109S076.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
Authors' Contribution
Muhterem Duyu and Burak Durmaz contributed equally to this work.
References
- 1.Robison LL. General principles of the epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 3rd edition. Philadelphia, Pa, USA: Lippincott-Raven; 1997. pp. 1–10. [Google Scholar]
- 2.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112(3):461–472. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 3.Vrooman LM, Silverman LB. Childhood acute lymphoblastic leukemia: update on prognostic factors. Current Opinion in Pediatrics. 2009;21(1):1–8. doi: 10.1097/MOP.0b013e32831f1f24. [DOI] [PubMed] [Google Scholar]
- 4.Kaspers GJL, Zwaan CN. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92(11):1519–1532. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 5.Balaga O, Friedman Y, Linial M. Toward a combinatorial nature of microRNA regulation in human cells. Nucleic Acids Research. 2012;40:9404–9416. doi: 10.1093/nar/gks759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Shi W, Wu C, Ju J, Jiang J. miR-181b as a key regulator of the oncogenic process and its clinical implications in cancer. Biomedical Reports. 2014;2:7–11. doi: 10.3892/br.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Zhang KY, Liu SM, Sen S. Tumor-associated circulating microRNAs as biomarkers of cancer. Molecules. 2014;19:1912–1938. doi: 10.3390/molecules19021912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Zhang Q, Deng M, et al. An analysis of human microRNA and disease associations. PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003420.e3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Yang J-H, Zheng Y-S, et al. Genome-wide analysis of small RNA and novel microRNA discovery in human acute lymphoblastic leukemia based on extensive sequencing approach. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0006849.e6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schotte D, Chau JCK, Sylvester G, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23(2):313–322. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Liang YN, Luo XQ, Liu XD, Guo HX. Association of miRNAs expression profiles with prognosis and relapse in childhood acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2011;32:178–181. [PubMed] [Google Scholar]
- 12.Han B-W, Feng D-D, Li Z-G, et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Human Molecular Genetics. 2011;20(24):4903–4915. doi: 10.1093/hmg/ddr428.ddr428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Oliveira JC, Scrideli CA, Brassesco MS, et al. Differential MiRNA expression in childhood acute lymphoblastic leukemia and association with clinical and biological features. Leukemia Research. 2012;36(3):293–298. doi: 10.1016/j.leukres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez-Camino A, Lopez-Lopez E, Martin-Guerrero I, et al. Non-coding RNAs-related polymorphisms in pediatric acute lymphoblastic leukemia susceptibility. Pediatric Research. 2014 doi: 10.1038/pr.2014.43. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Li D, Zhuang Y, Shi Q, Wei W, Ju X. Overexpression of miR-708 and its targets in the childhood common precursor B-cell ALL. Pediatric Blood & Cancer. 2013;60:2060–2067. doi: 10.1002/pbc.24583. [DOI] [PubMed] [Google Scholar]
- 16.Gu H, Li H, Zhang L, et al. Diagnostic role of microRNA expression profile in the serum of pregnant women with fetuses with neural tube defects. Journal of Neurochemistry. 2012;122:641–649. doi: 10.1111/j.1471-4159.2012.07812.x. [DOI] [PubMed] [Google Scholar]
- 17.Git A, Dvinge H, Salmon-Divon M, et al. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA. 2010;16(5):991–1006. doi: 10.1261/rna.1947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cammarata G, Augugliaro L, Salemi D, et al. Differential expression of specific microRNA and their targets in acute myeloid leukemia. The American Journal of Hematology. 2010;85(5):331–339. doi: 10.1002/ajh.21667. [DOI] [PubMed] [Google Scholar]
- 19.Xue H, Hua LM, Luo JM. Relationship between microRNA-155 and hematological malignancies. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:810–814. doi: 10.7534/j.issn.1009-2137.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 20.Dixon-McIver A, East P, Mein CA, et al. Distinctive patterns of microRNA expression associated with karyotype in acute myeloid leukaemia. PLoS ONE. 2008;3(5) doi: 10.1371/journal.pone.0002141.e2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcucci G, Radmacher MD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. The New England Journal of Medicine. 2008;358(18):1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 22.Vigorito E, Perks KL, Abreu-Goodger C, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27(6):847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thai T-H, Calado DP, Casola S, et al. Regulation of the germinal center response by MicroRNA-155. Science. 2007;316(5824):604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Li Z, He C, et al. MicroRNAs expression signatures are associated with lineage and survival in acute leukemias. Blood Cells, Molecules, and Diseases. 2010;44(3):191–197. doi: 10.1016/j.bcmd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justiniano SE, Elavazhagan S, Fatehchand K, et al. Fcγ receptor-induced soluble vascular endothelial growth factor receptor-1 (VEGFR-1) production inhibits angiogenesis and enhances efficacy of anti-tumor antibodies. Journal of Biological Chemistry. 2013;288:26800–26809. doi: 10.1074/jbc.M113.485185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havelange V, Stauffer N, Heaphy CCE, et al. Functional implications of microRNAs in acute myeloid leukemia by integrating microRNA and messenger RNA expression profiling. Cancer. 2011;117(20):4696–4706. doi: 10.1002/cncr.26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113(20):4914–4917. doi: 10.1182/blood-2008-11-189845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costinean S, Zanesi N, Pekarsky Y, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eμ-miR155 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Seminars in Cancer Biology. 2008;18(2):131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Fragoso R, Mao T, Wang S, et al. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genetics. 2012;8 doi: 10.1371/journal.pgen.1002855.e1002855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J-F, Luo Y-M, Wan X-H, Jiang D. Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle, and apoptosis. Journal of Biochemical and Molecular Toxicology. 2011;25(6):404–408. doi: 10.1002/jbt.20396. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharya A, Schmitz U, Wolkenhauer O, Schönherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene. 2013;32:3175–3183. doi: 10.1038/onc.2012.324. [DOI] [PubMed] [Google Scholar]
- 35.Zanette DL, Rivadavia F, Molfetta GA, et al. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Brazilian Journal of Medical and Biological Research. 2007;40(11):1435–1440. doi: 10.1590/s0100-879x2007001100003. [DOI] [PubMed] [Google Scholar]
- 36.Mi S, Lu J, Sun M, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):19971–19976. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu YD, Wang L, Sun C, et al. Distinctive microRNA signature is associated with the diagnosis and prognosis of acute leukemia. Medical Oncology. 2012;29:2323–2331. doi: 10.1007/s12032-011-0140-5. [DOI] [PubMed] [Google Scholar]
- 38.Schotte D, Lange-Turenhout EAM, Stumpel DJPM, et al. Expression of miR-196b is not exclusively MLL-driven but is especially linked to activation of HOXA genes in pediatric acute lymphoblastic leukemia. Haematologica. 2010;95(10):1675–1682. doi: 10.3324/haematol.2010.023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulci V, Colombo T, Chiaretti S, et al. Characterization of B- and T-lineage acute lymphoblastic leukemia by integrated analysis of microRNA and mRNA expression profiles. Genes Chromosomes and Cancer. 2009;48(12):1069–1082. doi: 10.1002/gcc.20709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Significant miRNA profile compared to control cases by microarray study in T-lineage ALL and B-lineage ALL and their fold changes are given. Upregulated miRNAs are shown in bold. No significant miRNA was detected after FDR correction in died ALL patients”


