Abstract
The activated B cell-like (ABC) subtype of diffuse large B cell lymphoma (DLBCL) is an aggressive lymphoma that is addicted to NF-κB signaling through the CARD11-BCL10-MALT1 complex. In this issue of Cancer Cell, two reports describe MALT1 inhibitors suitable for clinical use that are selectively toxic to this malignancy.
Aberrant activation of NF-κB is a feature shared by many human lymphomas due to the ability of NF-κB to promote tumor survival. In particular, constitutive NF-κB activity is a hallmark of the ABC DLBCL subtype. This DLBCL subtype is the most recalcitrant to current immunochemotherapy regimens, in part due to the anti-apoptotic properties of NF-κB activity. Hence, targeted therapeutic agents that shutdown NF-κB in ABC DLBCL are urgently needed.
ABC DLBCL tumors subvert normal B cell signaling pathways to activate NF-κB by acquiring somatic mutations that activate and/or amplify their signaling output (Figure 1). An RNA interference screen identified a central role for CARD11, BCL10 and MALT1 in the pathogenesis of ABC DLBCL cell lines (Ngo et al., 2006). These three signaling effectors form the “CBM” complex, which serves as a signaling scaffold that recruits TRAF6, TAK1 and the IKK complex to activate the IκB kinase β (IKKβ) and stimulate NF-κB through the “classical” pathway. In 10% of ABC DLBCL cases, somatic mutations affecting the coiled-coil domain of CARD11 spontaneously induce the formation of the CBM complex and NF-κB activity (Lenz et al., 2008). Other ABC DLBCL lymphomas lack CARD11 mutations but nevertheless rely upon wild type CARD11 fo activate NF-κB and sustain survival (Ngo et al., 2006). These ABC DLBCL tumors rely upon a “chronic active” form of BCR signaling to engage CARD11 and the NF-κB pathway (Davis et al., 2010). ABC DLBCLs with wild type CARD11 die upon knockdown or pharmacologic inhibition of any component of the BCR signaling cascade (Davis et al., 2010). Recurrent mutations in the BCR subunits CD79B and CD79A occur in roughly one fifth of ABC DLBCL cases that serve to amplify BCR signaling, providing genetic evidence that chronic active BCR signaling is important in ABC DLBCL pathogenesis (Davis et al., 2010).
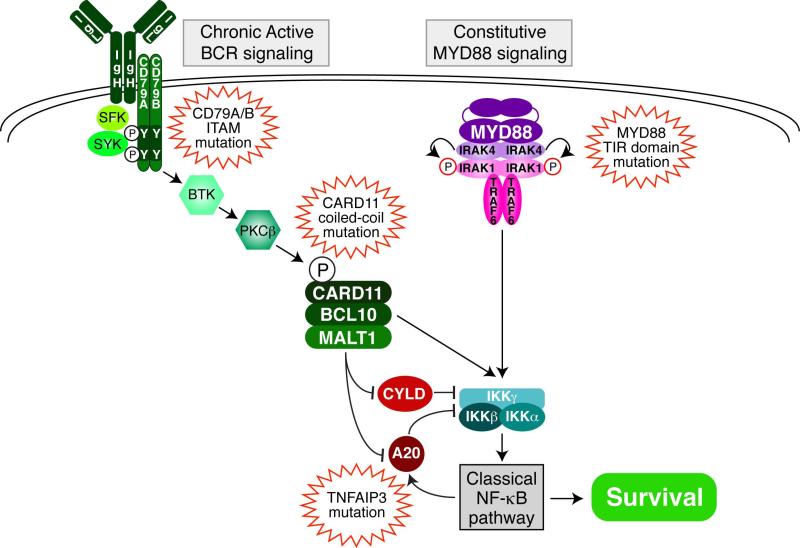
Figure 1.
Role of MALT1 in signaling to NF-κB in ABC DLBCL. Shown are two prominent pathways leading to NF-κB activation in ABC DLBCL: chronic active BCR signaling and constitutive MYD88 signaling. MALT1 plays a key role in the BCR pathway in two ways. First, as a component of the CBM complex with CARD11 and BCL10, MALT1 helps recruit and activate IκB kinase (IKK). Second, MALT1 protease activity potentiates NF-κB signaling by cleaving and inactivating two negative regulators of IKK, A20 (TNFAIP3) and CYLD. Shown are recurrent mutations in ABC DLBCL tumors that cause or intensify NF-κB activity.
BCR pathway inhibitors are currently being investigated in clinical trials to evaluate their efficacy against ABC DLBCL and other forms of B cell lymphoma. These inhibitors chiefly target either BCR proximal kinases, such as BTK and SYK, or the phosphatidylinositol 3-kinase pathway that emanates from the BCR. Promising responses have been observed, including complete and partial responses to the BTK inhibitor ibrutinib in ABC DLBCL. However, experiments in cell lines suggest that these upstream BCR pathway inhibitors will be unable to treat tumors that harbor oncogenic CARD11 mutations, necessitating alternative therapies for these patients.
The recently described proteolytic activity of MALT1 provides a new target for therapeutic development (reviewed in (McAllister-Lucas et al., 2011)). The caspase-like domain of MALT1 cleaves substrates following arginine residues, unlike conventional caspase that cleave after aspartate residues. MALT1 cleaves and disables A20 (TNFAIP3) and CYLD, both negative regulators of NF-κB, thereby potentiating NF-κB signaling. Based on these results in normal lymphocytes, two groups demonstrated that MALT protease activity is required for NF-κB activity and survival of ABC DLBCL cells (Ferch et al., 2009; Hailfinger et al., 2009). A peptide inhibitor of MALT1 paracaspase activity was toxic to ABC DLBCL cell lines, but not to models of other lymphoma subtypes. In theory, MALT1 should make an excellent therapeutic target. First, MALT1 knockout mice are defective in T and B cell activation but are otherwise healthy. Second, the paracaspase domain of MALT1 is unique within the human genome, suggesting that a MALT1 inhibitor might not cause significant off-target side effects.
In this issue of Cancer Cell, two groups report the discovery of small molecule inhibitors of MALT1 that represent a new class of lymphoma therapeutics. Both studies utilized in vitro MALT1 protease assays in high-throughput screens of small molecule libraries, yielding inhibitors of MALT1 activity at low micromolar concentrations in vitro. In a library of drugs approved for human use, Nagel et al. identified three phenothiazines, a class of anti-psychotic drugs, that inhibit MALT1 paracaspase activity and kill ABC DLBCL cells (Nagel, 2012). The doses necessary to inhibit growth of ABC DLBCL xenografts were equivalent to those achieved when humans are given these drugs, suggesting that they could be used off-label in clinical trials soon. While phenothizines may be tolerated for short term chemotherapy, their long-term use will be limited by the already known side effects characteristic of this drug class, for example tardive dyskinesia. Fontan et al. discovered a novel small molecule, termed MI-2, that notably inhibited MALT1 by forming a covalent linkage in the active site (Fontan, 2012). Although traditional drug development has shyed away from irreversible inhibitors because of potential crossreactivity and immunogenicity, they afford outstanding pharmacodynamic properties. Indeed, the Hepatitis C NS3/4A protease inhibitor Telaprevir and the proteasome inhibitor Carfilzomib are both irreversible. Likewise, the unusual potency of ibrutinib in many lymphoid malignancies may be due to its covalent attachment to BTK. While MI-2 is a lead compound that may require further optimization, it is notable that mice treated with MI-2 did not have detectable physiological, histological or biochemical signs of toxicity.
Clinical trials with correlative studies will be needed to determine the lymphoma phenotypes and genotypes that are best suited to MALT1-directed therapy. While the BCR pathway inhibitors ibrutinib is showing promising activity in clinical trials, it is too early to know what mechanisms of resistance may develop. From this perspective, we cannot really have too many targeted therapies, especially ones that have few if any side effects. An important niche not addressed by the BCR pathway inhibitors would be the ~10% of ABC DLBCL tumors with CARD11 mutations. MALT1 inhibitors might potentially also be useful in some cases of germinal center B cell-like (GCB) subtype of DLBCL, since CARD11 mutations occur in 5-10% of these tumors and are associated with elevated NF-κB activity compared to the majority of GCB DLBCLs (Lenz et al., 2008). Gastric MALT lymphomas with a t(11;18) translocation may be another venue since this translocation creates a fusion oncoprotein composed of protein interaction domains from c-IAP2 and the MALT1 paracaspase domain that is proteolytically active (McAllister-Lucas et al., 2011). Germ line CARD11 coiled-coil domain mutations have recently been identified in families with a congenital B lymphocytosis, which can progress to chronic lymphocytic leukemia; MALT1 inhibitors could prove useful in this setting if they can be tolerated long-term without side effects (Snow et al., 2012). Finally, it should be noted that a second prominent signaling pathway can activate NF-κB in ABC DLBCL via the signaling adapter MYD88. Recurrent somatic mutations of MYD88 occur in 39% of ABC DLBCLs, with one particularly potent point mutant, MYD88 L265P, occuring in 29% of cases (Ngo et al., 2011). Some ABC DLBCLs have both MYD88 L265P and CD79B mutations (Ngo et al., 2011), and cell line models of such cases rely on MALT1 for survival (Fontan, 2012). However, other ABC DLBCLs only have MYD88 mutations and are not dependent upon BCR signaling (Ngo et al., 2011), and these do not respond to MALT1 inhibition (Fontan, 2012). Thus, the optimum deployment of MALT1 inhibitors awaits a precise definition of which ABC DLBCL tumors rely upon BCR signaling through MALT1 for survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferch U, Kloo B, Gewies A, Pfander V, Duwel M, Peschel C, Krappmann D, Ruland J. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2009;206:2313–2320. doi: 10.1084/jem.20091167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan L. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 2012 doi: 10.1016/j.ccr.2012.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailfinger S, Lenz G, Ngo V, Posvitz-Fejfar A, Rebeaud F, Guzzardi M, Penas EM, Dierlamm J, Chan WC, Staudt LM, Thome M. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2009;106:19946–19951. doi: 10.1073/pnas.0907511106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas LM, Baens M, Lucas PC. MALT1 protease: a new therapeutic target in B lymphoma and beyond? Clin Cancer Res. 2011;17:6623–6631. doi: 10.1158/1078-0432.CCR-11-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel D. Pharmacological inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell. 2012 doi: 10.1016/j.ccr.2012.11.002. in press. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, Staudt LM. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow AL, Xiao W, Stinson JR, Lu W, Chaigne-Delalande B, Zheng L, Pittaluga S, Matthews HF, Schmitz R, Jhavar S, et al. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med. 2012 doi: 10.1084/jem.20120831. [DOI] [PMC free article] [PubMed] [Google Scholar]