Abstract
Ricin, a member of the A-B family of ribosome-inactivating proteins, is classified as a Select Toxin by the Centers for Disease Control and Prevention because of its potential use as a biothreat agent. In an effort to engineer therapeutics for ricin, we recently produced a collection of alpaca-derived, heavy-chain only antibody VH domains (VHH or “nanobody”) specific for ricin’s enzymatic (RTA) and binding (RTB) subunits. We reported that one particular RTB-specific VHH, RTB-B7, when covalently linked via a peptide spacer to different RTA-specific VHHs, resulted in heterodimers like VHH D10/B7 that were capable of passively protecting mice against a lethal dose challenge with ricin. However, RTB-B7 itself, when mixed with ricin at a 1∶10 toxin:antibody ratio did not afford any protection in vivo, even though it had demonstrable toxin-neutralizing activity in vitro. To better define the specific attributes of antibodies associated with ricin neutralization in vitro and in vivo, we undertook a more thorough characterization of RTB-B7. We report that RTB-B7, even at 100-fold molar excess (toxin:antibody) was unable to alter the toxicity of ricin in a mouse model. On the other hand, in two well-established cytotoxicity assays, RTB-B7 neutralized ricin with a 50% inhibitory concentration (IC50) that was equivalent to that of 24B11, a well-characterized and potent RTB-specific murine monoclonal antibody. In fact, RTB-B7 and 24B11 were virtually identical when compared across a series of in vitro assays, including adherence to and neutralization of ricin after the toxin was pre-bound to cell surface receptors. RTB-B7 differed from both 24B11 and VHH D10/B7 in that it was relatively less effective at blocking ricin attachment to receptors on host cells and was not able to form high molecular weight toxin:antibody complexes in solution. Whether either of these activities is important in ricin toxin neutralizing activity in vivo remains to be determined.
Introduction
There are ongoing initiatives to develop countermeasures against ricin, a Select Toxin, as classified by the Centers for Disease Control and Prevention (CDC), and which has been the subject of a number of recent high profile bioterrorism incidents in the United States [1], [2]. Ricin is a glycoprotein derived from the castor bean plant, Ricinus communis, and a member of the medically important family of A-B toxins [3]. Ricin’s enzymatic subunit (RTA) is an RNA N-glycosidase that inactivates eukaryotic ribosomes by catalyzing the hydrolysis of a universally conserved residue within the so-called sarcin/ricin loop (SRL) of 28S rRNA [4], [5]. Ricin’s B subunit (RTB) is a galactose- and N-acetylgalactosamine (Gal/GalNAc)-specific lectin that has two important functions in cytotoxicity. First, RTB promotes ricin attachment and endocytosis of ricin into all mammalian cell types, including epithelial cells, sinusoidal endothelial cells, and macrophages [6], [7]. Second, following endocytosis, RTB mediates the retrograde transport of RTA from the plasma membrane to the trans-Golgi network (TGN) and endoplasmic reticulum (ER), where RTA is liberated from RTB and retro-translocated into the cell cytoplasm [8], [9].
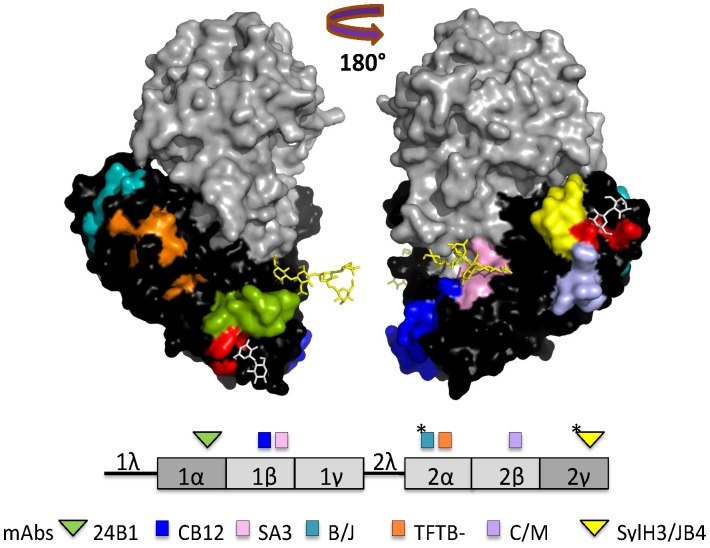
Considering its essential role in toxin uptake and trafficking, RTB is an appealing target for antibody-based therapeutics. Structurally, RTB is composed of two globular domains (1 and 2) each containing three homologous sub-domains (α, β and γ), although only the external sub-domains (1α and 2γ) retain functional carbohydrate recognition activity ( Figure 1 ) [6], [10]. Sub-domain 1α (residues 17–59) is Gal-specific and is considered a “low affinity” carbohydrate recognition domain (CRD), whereas sub-domain 2γ (residues 228–262) binds both Gal and GalNAc and is considered a “high affinity” CRD [11]–[13]. RTB has four intramolecular disulfide bonds, in addition to the single intermolecular disulfide bond that joins it to RTA [14], [15]. Finally, RTB has two N-linked mannose side chains that have been postulated to interact with mannose-binding protein(s) during ricin intracellular transport and/or influence the intracellular stability of RTB [16]–[19].
Figure 1. Previously identified and characterized epitopes on RTB recognized by neutralizing and non-neutralizing mAbs.
X-ray crystal structure of ricin holotoxin visualized using PyMOL and based on PDB file 2AAI [55]. RTA (grey), RTB (black), ricin’s N-linked mannose side chains (yellow sticks) and lactose moieties (white sticks) are shown in the upper panel. Confirmed and putative epitopes (*) recognized by neutralizing (triangles) and non-neutralizing (squares) RTB-specific mAbs are color-coded on the holotoxin structure to match RTB’s linear subdomain organization in the lower panel. This figure was modified from an earlier version [26].
A number of RTB-specific murine monoclonal antibodies (mAbs) have been described in the literature, although only a handful have been shown to have toxin-neutralizing activity [20]–[26]. The two most well characterized mAbs from our laboratory are SylH3 and 24B11. Although SylH3 and 24B11 are each able to neutralize ricin in vitro and in vivo, they apparently do so by different mechanisms [27]–[29]. SylH3 virtually abolishes ricin’s ability to adhere to host cell receptors, and is therefore postulated to neutralize ricin by steric hindrance. 24B11 also affects ricin binding to host cell surfaces, although this activity alone does not fully account for 24B11’s potent toxin-neutralizing activity. Rather, we recently reported that 24B11 is able to associate with ricin in solution or after the toxin has adhered cell surface receptors. Surface-bound 24B11 is subsequently endocytosed as a toxin:antibody complex and interferes with retrograde transport of ricin to the TGN [27]. We have also reported that SylH3 and 24B11 Fab fragments were as effective as the full length IgGs at neutralizing ricin in vitro and in vivo, indicating that in the case of SylH3 and 24B11, neither bivalency nor Fc-domains are necessary determinants of toxin-neutralizing activity [28].
However, the relationships between affinity, avidity, epitope specificity, Fc-mediated effector functions, and toxin-neutralizing activity in vitro and in vivo remains poorly understood in the case of ricin. Defining mechanisms of toxin-neutralizing activity by RTB-specific antibodies has been particularly challenging because only a limited number of conventional murine mAbs besides 24B11 and SylH3 are available [21], [23], [25]–[27], [29]. Moreover, conventional mAbs like 24B11 and SylH3 are not easily reengineered or modified to permit a systematic analysis of the factors that render an antibody effective at neutralizing ricin. Such versatility can only be achieved with single-domain camelid-derived antibodies, referred to as VHHs or simply “nanobodies”, which are small, generally highly stable, and easily expressed in Escherichia coli or on the surface of filamentous bacteriophages like M13 [30]. For example, RTA- and RTB-specific single chain nanobodies were affinity isolated from a phage-displayed semisynthetic llama library and have proven useful for a number of diagnostic applications [31]–[34]. Camelid-derived, single domain antibodies against Shiga toxin, botulinum neurotoxins (BoNT) and Clostridium difficile toxins have also been described [35]–[38].
We recently produced and partially characterized a collection of ricin-specific VHHs from alpacas [39]. We identified 11 unique RTA-specific VHHs and 9 unique RTB-specific VHHs. Among the nine unique RTB-specific VHHs, only one, RTB-B7, had demonstrable toxin-neutralizing activity (TNA) in a Vero cell cytotoxicity assay, although a number of others like RTB-D12 had apparent affinities for ricin that were equal to or less than RTB-B7’s [39]. RTB-B7 was covalently linked via a short peptide spacer (GGGGS)3 to three different RTA-specific VHHs, including RTA-D10, resulting in three different VHH “heterodimers” that each proved capable of passively protecting mice against a lethal dose ricin challenge. We have subsequently characterized two of the three RTA-specific VHH components of the three heterodimers in great detail, including solving the X-ray crystal structures of the VHH monomers in complex with RTA (MJ Rudolph, DJ Vance, J Cheung, MC Franklin, F Burshteyn, MS Cassidy, EN Gary, C Herrera, CB Shoemaker, and NJ Mantis, manuscript in revision). However, very little is known about RTB-B7. It is not known if RTB-B7, at high doses, is able to passively protect mice from ricin toxin, or how RTB-B7 compares to RTB-specific murine mAbs in terms of toxin-neutralizing activity. As only a few RTB-specific toxin-neutralizing antibodies have been identified, we deemed it important to characterize each of them as fully as possible as a means to identify which specific attributes (e.g., affinity, epitope specificity, inhibition of attachment) are critical for antitoxin activity. Therefore, in this study we undertook a detailed in vitro characterization of RTB-B7.
Materials and Methods
2.1 Chemicals, Biological Reagents and Cell Lines
Ricin toxin (Ricinus communis agglutinin II), FITC (Fluorescein isothiocyanate)-labeled ricin, biotinylated ricin toxin, Ricinus communis agglutinin I (RCA-I) and ricin toxin B subunit (RTB) were purchased from Vector Laboratories (Burlingame, CA). Ricin was dialyzed as described [29] against phosphate buffered saline (PBS) at 4°C in 10,000 MW cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL), prior to use in cytotoxicity studies. D-(+)- Lactose, was obtained from J.T. Baker (Center Valley, PA) and asialofetuin (ASF) from Sigma-Aldrich (St. Louis, MO). Goat serum was purchased from Gibco-Invitrogen (Carlsbad, CA). Anti-E-tag Horseradish peroxidase (HRP) conjugated mAb was purchased from Bethyl Laboratories, Inc (Montgomery, TX) and goat-anti-mouse IgG HRP conjugated and streptavidin HRP conjugated were purchased from Fisher Scientific (Pittsburgh, PA). Unless noted otherwise, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Cell lines and cell culture media were obtained from the tissue culture media core facility at the Wadsworth Center. THP-1 cells were grown in RPMI +10% Fetal Bovine Serum (FBS) and Vero cells, a fibroblast-like kidney cell line derived from the African green monkey, were grown in DMEM +10% FBS. All cell lines were maintained in 37°C with 5% CO2 incubators, unless noted otherwise. Single chain camelid antibodies (VHHs) which were E-tagged for ELISA purposes have been previously described [39] ( Table 1 ).
Table 1. Characteristics of RTB-specific VHHs and mAbs used in this study.
| VHH/mAbs | GenBanka | Length (AA) | Verob | THP-1 Prec | THP-1 Postc | Protection |
| TFTB-1d | NA | NA | ND | ND | ND | – |
| 24B11d | NA | NA | 1.2 | 0.25 | 0.25 | + |
| RTB-B7 | KF746034 | 132 | 1.5 | 0.25 | 1.4 | – |
| RTB-D12 | KF746031 | 130 | – | – | 330 | – |
| RTB-D8 | KF746036 | 124 | – | – | 330 | – |
| RTA-G12 | KF746020 | 137 | ND | ND | ND | – |
GenBank accession numbers; bVero cytotoxicity assay performed as described in Materials and Methods; value shown are in nM; cPre-bound and post-bound THP-1 cytotoxicity assays performed in Materials and Methods; values shown are in nM. dAnti-RTB mAbs previously characterized in [26], [29]. Abbreviations: ND, not determined; NA, not applicable.
2.2 Mouse Strains, Animal Care and Ricin Toxin Challenge Studies
Mouse experiments were performed as described [39]. Briefly, groups of female BALB/c mice (5 mice per group) approximately 8–10 weeks of age were purchased from Taconic Labs (Hudson, NY). Animals were housed under conventional, specific pathogen-free conditions and were treated in compliance with the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC) guidelines. For the challenge experiments, mice were injected by the intraperitoneal (i.p.) route on day 0 with pre-mixed ricin toxin (RT; 2 µg per mouse) and the corresponding VHH (RTB-B7 at 20 µg and 100 µg per mouse; RTB-D8 and RTB-D12 at 20 µg per mouse; D10/B7 at 30 µg per mouse) in a final volume of 0.4 mL PBS. Following challenge mice were monitored at least two times per day for overt signs of discomfort, including lethargy, hunching, and failure to resume normal feeding behavior, ruffled fur and absence of grooming. In addition, every 24 h, mice were assessed for the onset of hypoglycemia, a well-established surrogate marker of ricin intoxication. A drop of blood (5 µl) was collected from the lateral tail vein of each animal and blood glucose levels were measured using a hand-held glucometer (Accu-Chek Advantage, Roche, Indianapolis, IN). Mice were euthanized by carbon dioxide (CO2) asphyxiation when they became overtly moribund and/or blood glucose levels fell below 25 mg/dL. At no point in the study were the animals administered analgesics or anesthetics so as not to confound the effects of the antibody treatment. Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software, San Diego, CA). For survival studies, statistical significance was determined using the Log-Rank (Mantel-Cox) test.
2.3 Ethics Statement
Experiments described in this study that involved mice were reviewed and approved by the Wadsworth Center’s IACUC under protocol #13-384. The Wadsworth Center complies with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and was issued assurance number A3183-01. Moreover, the Wadsworth Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Obtaining this voluntary accreditation status reflects that Wadsworth Center’s Animal Care and Use Program meets all of the standards required by law, and goes beyond the standards as it strives to achieve excellence in animal care and use.
2.4 ELISAs for Determining VHHs Specificity
ELISAs were performed as described [29]. Briefly, Nunc Immuno MicroWell 96 well plates from ThermoFisher Scientific (Rochester, NY) were coated overnight with 0.1 µg/well of ricin (15 nM), RTB (29 nM), RCA-I (16 nM) or 0.4 µg/well of ASF (82 nM) all in PBS (pH 7.4). The following day the plates were blocked with 2% PBS-goat serum (pH 7.4) for 2 h, then plates were treated with antibodies (VHHs at 10 µg/mL, 330 nM; 24B11 at 22.75 µg/mL, 151.67 nM; TFTB-1 at 2 µg/mL, 13.33 nM) in 5-or 2-fold serial dilutions or with 0.1 µg/well of bovine serum albumin (BSA) (8 nM) for 1 h. For competition ELISAs, the antibodies were pre-mixed with ricin at 200 µg/mL and incubated for at least 30 min prior to adding to the plates for 1 h. Secondary antibodies, HRP-anti-E-tag, HRP-goat-anti-mouse IgG, or avidin-HRP were incubated for 1 h and developed using SureBlue Peroxidase Substrate, TMB (KPL, Gaithersburg, MD). The reactions were stopped using 1 M phosphoric acid and absorbance was read at 450 nm using the VersaMax Microplate Reader with Softmax Pro 5.2 software (Molecular Devices, Sunnyvale, CA). All samples were performed at least in triplicate.
2.5 Vero Cell Cytotoxicity Assays
Vero cell cytotoxicity assays were performed as described [29]. In brief, Vero cells were trypsinized, adjusted to ∼5 ×104 cells per mL and seeded (100 µl/well) into white bottom 96-well plates (Corning Life Sciences, Corning, NY), and allowed to adhere overnight. Vero cells were then treated with ricin (0.01 µg/mL; 154 pM), ricin:Ab mixtures, or medium alone (negative control) for 2 h at 37°C. Cells were washed to remove non-internalized toxin or ricin:Ab mixtures, and 100 µl of fresh medium was added to the wells. Fresh medium was allowed to incubate for 48 h and cell viability was measured using CellTiter-Glo (Promega, Madison, WI). All samples were performed in quadruplicate and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only.
2.6 THP-1 Cell Cytotoxicity Assays
THP-1 cell cytotoxicity assays were done as described [40]. Briefly, THP-1 cells were spun (5 min at 400×g) and adjusted to ∼5 ×104 cells per mL and seeded (100 µl/well) into clear U-bottom 96-well plates (BD Bioscience, San Jose CA) and allowed to grow overnight. The next day, THP-1 cells were spun to remove medium and were then treated with ricin (0.01 µg/mL; 154 pM), ricin:Ab mixtures, or medium alone for 2 h at 37°C. Cells were then subjected to centrifugation and washed to remove non-internalized toxin or ricin:Ab mixtures. Fresh medium was added to the wells and allowed to incubate for 48 h. Cell viability was determined using CellTiter-Glo after the content of each plate was transferred into white bottom 96-well plates. In order to address post-attachment experiments, THP-1 cells were kept on ice at 4°C, to allow for ricin attachment but prevented internalization of the toxin prior to antibody treatment. The cytotoxicity assay was performed as described above but cells were kept on ice until they were transferred to 37°C for the 48 h incubation period. All samples were performed in quadruplicate and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only.
2.7 Ricin Binding Assays using THP-1 Cells and Flow Cytometry
Ricin binding to cell surfaces was performed as described [26]. In brief, THP-1 cells were collected by gentle, low speed centrifugation (5 min at 400×g). The resulting cell pellets were suspended to ∼5 ×106 cells per mL and then seeded (200 µl/well) into clear U-bottom 96-well plates (BD Bioscience, San Jose CA). FITC-labeled ricin (3 µg/mL) was mixed with Abs or lactose (30 mg/mL) for 30 min on ice in the dark prior to being added to THP-1 cells. Cells were then washed twice with PHEM buffer to remove unbound toxin:Ab complexes and fixed with 4% paraformaldehyde (PFA) in PHEM for 15 min. Ricin binding to the surfaces of THP-1 cells was measured using FACS Calibur flow cytometer (BD Bioscience, San Jose CA). A minimum of 10,000 events was analyzed per sample.
2.8 Analytical Ultracentrifugation (AUC)
Ricin and Ab samples were dialyzed overnight in PBS (pH 7.4) at 4°C in 10,000 MW cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL) prior to being subject to AUC. Sedimentation Velocity (SV) experiments were conducted in a Beckman Optima XL-I analytical ultracentrifuge at 20°C at a rotor speed of 50,000 rpm. Double-sector charcoal-filled epon centerpieces were filled with a sample volume of 400 µl and the reference volume of dialysis buffer was 420 µl. Absorption measurements were made at 280 nm or 230 nm, dependending on the protein concentration. Samples were run in an An-60 Ti four-hole rotor with zero time between scans. Care was taken to have the rotor at thermal equilibrium for an hour before accelerating directly to the speed of the experiment. The data were analyzed by the c(s) method found in SEDFIT [41]. The experimentally calculated sedimentation coefficients were converted to s20,w values within the SEDFIT software and graphed using Origins (OriginLab Corporation, Northampton, MA).
2.9 Statistical Analyses and Modeling Software
Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software, San Diego, CA). The open-source molecular visualization software PyMOL (DeLano Scientific LLC, Palo Alto, CA) was used for modeling of ricin.
Results
3.1 Passive Protection Studies with VHH RTB-B7
We previously reported that three different heterodimeric VHHs, each containing RTB-B7, were capable of passively protecting mice against ricin toxin [39]. However, in that same study we reported that monomeric RTB-B7 (10 µg per mouse; 10∶1 VHH:toxin molar ratio) afforded no benefit to mice in the face of a 10xLD50 ricin challenge. To investigate whether the failure of RTB-B7 to passively protect mice was simply an issue of insufficient antibody, we repeated the passive protection studies using 2-fold (20 µg) and 10-fold (100 µg) higher amounts of RTB-B7. For comparison purposes, two other RTB-specific VHHs were tested in parallel: RTB-D8 and RTB-D12. We chose RTB-D12 because its apparent affinity (EC50) for ricin toxin, as determined by ELISA, is virtually identical to that of RTB-B7’s (0.8 nM versus 0.6 nM, respectively). RTB-D8 was chosen because it binds to ricin with a relatively low affinity (EC50 3.6 nM) and was therefore not expected to provide an in vivo toxin-neutralizing activity. As a positive control for these studies, groups of mice were treated with the VHH heterodimer D10/B7. Following ricin challenge, animals were monitored for a period of 7 days for the onset of hypoglycemia, a well-established surrogate marker of ricin intoxication, as well as mortality.
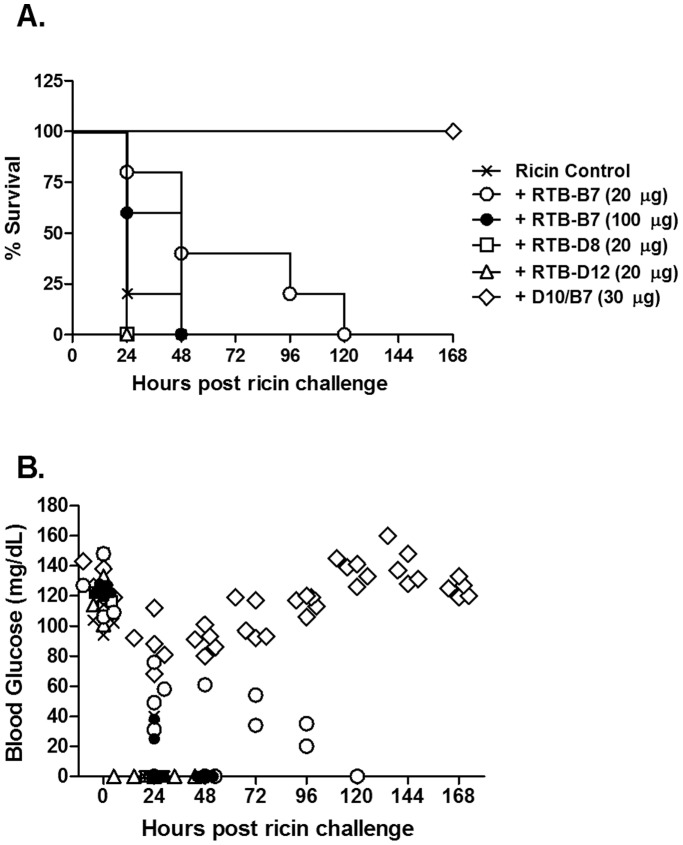
As expected, mice that received the heterodimer D10/B7 (30 µg; 20∶1 VHH heterodimer:toxin molar ratio) survived ricin challenge and experienced only minor declines in blood glucose levels ( Fig. 2 ). Conversely, neither RTB-D8 nor RTB-D12 (each at 20 µg; 20∶1 VHH:toxin molar ratio) afforded any protection against ricin toxin, as evidenced by the fact that the VHH-treated mice experienced a rapid decline in blood glucose levels and died within 24 h ( Fig. 2 ). Surprisingly, even relatively high amounts of RTB-B7 (20 µg and 100 µg, equivalent to 20∶1 and 100∶1 VHH:toxin molar ratio, respectively) had no impact on survival (0/5 mice survived in both groups) following ricin challenge ( Fig. 2 ). Overall, these data demonstrate that RTB-B7, as a monomer, does not elicit protection in vivo, but as a heterodimer (D10/B7) is capable of passively protecting mice against 10xLD50 of ricin.
Figure 2. Monomeric RTB-B7 does not passively protective mice from ricin challenge.
Antibodies D10/B7, RTB-B7, RTB-D8 or RTB-D12 were mixed with ricin (2 µg; equivalent to 10xLD50) and then administered to adult BALB/c mice (n = 5 per group) by i.p. injection. Mice were monitored for seven days for (A) survival and (B) hypoglycemia.
3.2 RTB-B7 Neutralizes Ricin in vitro as Effectively as 24B11
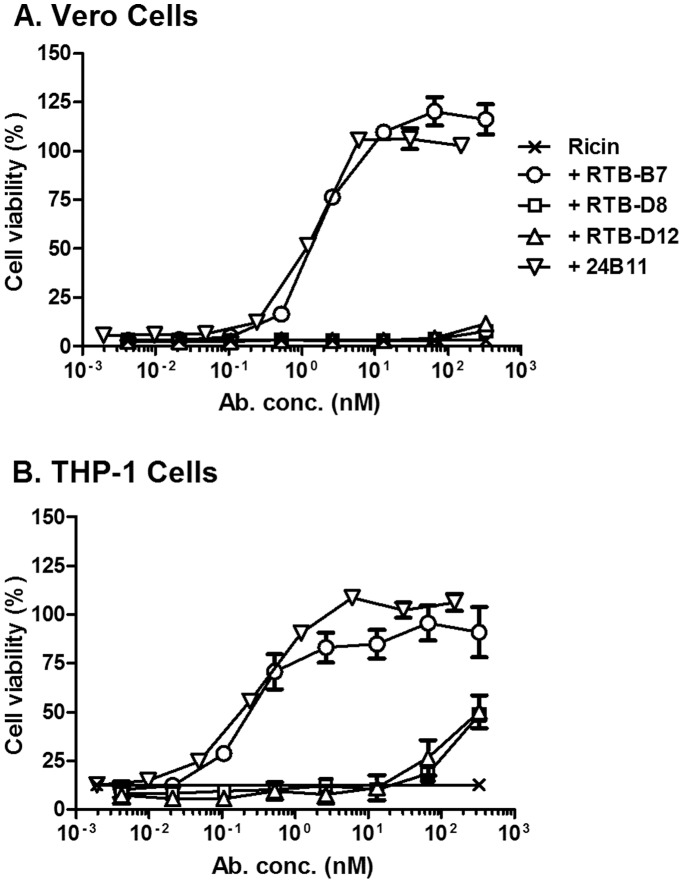
The inability of RTB-B7 (even at 100 fold molar excess over ricin) to passively protect mice against a 10xLD50 ricin challenge was unexpected, considering that we previously reported that RTB-B7 was highly effective at neutralizing ricin in a Vero cell cytotoxicity assay [39]. We therefore revisited the Vero cell cytotoxicity assay and compared RTB-B7 side-by-side with 24B11, as well as the non-neutralizing VHHs, RTB-D8 and RTB-D12. As reported previously, we found that RTB-B7 neutralized ricin in a dose-dependent manner. Moreover, RTB-B7’s estimated IC50 (∼1.5 nM) was virtually identical to 24B11’s IC50 when the two antibodies were compared in the same assay ( Fig. 3A ). In contrast, neither RTB-D8 nor RTB-D12 (which has the same apparent affinity for ricin as RTB-B7) had any detectable toxin-neutralizing activity ( Fig. 3A ).
Figure 3. RTB-B7 neutralizes ricin in a dose-dependent manner.
Ricin was mixed with 2-fold serial dilutions of indicated antibodies and then applied to (A) Vero cells or (B) THP-1 cells for 2 h. The cells were then washed and cell viability was measured 48 h later, as described in Materials and Methods. The data (mean ± SD) represent a single experiment in which each sample was done in quadruplicate. The experiment was repeated at least twice with identical results.
We next examined the capacity of RTB-B7 to neutralize ricin in vitro using THP-1 cells, a human monocyte/macrophage-derived cell line that is potentially more representative of ricin’s primary target cell in vivo [42], [43]. In the THP-1 assay, RTB-B7 also neutralized ricin with a dose-dependent profile that was nearly identical to 24B11’s ( Fig. 3B ), thereby demonstrating that RTB-B7 is among the most potent in vitro toxin-neutralizing antibodies described to date. In the THP-1 assay, RTB-D8 and RTB-D12 had some detectable toxin-neutralizing activity at very high concentrations, suggesting these two VHHs should be classified as partially neutralizing (not non-neutralizing) antibodies ( Fig. 3B ).
3.3 Characteristics of RTB-B7 Recognition of Ricin and RTB
The capacity of RTB-B7 to neutralize ricin in vitro but not in vivo led us to investigate in more detail the specific in vitro properties of RTB-B7, particularly with respect to specificity of toxin binding and recognition. In an effort to define the epitope recognized by RTB-B7, we subjected RTB-B7 (as well as RTB-D8 and RTB-D12) to the following previously established assays: RCA-I ELISA, pepscan analysis [29], panning with a 12-mer phage-displayed peptide library [28], [44], Western blot analysis, and competitive ELISAs with a collection of well-characterized RTB-specific neutralizing and non-neutralizing murine mAbs [26].
RCA-I is a tetrameric glycoprotein from Ricinus communis consisting of two ricin-like heterodimers whose B subunit (RCB) shares 84% sequence identity with RTB [29], [45], [46]. We found that RTB-B7 bound ricin and RCA-I with similar EC50s, demonstrating that RTB-B7’s epitope is likely conserved between the two closely related proteins (Fig. S1). RTB-D8 and RTB-D12 were similar to RTB-B7 in that they bound equally well to RCA-I and ricin. However, RTB-B7’s epitope is likely discontinuous in nature, as neither pepscan or affinity enrichment using a 12-mer phage-displayed library identified peptides that specifically bound RTB-B7. Moreover, Western blot analysis indicated that RTB-B7 reactivity was abolished when ricin (or RTB) was treated with β-mercaptoethanol (BME) (data not shown).
It was previously reported that neither SylH3 nor 24B11, two RTB-specific neutralizing mAbs, were able to competitively inhibit RTB-B7 from binding to ricin [39]. We extended these previous findings by performing competitive ELISAs with additional neutralizing (JB4) and non-neutralizing (TFTB-1, B/J F9, C/M A2, SA3, CB12, and JB11) murine mAbs. Ricin-coated ELISA plates were incubated with saturating amounts of murine mAbs and then probed with the RTB-B7, RTB-D8 or RTB-D12 (1 µg/mL; 33 nM). The EC50s of RTB-B7, RTB-D8 and RTB-D12 were unaffected by any of the murine mAbs tested (data not shown), suggesting that the three VHHs recognize distinct epitopes on RTB.
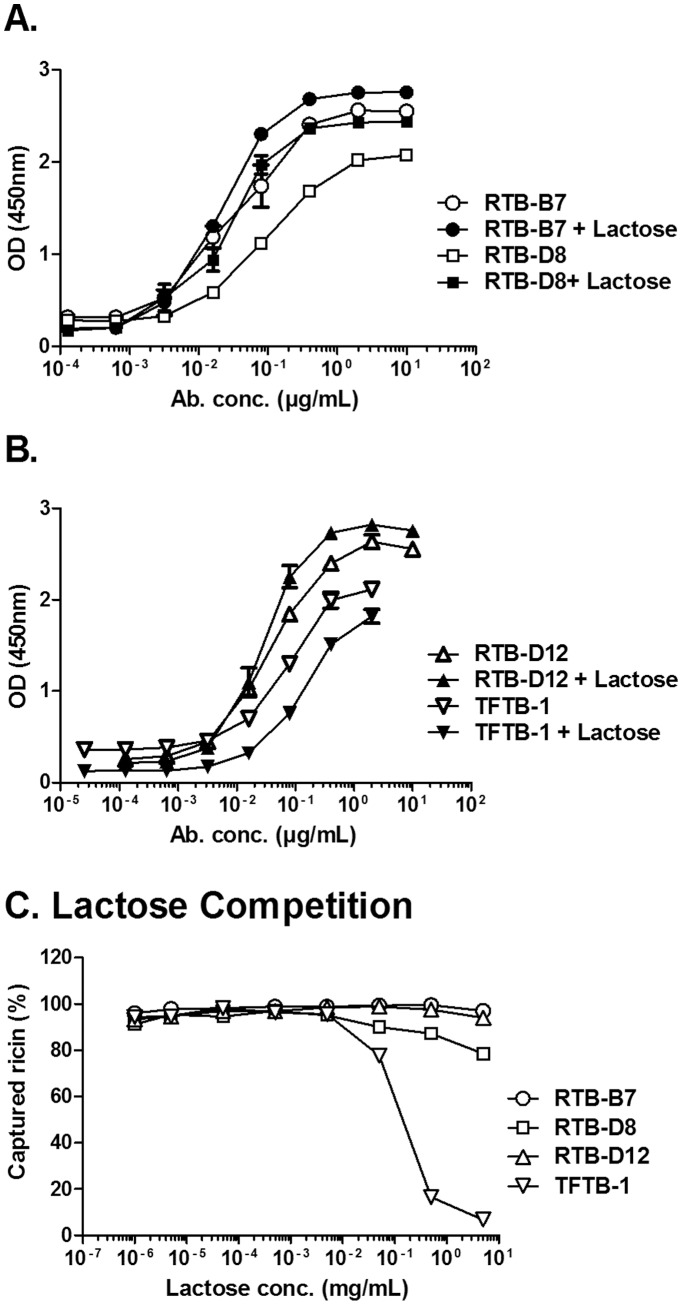
To determine whether RTB-B7 recognizes an epitope on RTB that is influenced by ligand engagement, we performed ricin ELISAs in the absence or presence of saturating amounts of lactose (10 mg/mL). The apparent affinity of RTB-B7 for ricin was unchanged (or even slightly enhanced) in the presence of lactose ( Fig. 4A, B ). This is in contrast to the non-neutralizing murine mAb, TFTB-1, whose capacity to recognize ricin was negatively impacted (∼20%) by lactose ( Fig. 4B ). The effect of ligand binding was even more pronounced when the antibodies were tested for their ability to “capture” soluble, biotinylated ricin in the presence of increasing concentrations of lactose ( Fig. 4C ). Lactose concentrations greater than 0.1 mg/mL resulted in a precipitous drop in TFTB-1′s ability to capture soluble ricin, whereas the abilities of VHHs RTB-B7, RTB-D8 and RTB-D12 to capture ricin were not altered ( Fig. 4C ). These data indicate that RTB-B7 recognizes an epitope on RTB that is not influenced by ligand engagement.
Figure 4. Impact of lactose on RTB-B7’s recognition of ricin.
To examine the impact of lactose on the ability of antibody to recognize ricin, ELISA plates were coated with ricin overnight. Plates were then probed with lactose for 1-mixed lactose with serial diluted concentrations of (A) RTB-B7 and RTB-D8 or (B) RTB-D12 and TFTB-1. Plates were incubated with mixtures of lactose:Ab and developed as described in Materials and Methods. (C) In the competition ELISA, plates were coated with the indicated antibodies at a constant concentration (VHHs at 10 µg/mL and TFTB-1 at 2 µg/mL) overnight. Serial dilutions of lactose (5 mg/mL) were incubated with biotinylated ricin before being applied to plates and developed. Binding was normalized to ricin bound to antibody in the absence of lactose. The data shown represent a single experiment in which each sample was done in triplicate and repeated at least twice. Data are expressed as the mean ± SD.
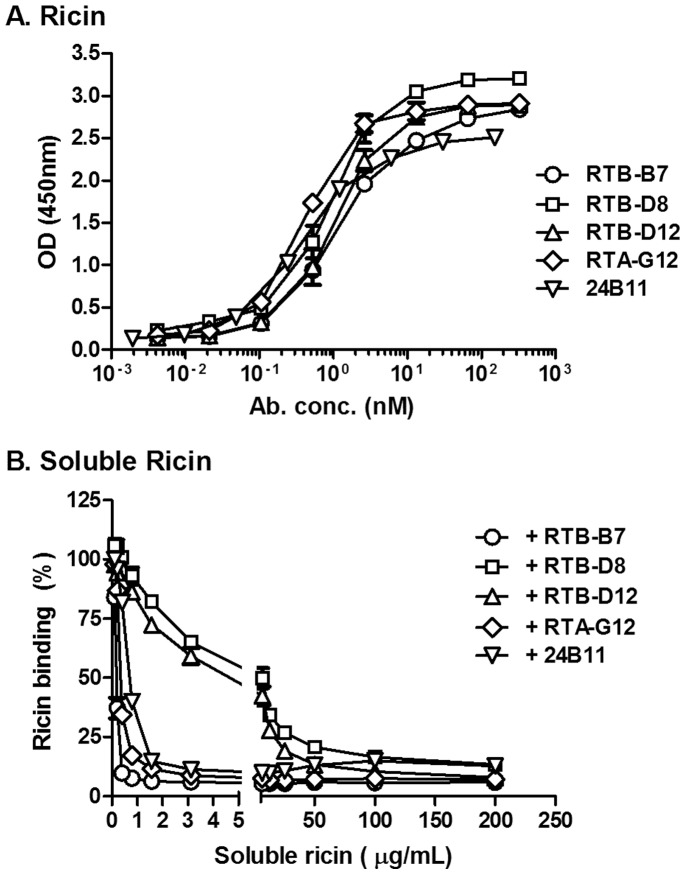
We have recently noted that certain mAbs recognize plate-bound ricin with high affinity, but bind poorly to ricin in solution (J. O’Hara, A. Yermakova, E. Sully, and N. Mantis, manuscript in preparation). Based on these and other observations, we speculate that adsorption of ricin to polystyrene microtiter plates results in the exposure of normally cryptic or subdominant epitopes (R’-form). To examine accessibility of RTB-B7’s epitope, we performed ELISAs in the absence or presence of soluble ricin toxin ( Figure 5 ). Antibodies 24B11 and RTA-G12, an RTA-specific VHH with toxin-neutralizing activity, were examined in parallel. We confirmed that RTB-B7, RTB-D8 and RTB-D12, as well as 24B11 and RTA-G12, recognized plate-bound ricin with roughly equal EC50s ( Figure 5A ). On the other hand, attachment of RTB-B7, 24B11 and RTA-G12 to plate bound ricin was completely inhibited by very low amounts of soluble native ricin (IC50<0.1 µg/mL), whereas inhibition of RTB-D8 and RTB-D12 required very high concentrations (>50 µg/ml) of toxin ( Figure 5B ). These data demonstrate that RTB-B7’s epitope is presented on the surface of native ricin, whereas the epitopes recognized by RTB-D8 and RTB-D12 are significantly (>500 fold) less accessible.
Figure 5. Recognition of plate bound and soluble ricin by RTB-B7.
(A) ELISA plates were coated overnight with ricin. Antibodies 24B11 and VHHs were serial diluted and added to the coated plates and developed as described in Materials and Methods. Shown on the Y-axis is optical density (OD). (B) For the competition ELISA, plates were coated with ricin overnight. Ricin (200 µg/mL) was serial diluted and pre-mixed with constant concentration of the indicated antibodies at equal molar amounts taking into account the number of binding sites (VHHs at 10 µg/mL and 24B11 at 22.75 µg/mL). Ricin:Ab solutions were then added to the plates and developed. The data shown represent a single experiment in which each sample was done in triplicate and repeated at least twice. Data are expressed as the mean ± SD.
3.4 RTB-B7 is Able to Neutralize Ricin after the Toxin has attached to Host Cell Receptors
There is evidence that RTB-specific toxin-neutralizing antibodies can be loosely classified into one of two categories based on the degree to which they prevent ricin from binding to cellular receptors and whether or not they neutralize ricin after the toxin has pre-bound to host cells [21], [23], [25]–[29]. For example, SylH3 is a potent inhibitor of ricin-receptor interactions but only marginally effective at neutralizing ricin when pre-bound to cell surfaces, while 24B11 only partially inhibits toxin-receptor interactions but is highly effective at neutralizing ricin when toxin is pre-bound to cells [27], [28]. In an effort to better characterize RTB-B7, we first examined its ability to inhibit ricin from binding to the surrogate receptor ASF. Biotinylated ricin was mixed with RTB-B7 at a range of concentrations and then applied to ELISA plates coated with ASF. SylH3 and 24B11, as well as RTB-D8 and RTB-D12, were examined in parallel. As expected, SylH3 almost completely blocked (>90%; EC50 ∼1 nM) the ability of ricin to bind to ASF, while 24B11 only marginally (<30%) impacted toxin-ASF interactions ( Fig. 6 ). In contrast, neither RTB-B7 nor the partially neutralizing VHHs, RTB-D8 and RTB-D12, affected the ability of ricin to associate with ASF, even at the highest concentrations tested.
Figure 6. RTB-B7 does not inhibit ricin from biding to ASF.
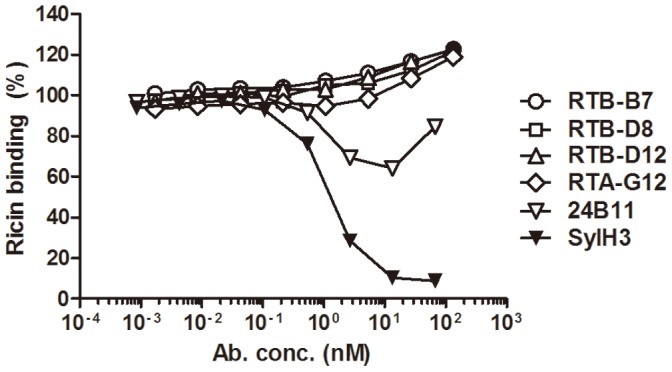
ELISA plates where coated with ASF overnight. Plates were then probed with pre-mixed ricin with the indicated VHHs (4.4 µg/mL) or mAbs 24B11 and SylH3 (10 µg/mL). Binding was normalized to ricin in the absence of antibody. The data shown represent a single experiment in which each sample was done in triplicate and repeated at least twice. Data are expressed as the mean ± SD. In most instances error bars are masked by symbol and therefore not visible.
To assess antibody effects on ricin’s interactions with cell surfaces, FITC-ricin was incubated with approximately equimolar amounts of RTB-B7 or other VHHs/mAbs and then applied to THP-1 cells at 4°C. The cells were then washed and subjected to flow cytometry to quantitate the amount of FITC-ricin bound to the cells. Lactose (30 mg/mL; 88 mM) was used as a positive control for these studies, as saturating amounts of the disaccharide are known to occupy RTB’s carbohydrate recognition domains and competitively inhibit ricin binding to cell surfaces [7]. The non-neutralizing mAb TFTB-1 was used as a negative control [26]. As expected, lactose inhibited ricin from binding to THP-1 cells, whereas TFTB-1 did not (<5%) ( Table 2 ). VHHs RTB-D8, RTB-D12 and RTB-B7 partially (20–30%) inhibited ricin from binding to THP-1 cells, while D10/B7 and 24B11 reduced ricin binding to THP-1 cells by >85% and >65%, respectively. Thus, RTB-B7 does not inhibit ricin binding to cell surfaces as efficiently as D10/B7.
Table 2. Antibody-mediated inhibition of ricin binding to the surface of THP-1 cells.
| Ricin | Treatment | Conc. | Inhibition (%)a |
| + | – | – | 0 |
| + | Lactose | 88 mM | 75 |
| + | RTB-B7 | 50 nM | 29 |
| + | RTB-D8 | 50 nM | 18 |
| + | RTB-D12 | 50 nM | 28 |
| + | D10/B7 | 50 nM | 86 |
| + | 24B11 | 50 nM | 66 |
| + | TFTB-1 | 50 nM | 2.5 |
FITC-ricin (46 nM) was incubated with lactose or indicated antibodies for 30 min before being applied to THP-1 cells and then subjected to flow cytometry, as described in Materials and Methods. The percent (%) inhibition of ricin binding was calculated by dividing the experimental geometric mean fluorescence intensity (MFI) by the control (FITC-ricin only) geometric MFI and then multiplying by 100.
We next examined the ability of RTB-B7 to recognize receptor-bound ricin and to neutralize toxin when pre-bound to cell surfaces. ELISA plates coated with ASF were incubated with a saturating concentration of ricin and then probed with RTB-B7 across a range of concentrations. RTB-B7 ( Fig. 7A ), as well as RTB-D8, RTB-D12 and D10/B7 (data not shown), recognized ASF-ricin in a dose-dependent manner. RTB-B7 was next tested for the ability to neutralize ricin after the toxin was pre-bound to cell surfaces. In order to do so, THP-1 cells were cooled on ice to arrest toxin endocytosis and treated with ricin for 30 min before the addition of RTB-B7, RTB-D8, RTB-D12 or 24B11, as described in the Materials and Methods. We found that 24B11 (IC50 0.25 nM) and RTB-B7 (IC50 1.4 nM) were each able to effectively neutralize ricin in this assay ( Fig. 7B ), whereas RTB-D8 and RTB-D12 were relatively ineffective, as they had detectable toxin-neutralizing activities only at very high concentrations (>330 nM) ( Fig. 7B ). These data demonstrate that RTB-B7, like 24B11, has the capacity to neutralize ricin after the toxin has adhered to cell surface receptors.
Figure 7. RTB-B7 neutralizes ricin when toxin is pre-bound to receptor.
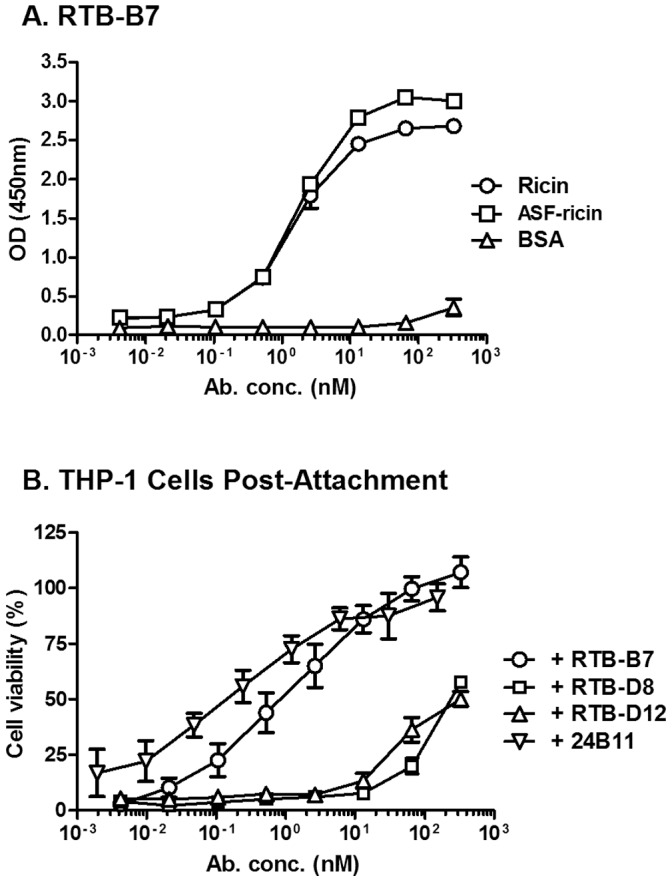
(A) To examine antibody recognition of receptor bound ricin, ELISA plates were coated with ricin or ASF overnight. ASF coated plates where then incubated with ricin while the remaining wells were incubated with 2% PBS-goat serum. RTB-B7 was serial diluted and added to the wells and developed as described in Materials and Methods. BSA wells were used to control for background. (B) To examine toxin neutralizing activity, THP-1 cells were treated with ricin for 30 min at 4°C, then washed and incubated with antibody or medium alone for 30 min at 4°C. Then, THP-1 cells where transferred to 37°C and 48 h later cell viability was measured as described in Materials and Methods. Viability was normalized to medium only treated cells and ricin-only treated cells determined the potency of the toxin in this assay. The ricin-only control cells were <20% viable. The data shown represent a single experiment in which each sample was done in triplicate or quadruplicate and repeated at least twice. Data are expressed as the mean ± SD.
3.6 Antibodies D10/B7 and 24B11 Promote Ricin Aggregation in Solution, Whereas RTB-B7 does not
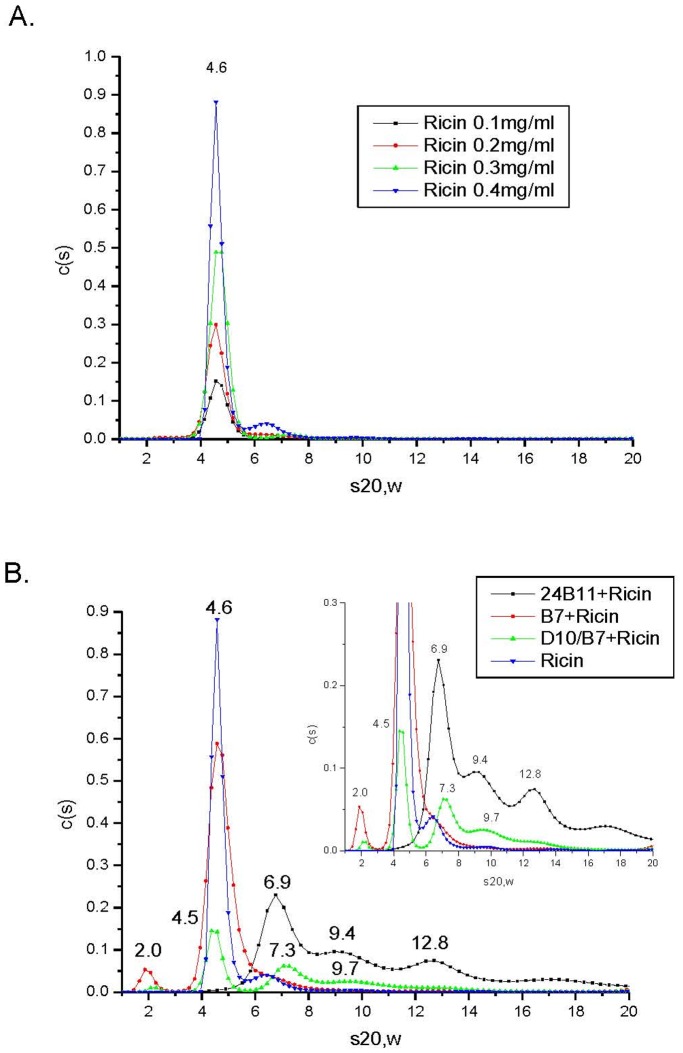
VHH heterodimer D10/B7 consists of two ricin-specific single chain VHH monomers, RTA-D10 and RTB-B7, joined by a flexible peptide linker [39]. By virtue of its ability to bind epitopes on RTA and RTB, D10/B7 can theoretically crosslink ricin and promote the formation of heterogeneous toxin-antibody complexes. Although monomeric in nature, we cannot formally exclude the possibility that RTB-B7 may homodimerize and therefore promote ricin aggregation to some degree. Because the formation of toxin:antibody complexes may contribute to toxin-neutralizing activity, we subjected our antibody samples to AUC analysis. Sedimentation coefficients for ricin, 24B11, D10/B7 and RTB-B7 were examined at a range of concentrations and were determined to be 4.6S, 6.7S, 2.9S and 2.3S, respectively ( Fig. 8 A ; S2A–C ). The sedimentation coefficient for ricin (s20,w = 4.6S) was identical to what is reported in the literature [47].
Figure 8. Sedimentation coefficients for ricin and ricin:antibody complexes.
Ricin and ricin:Ab complexes were subject to AUC, as described in the Materials and Methods. (A) Sedimentation coefficients of ricin were determined at indicated toxin concentrations (0.1–0.4 mg/ml). (B) Sedimentation coefficients of ricin:Ab complexes. Ricin was mixed with 24B11 (3.5 µM), D10/B7 (3.5 µM) and RTB-B7 (7 µM) to achieve an equimolar ratio between ricin and ricin-binding sites on each of the three different antibodies. The mixtures were incubated at room temperature for 120–180 min and then subjected to AUC (20°C). For convenience, the corrected sedimentation coefficients (s20,w) are denoted above each sedimentation distribution in the plot. The inset in panel B is simply a magnification of the figure sedimentation profiles to enhance resolution of the X-axis.
In order to characterize the aggregation potential of the individual antibodies, RTB-B7, D10/B7 and 24B11 were mixed at a 1∶1 molar ratio with ricin and then subjected to AUC. As shown in Figure 8B , D10/B7 and 24B11 promoted ricin aggregation, whereas RTB-B7 did not. Specifically, ricin:24B11 mixtures resulted in three defined sedimentation coefficient distributions at 6.9S, 9.4S and 12.8S, which we interpreted as corresponding to 24B11 (s20,w = 6.9S), a 1∶1 ricin:24B11 complex (s20,w = 9.4S) and 2∶1 ricin:24B11 complex (s20,w = 12.8S). The sedimentation coefficients for ricin:D10/B7 mixture revealed distributions at 4.5S and 7.3S, followed by a long tail with a larger complex identified at 9.7S. We interpret this distribution profile as corresponding to free ricin (s20,w = 4.5S), a 1∶1 mixture of ricin:D10/B7 (s20, w = 7.3S), and heterogeneous ricin:D10/B7 aggregates of various sizes. Due to RTB-B7’s small size, its effect on ricin aggregation was difficult to discern. However, the peak distribution around 4.6S had a much broader shoulder than the ricin control sample, suggesting the presence of free ricin plus a 1∶1 ricin:RTB-B7 complexes, not higher in molecular weight complexes.
Discussion
The antibody response to ricin toxin is surprisingly complex and the specific correlates of protection to this biothreat agent remain poorly defined, especially as compared to other protein toxins. For example, tetanus and diphtheria vaccines have been in clinical use for decades and it is well established that the primary correlate of protection is associated with a specific threshold of serum IgG toxin-neutralization activity [48]. In the case of ricin, however, protection (in mice) does not necessarily correlate with either pre-existing toxin-neutralizing activities or total antitoxin antibody levels [49], [50]. Similarly, analysis of serum IgG responses in humans that received a candidate ricin toxin subunit vaccine revealed a disconnect between total antibody titers and toxin-neutralizing activities (i.e., several individuals with high ricin-specific serum IgG titers had low or undetectable toxin-neutralizing activities) [51], [52]. The absence of definitive correlates of immunity to ricin has hindered evaluation of candidate ricin subunit vaccines and made the development of immunotherapeutics challenging. Therefore, as a strategy to “deconvolve” the polyclonal antibody response to ricin, we have focused on characterizing a large number of murine mAbs and camelid nanobodies with the goal of identifying the specific determinants that are associated with toxin-neutralizing activity in vivo [39], [53].
In this study, we undertook a comprehensive in vitro characterization of the camelid antibody RTB-B7. RTB-B7 is of interest as it was the only antibody with toxin-neutralizing activity among a collection of nine recently described RTB-specific VHHs [39]. Moreover, heterodimers consisting of RTB-B7 linked to one of three different RTA-specific VHHs (i.e., RTA-D10) were able to passively protect mice against ricin challenge, even though 100-fold molar excess of monomeric RTB-B7 does not alter the toxicity of ricin in vivo ( Figure 2 ). The main findings of the current study are that RTB-B7 (i) has potent in vitro toxin-neutralizing activity in two well-established cell-based cytotoxicity assays; (ii) recognizes a conformational epitope that is conserved between RTB and RCB (see below); (iii) binds to and neutralizes ricin in its soluble and receptor-bound forms; but, (iv) only partially inhibits ricin attachment to cell surface receptors. Thus, at least in vitro, RTB-B7 and 24B11 are virtually indistinguishable. Yet, 24B11 is able to passively protect mice against ricin toxin, whereas RTB-B7 is not [28]. The reason why RTB-B7 is devoid of detectable toxin-neutralizing activity in vivo remains unknown, but the fact that 24B11 Fab fragments are able to passively immunize mice demonstrates that neither antibody avidity or Fc-mediated clearance is absolutely required for protection in vivo [28]. Based on these data, we postulate that epitope specificity, and not affinity or avidity per se is the primary determinant of ricin toxin-neutralizing activity. The importance of epitope specificity in ricin toxin neutralization in vivo is supported as well by other studies with murine mAbs and VHH heterodimers [26], [39], [54].
While RTB-B7’s epitope was not definitively identified in this study, it can be tentatively localized to a limited region on RTB. For example, RTB-B7 bound equally well to RTB and RCB, indicating that RTB-B7’s epitope is conserved between the two proteins, which are only 84% identical at the amino acid level [45]. The differences between RTB and RCB are concentrated within domain 1, particularly within subdomains 1α and 1β, suggesting that RTB-B7 may recognize an epitope in RTB’s domain 2. Antibody competition studies with a collection of neutralizing (24B11, SylH3 and JB4) and non-neutralizing mAbs (TFTB-1, B/J F9, C/M A2, SA3, CB12 and JB11) is also consistent with RTB-B7 being targeted to domain 2, particularly a stretch of residues (200–240) within subdomain 2γ that is conserved between RTB and RCB. The fact that RTB-B7 was not particularly effective at inhibiting ricin attachment to cell surfaces would suggest that RTB-B7’s epitope is spatially distinct from key residues of RTB involved in Gal/GalNAc recognition (e.g., residues 248 and 234 in domain 2). Finally, in taking in account solvent accessibility, we postulate that RTB-B7 recognizes a conformational epitope localized within residues 200–230 of RTB.
It is interesting to note that the heterodimer VHH D10/B7, which consists of RTB-B7 covalently linked via a short peptide spacer to RTA-D10, was highly effective (>85%) at blocking attachment of ricin to the surface of THP-1 cells and, as AUC analysis revealed, promoting ricin aggregation in solution. It is tempting to speculate that one or both of these attributes are important in neutralizing ricin in vivo. RTA-D10 has moderate toxin-neutralizing activity in vitro, but like RTB-B7 is unable to protect mice against ricin challenge. We recently solved the X-ray crystal structure of RTA-D10 in complex with RTA (MJ Rudolph, DJ Vance, J Cheung, MC Franklin, F Burshteyn, MS Cassidy, EN Gary, C Herrera, CB Shoemaker, and NJ Mantis, manuscript resubmitted), revealing that RTA-D10 makes contact with a face of RTA that is almost diametrically opposed to RTB. Considering that the spacer between RTB-B7 and RTA-D10 is (at least) theoretically too short to enable intra-molecular RTA-RTB interaction, it is likely that D10/B7 promotes the formation of ricin intermolecular crosslinking, which is consistent with the aggregation seen in the AUC experiments. It is unclear at this point whether toxin aggregation and inhibition of toxin binding to cell surfaces are separate phenomena or whether aggregation itself results in the inability of ricin to attach to membrane bound Gal/GalNAc moieties. In future studies we will systematically construct and characterize additional anti-ricin VHH heterodimers and compare them with their respective monomeric constitutes as a means to dissect the functional properties of antibodies that are important in toxin neutralization in vivo. Only then will it be possible to rationally design effective therapeutics against ricin.
Supporting Information
Reactivity of RTB-B7, RTB-D8 and RTB-D12 with RCA-I. ELISA plates were coated with ricin or RCA-I overnight. (A) RTB-B7, (B) RTB-D8 and (C) RTB-D12 were serial diluted and added to the wells and reactivity was determined as mentioned in Materials and Methods. BSA wells were used to control for background. The data shown represent a single experiment in which each sample was done in triplicate and repeated at least twice. Data are expressed as the mean ± SD.
(TIF)
Sedimentation coefficients for 24B11, D10/B7 and RTB-B7. AUC was used to determine sedimentation coefficients for antibodies (A) RTB-B7, (B) D10/B7 and (B) 24B11 at indicated concentrations, as described in the Materials and Methods. For convenience, the corrected sedimentation coefficients (s20,w) are denoted above each sedimentation distribution.
(TIFF)
Acknowledgments
We would like to acknowledge Drs. Anastasiya Yermakova and Joanne O’Hara for technical assistance and providing valuable feedback and scientific insight related to this project. We thank Jacqueline M. Tremblay (Tufts University) for providing us with purified VHHs. We thank Mr. Renjie Song of the Wadsworth Center Immunology Core for assistance with flow cytometry, the Wadsworth Center Biochemistry Core for assistance with analytical ultracentrifugation, and Dr. Karen Chave of the Wadsworth Center Protein Expression Core for murine monoclonal antibody purification.
Funding Statement
CH gratefully acknowledges the support of a Carson Carr Diversity Scholarship from the University at Albany. This project was supported in part by a grant from the National Institutes of Health (AI097688) to NJM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reisler RB, Smith LA (2012) The need for continued development of ricin countermeasures. Adv Prev Med 2012: 149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe DN, Florence W, Bryant P (2013) Current biodefense vaccine programs and challenges. Hum Vaccin Immunother 9. [DOI] [PubMed]
- 3. Audi J, Belson M, Patel M, Schier J, Osterloh J (2005) Ricin poisoning: a comprehensive review. JAMA 294: 2342–2351. [DOI] [PubMed] [Google Scholar]
- 4. Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem 262: 5908–5912. [PubMed] [Google Scholar]
- 5. Endo Y, Tsurugi K (1987) RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem 262: 8128–8130. [PubMed] [Google Scholar]
- 6. Rutenber E, Ready M, Robertus JD (1987) Structure and evolution of ricin B chain. Nature 326: 624–626. [DOI] [PubMed] [Google Scholar]
- 7. Sandvig K, Olsnes S, Pihl A (1976) Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem 251: 3977–3984. [PubMed] [Google Scholar]
- 8.Sandvig K, Skotland T, van Deurs B, Klokk TI (2013) Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. [DOI] [PubMed]
- 9. Spooner RA, Lord JM (2012) How Ricin and Shiga Toxin Reach the Cytosol of Target Cells: Retrotranslocation from the Endoplasmic Reticulum. Curr Top Microbiol Immunol 357: 19–40. [DOI] [PubMed] [Google Scholar]
- 10. Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, et al. (1987) The three-dimensional structure of ricin at 2.8 A. Journal of Biological Chemistry 262: 5398–5403. [PubMed] [Google Scholar]
- 11. Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, et al. (1992) Cell surface and intracellular functions for ricin galactose binding. J Biol Chem 267: 11917–11922. [PubMed] [Google Scholar]
- 12. Rutenber E, Robertus JD (1991) Structure of ricin B-chain at 2.5 A resolution. Proteins 10: 260–269. [DOI] [PubMed] [Google Scholar]
- 13. Zentz C, Frenoy JP, Bourrillon R (1978) Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta 536: 18–26. [DOI] [PubMed] [Google Scholar]
- 14. Lewis MS, Youle RJ (1986) Ricin subunit association. Thermodynamics and the role of the disulfide bond in toxicity. J Biol Chem 261: 11571–11577. [PubMed] [Google Scholar]
- 15. Villafranca JE, Robertus JD (1981) Ricin B chain is a product of gene duplication. J Biol Chem 256: 554–556. [PubMed] [Google Scholar]
- 16. Kimura Y, Hase S, Kobayashi Y, Kyogoku Y, Ikenaka T, et al. (1988) Structures of sugar chains of ricin D. J Biochem (Tokyo). 103: 944–949. [DOI] [PubMed] [Google Scholar]
- 17. Slominska-Wojewodzka M, Gregers TF, Walchli S, Sandvig K (2006) EDEM is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol Biol Cell 17: 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wales R, Richardson PT, Roberts LM, Woodland HR, Lord JM (1991) Mutational analysis of the galactose binding ability of recombinant ricin B chain. J Biol Chem 266: 19172–19179. [PubMed] [Google Scholar]
- 19. Zhan J, de Sousa M, Chaddock JA, Roberts LM, Lord JM (1997) Restoration of lectin activity to a non-glycosylated ricin B chain mutant by the introduction of a novel N-glycosylation site. FEBS Lett 407: 271–274. [DOI] [PubMed] [Google Scholar]
- 20. Colombatti M, Pezzini A, Colombatti A (1986) Monoclonal antibodies against ricin: effects on toxin function. Hybridoma 5: 9–19. [DOI] [PubMed] [Google Scholar]
- 21. Colombatti M, Johnson VG, Skopicki HA, Fendley B, Lewis MS, et al. (1987) Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. JImmunol 138: 3339–3344. [PubMed] [Google Scholar]
- 22. Foxwell BM, Detre SI, Donovan TA, Thorpe PE (1985) The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology 34: 79–88. [DOI] [PubMed] [Google Scholar]
- 23. Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, et al. (2004) Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol 172: 6221–6228. [DOI] [PubMed] [Google Scholar]
- 24. McGuinness CR, Mantis NJ (2006) Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun 74: 3463–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prigent J, Panigai L, Lamourette P, Sauvaire D, Devilliers K, et al. (2011) Neutralising antibodies against ricin toxin. PLoS One 6: e20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yermakova A, Mantis NJ (2011) Protective immunity to ricin toxin conferred by antibodies against the toxin’s binding subunit (RTB). Vaccine 29: 7925–7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yermakova A, Klokk TI, Cole R, Sandvig K, Mantis NJ (2014) Antibody-mediated inhibition of ricin toxin retrograde transport. MBio 5. [DOI] [PMC free article] [PubMed]
- 28. Yermakova A, Mantis NJ (2013) Neutralizing activity and protective immunity to ricin toxin conferred by B subunit (RTB)-specific Fab fragments. Toxicon 72: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yermakova A, Vance DJ, Mantis NJ (2012) Sub-Domains of Ricin’s B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS One 7: e44317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82: 775–797. [DOI] [PubMed] [Google Scholar]
- 31. Anderson GP, Matney R, Liu JL, Hayhurst A, Goldman ER (2007) Multiplexed fluid array screening of phage displayed anti-ricin single domain antibodies for rapid assessment of specificity. Biotechniques 43: 806–811. [DOI] [PubMed] [Google Scholar]
- 32. Goldman ER, Anderson GP, Liu JL, Delehanty JB, Sherwood LJ, et al. (2006) Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Anal Chem 78: 8245–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldman ER, Liu JL, Bernstein RD, Swain MD, Mitchell SQ, et al. (2009) Ricin detection using phage displayed single domain antibodies. Sensors (Basel) 9: 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson GP, Glaven RH, Algar WR, Susumu K, Stewart MH, et al. (2013) Single domain antibody-quantum dot conjugates for ricin detection by both fluoroimmunoassay and surface plasmon resonance. Anal Chim Acta 786: 132–138. [DOI] [PubMed] [Google Scholar]
- 35. Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, et al. (2012) A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS One 7: e29941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB (2010) Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect Immun 78: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, et al. (2010) Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56: 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay JM, Mukherjee J, Leysath CE, Debatis M, Ofori K, et al. (2013) A single VHH-based toxin neutralizing agent and an effector antibody protects mice against challenge with Shiga toxins 1 and 2. Infect Immun. [DOI] [PMC free article] [PubMed]
- 39. Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB (2013) Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J Biol Chem 288: 36538–36547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gage E, Hernandez MO, O’Hara JM, McCarthy EA, Mantis NJ (2011) Role of the Mannose Receptor (CD206) in immunity to ricin. Toxins (Basel) 3(9): 1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schuck P (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J 78: 1606–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Auwerx J (1991) The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 47: 22–31. [DOI] [PubMed] [Google Scholar]
- 43. Korcheva V, Wong J, Lindauer M, Jacoby DB, Iordanov MS, et al. (2007) Role of apoptotic signaling pathways in regulation of inflammatory responses to ricin in primary murine macrophages. Mol Immunol 44: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vance DJ, Mantis NJ (2012) Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin’s enzymatic subunit. Toxicon 60: 874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roberts LM, Lamb FI, Pappin DJ, Lord JM (1985) The primary sequence of Ricinus communis agglutinin. Comparison with ricin. J Biol Chem 260: 15682–15686. [PubMed] [Google Scholar]
- 46. Stirpe F (2004) Ribosome-inactivating proteins. Toxicon 44: 371–383. [DOI] [PubMed] [Google Scholar]
- 47. Frenoy JP (1986) Effect of physical environment on the conformation of ricin. Influence of low pH. Biochem J 240: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plotkin SA (2008) Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47: 401–409. [DOI] [PubMed] [Google Scholar]
- 49. Greene CJ, Chadwick CM, Mandell LM, Hu JC, O’Hara JM, et al. (2013) LT-IIb(T13I), a non-toxic type II heat-labile enterotoxin, augments the capacity of a ricin toxin subunit vaccine to evoke neutralizing antibodies and protective immunity. PLoS One 8: e69678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES (2010) Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine 28: 5315–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, et al. (2006) A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A 103: 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitetta ES, Smallshaw JE, Schindler J (2012) A Small Phase IB Clinical Trial of an Alhydrogel-Adsorbed Recombinant Ricin Vaccine (RiVax). Clin Vaccine Immunol. [DOI] [PMC free article] [PubMed]
- 53. O’Hara JM, Yermakova A, Mantis NJ (2012) Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol 357: 209–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O’Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN 3rd, et al. (2010) Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 28: 7035–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Katzin BJ, Collins EJ, Robertus JD (1991) Structure of ricin A-chain at 2.5 A. Proteins 10: 251–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reactivity of RTB-B7, RTB-D8 and RTB-D12 with RCA-I. ELISA plates were coated with ricin or RCA-I overnight. (A) RTB-B7, (B) RTB-D8 and (C) RTB-D12 were serial diluted and added to the wells and reactivity was determined as mentioned in Materials and Methods. BSA wells were used to control for background. The data shown represent a single experiment in which each sample was done in triplicate and repeated at least twice. Data are expressed as the mean ± SD.
(TIF)
Sedimentation coefficients for 24B11, D10/B7 and RTB-B7. AUC was used to determine sedimentation coefficients for antibodies (A) RTB-B7, (B) D10/B7 and (B) 24B11 at indicated concentrations, as described in the Materials and Methods. For convenience, the corrected sedimentation coefficients (s20,w) are denoted above each sedimentation distribution.
(TIFF)