Abstract
One limitation in the development of biosensors for the early detection of disease is the availability of high specificity and affinity ligands for biomarkers that are indicative of a pathogenic process. Within the past ten years, biopanning of phage displayed peptide libraries on intact cells has proven to be a successful route to the identification of cell-specific ligands. The peptides selected from these combinatorial libraries are often able to distinguish between diseased cells and their normal counterparts as well as cells in different activation states. These ligands are small and chemical methodologies are available for regiospecific derivatization. As such, they can be incorporated into a variety of different diagnostic and therapeutic platforms. Here we describe the methods utilized in the selection of peptides from phage displayed libraries by biopanning. In addition, we provide methods for the synthesis of the selected peptides as both monomers and tetramers. Downstream uses for the peptides are illustrated.
Keywords: Phage Display, Peptides, Cell-Targeting, Biopanning, Combinatorial Library, Diagnostics, Therapeutics, Quantum Dots
1. Introduction
The development of biosensors for the detection of different disease states is dependent on the availability of high affinity and specificity ligands for the desired cell type and/or biomarker. In many applications, the accessibility of such ligands has been the limiting factor in the development of the technology. To date, antibodies have been the most common class of ligands utilized. However, antibodies are expensive and can be difficult to modify. Additionally, if the down-stream application is to detect particular cell types (i.e. a cancerous cell versus its normal counterpart), the antibody must bind to its target in the context of an intact cell.
As such, our lab and others have turned towards peptide libraries as a source of cell-specific ligands(1–16). In the same fashion that phage displayed peptide libraries can be panned on purified biomolecules, whole cells can be used as the bait for the peptide library. This approach, often referred to as biopanning, results in the isolation of peptides that display high cell-specificity; ligands can be isolated that discriminate between cell types and disease states. Furthermore, cell-specific peptides can be obtained without knowledge of a suitable cell surface biomarker. The protocol is amenable to a variety of different cell types, including primary cells. To date, we have identified cell-specific peptides for many different cell types including cells of the immune system(2, 4), pancreatic β-cells(7), cardiac cells(3), tumor cells(5, 6), and pathogen-infected cells(8). Importantly, most peptides selected in this manner are active outside of context of the phage, retaining their cell-specificity and affinity. Furthermore, we have shown that tetramerizing the peptides on a branched scaffold can greatly enhance the peptides affinity for its target cell type(5, 6, 8, 17, 18). These peptides can be employed for the delivery of fluorescent nanoparticles, as cell capture reagents for cell enrichment, and as antibody replacements for flow cytometry. As peptides are amenable to derivatization, we anticipate that these cell-specific ligands will find utility in a variety of different biosensor platforms.
2. Materials
2.1 Cell culture and phage panning (methods outlined in 3.1 and 3.2)
Tissue culture cell line or primary cell of interest
12 well tissue culture plates for adherent cells
15 mL and 50 mL polypropylene centrifuge tubes for non-adherent cells
1.5 mL microcentrifuge tubes
Cell scrapers
Phage library (see Note 1) or amplification stock for each round of panning.
RPMI media (or any cell-specific media) without serum
100 × Chloroquine stock: Dissolve 55 mg chloroquine in 10 mL PBS for a final concentration of 10 mM. Filter sterilize the solution
25 × Protease Inhibitor without EDTA (Roche)
Phosphate buffered saline (PBS): 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4
PBS+: Add 0.5 mM CaCl2 and 10 mM MgCl2 to PBS in this order with stirring.
PBS+ with 0.1% BSA: Add 0.1 g bovine serum albumin per 100 mL PBS+
0.1 M HCl-Glycine, 0.9% NaCl pH adjusted to 2.2 with glycine
1.5 M Tris-HCl, pH 8.8
30 mM Tris-HCl, pH 8.0
2.2 Bacterial culture and phage amplification and titering (methods outlined in 3.3–3.4)
Selective media for K91 bacterial stocks. We use M9-Pro minimal medium prepared as follows: Mix 7.5 g agar + 430 mL water and autoclave solution. Cool agar solution to ~55° C. Add 25 mL 20 × M9 Salts, 5 mL 20 % glucose, 50 µl 1 M CaCl2, 500 µl 1 M MgSO4, 100 µl 0.1% Thiamine, 2.5 mL 0.2 mg/mL biotin, 2.5 mL 1% uridine, 8 mL 1 % leucine, 8 mL 1 % phenylalanine, 8 mL 1% threonine, 8 mL 1% methionine, 8 mL 1% histidine, 8 mL 1% Tryptophan, and 8 mL 1 % lysine. 20 × M9 salts consist of 60 g Na2HPO4, 30 g KH2PO4, 5 g NaCl, and 10 g NH4Cl
LB media
100 mm and 150 mm LB-tet plates (12 µg/mL tetracycline)
Culture flasks for expansion and isolation of phage clones
20% PEG-8000 (Fisher Chemical) in 0.9% NaCl (see Note 2)
65° C heating block
Bacterial incubator with shaker
Various centrifuge tubes and bottles
Low (3000 × g) and high speed (11,000 × g) centrifuges for concentrating bacterial stocks and phage isolation
Spectrophotometer to monitor bacterial cultures
PBS prepared as described in 2.1
2.3 Quantitative real-time PCR for titering (method outlined in 3.5)
BioRad iCycler or similar apparatus
2× Sybr® green mastermix (see Note 3)
Optical PCR plates for real-time PCR
Optical sealing tape for real-time PCR
8-channel pipet, 5–50 µl
Serial dilutions of previously characterized phage preparation to generate a standard curve
- Specific primers to tetracycline resistance gene (see Note 4)
- Forward primer (tetR-F1): 5’-CGAATAAGAAGGCTGGCTCTGC-3’
- Reverse primer (tetR-R1): 5’-GCTGTGGGGCATTTTACTTTAGG-3’
2.4 Colony PCR for sequence determination (outlined in 3.6)
General materials for PCR mastermix preparation: 10× polymerase buffer, 25 mM MgCl2, 10 mM dNTP mix, Taq polymerase (GoTaq® DNA Polymerase 5 units/µl, Promega Corp, or Choice™ Taq, Denville Scientific).
Thermocycler
- Specific primers that flank library site
- Forward primer (fd-tet F1): 5’-GGGCGATGGTTGTTGTCATTG-3’
- Reverse primer (fd-tet B1): 5’-CTCATTTTCAGGGATAGCAAGCC-3’
Agarose gel apparatus
100 bp ladder standards (Promega Corp, catalog # G2101 or similar)
Exonuclease I (10 units/µl, New England Biolabs, or other suitable vendor)
Shrimp alkaline phosphatase (1 unit/µl, New England Biolabs or other suitable vendor)
BigDye® Terminator v3.1 (Applied Biosystems Inc)
70% Ethanol
Hi-Di™ Formamide (Applied Biosystems Inc)
Sequencing Stop/precipitation Reagent: prepare by mixing 125 mL 95% ethanol, 29 mL water and 6 mL 3 M sodium acetate, pH 5.2.
2.5 Selectivity and specificity determinations (outlined in 3.7)
Materials outlined in section 2.1 for the panning and 2.2 or 2.3 for titering
Isolated phage clones and a control phage clone displaying an irrelevant peptide sequence. Alternatively, a phage clone that displays no peptide (referred to as an “empty” phage) can be employed.
Cell lines or primary cells of interest.
2.6 Peptide synthesis (outlined in 3.8–3.11)
Symphony Synthesizer (Rainin Instruments, Protein Technologies, Inc. Woburn, MA) or other standard solid phase peptide synthesizer.
Resins for solid phase synthesis: Rink Amide AM resin (substitution level 0.71 mmol/g, Novabiochem, EMD Biosciences, San Diego, CA); Fmoc4-Lys2-Lys-β-Ala-CLEAR™ Acid Resin, Fmoc4-Lys2-Lys-Lys(Biotin-PEG)-β-Ala-CLEAR™ Acid Resin and Fmoc4-Lys2-Lys-Cys(Acm)-β-Ala-CLEAR™ Acid Resin (substitution level 0.21 mmol/g, Peptides International, Louisville, KY).
Fmoc amino acids required to synthesize desired peptide. Prepare 200 mM amino acid solutions by dissolving 20 mmol Fmoc-protected amino acids in DMF to final volume of 100 mL.
Coupling Reagents: 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), 1-Hydroxybenzotrizole (HOBt) all available from Novabiochem. Prepare 200 mM solutions as follows: Weigh out 18.965g HBTU,6.755g HOBt and 11 mL NMM, Add DMF to final volume of 250 mL.
If desired non-natural amino acids can be incorporated into the peptide. We routinely incorporate Fmoc-NH-(PEG)11-COOH (C42H65NO16) (Polypure, Oslo, Norway), Fmoc-Glu(biotinyl-PEG)-OH (C40H55N5O10S) and Fmoc-Lys(biotin)-OH (C31H38N4O6S) (Novabiochem, EMD Biosciences, San Diego, CA) (see Notes 5 and 6)
Piperidine in DMF (20%): 200 mL piperidine, 800 mL DMF.
- Cleavage cocktails (see Note 7):
- TFA: H2O:TIS (95%:2.5%:2.5%) prepared by mixing 9.5 mL trifluoroacetic acid (TFA), 0.25 mL H2O, 0.25 mL triisopropylsilane (TIS). This cleavage cocktail is used for the cleavage of linear synthesized tetrameric peptide and maleimido activated cores.
- TFA: EDT:H2O:TIS (94%:2.5%:2.5%:1%) prepared by mixing 9.4 mL TFA, 0.25 mL ethanedithiol (EDT), 0.25 mL H2O, 0.1 mL TIS. This cocktail is employed for peptides containing a cysteine residue.
Diethyl ether
Dichloromethane (DCM)
Dimethylformamide (see Note 8)
3-maleimidopropionic acid (Sigma-Aldrich Inc, St. Louis, MO)
2.7 Removal of group from selectively protected cysteine residues (outlined in 3.12)
PBS containing 0.01 M EDTA
Argon for flushing solutions
TFA:Anisole mixture (99:1)
Silver acetate (Sigma-Aldrich or other vendor)
Diethyl ether
0.2 M dithiothreitol prepared in 1 M acetic acid
8M guanidine hydrochloride
2.8 Peptide purification and characterization (outlined in 3.13)
HPLC solvent delivery system with binary gradient capability and a UV detector.
Reversed-phase octadecylsilica (C18) column. In our laboratory we use the following columns: Preparative column: Vydac RP-C18 column (250 mm length×22 mm diameter, 10 µm particle size). Analytical column: Varian RP-C18 column (250 mm length×4.6 mm diameter, 5 µm particle size)
Solvent filtration apparatus equipped with a 0.45 µm Teflon filter (Such as Ultra-ware filter apparatus 300/1000 mL from Kontes glass company and 0.45 µm Teflon filters from Millipore Corp.)
Syringe driven filter units, 0.22 µm porosity, 13mm (Millipore Corp.)
Eluent A: H2O/0.1% TFA and eluent B: acetonitrile/0.1%TFA
Access to mass spectrometer and/or peptide sequencing facility
2.9 Inhibition of selected phage clone by cognate peptide (outlined in 3.14)
Selected phage clone and corresponding synthetic peptide
Reagents indicated in 2.5
2.10 Applications of synthetic peptides (outlined in 3.15–3.18)
Synthetic peptide prepared with incorporation of biotin
Streptavidin-conjugated Qdots (emission wavelengths chosen to match microscope emission filter set)
Microscope slides and coverslips
8-well chamber slides (for example, VWR, Inc., catalog # 62407-296) can be used to culture adherent cells prior to staining.
Prolong® Gold antifade reagent with DAPI (Invitrogen)
Fluorescence microscope
Streptavidin-coated magnetic beads (Dynabeads M280-SA, Invitrogen, 6.7 × 108 beads/mL, 1 mg beads binds 700 pmoles free biotin)
Cell isolation magnet (or strong magnet)
Streptavidin-phycoerythrin or streptavidin-FITC
Flow cytometer (Cell Lab Quanta, Beckman Coulter or other suitable instrument)
PBS+
0.4% formalin: 37 % formaldehyde, Sigma Chemical, diluted 1:10 in PBS, immediately prior to use
70% Ethanol
Fingernail polish (any color)
3. Methods
The following information is based on our protocols for selection of peptide ligands for cell recognition and delivery. Selection of a peptide ligand, using our protocol, should be expected to take 5–6 rounds of biopanning. During each round, cells are incubated with a mixture of phage displaying different peptides. Phage that do not bind or bind only to the surface of the cell are washed away. Phage that bind to the cell and are internalized by the cell are retained. These cell-internalized phage are amplified in bacteria, isolated, and used as the input in the next round of biopanning. In each round of selection, the diversity of the phage sample is reduced while the proportion of phage displaying a peptide that mediates cell-specific binding is increased.
Once a phage displayed peptide has been selected using the biopanning protocol, we characterize the binding selectivity and cell specificity of that phage clone. Our determination of selectivity compares the binding and uptake of a cell-selected phage clone with binding and uptake of a control phage clone that was randomly selected from the library. This provides a rough estimate of the affinity of the peptide. Additionally, it assures that the cellular binding is due to the selected peptide and is not the result of non-specific phage binding. The measurement of cell specificity involves comparison of the selectivity index of a specific phage over a variety of different cell types. During the characterization process, we also prepare chemically synthesized versions of the specific peptide, monomeric and tetrameric, and test the utility of these constructs as cell binding reagents out of the context of phage presentation. Depending on the down-stream applications of the ligand, we will incorporate a unique cysteine for chemical modification or a biotin moiety for use with streptavidin-based reagents.
3.1 Phage panning for adherent cell lines
Cells are seeded onto tissue culture wells 24–48 hours before panning. Only a single well is required for each panning round. Once started, each round of the panning procedure requires approximately 4–5 hours to complete. Additional time is required for bacterial plating for titer determination and phage amplification.
24 – 48 hours before the phage biopanning will be conducted, trypsinize cells from a propagation flask and seed cells in 12-well plate. On the day of panning, one well should be ≈ 90% confluent. The proper level of confluence is generally obtained by seeding 100,000 to 150,000 cells in a well.
Begin the biopanning procedure by gently removing media from the well. Wash cells with 1mL RPMI media (or other cell-appropriate media) without serum (tip plate to accumulate liquid on one side of the well so that media can be aspirated without disturbing attached cells. Pipette wash media gently to avoid dislodging cells. Remove wash media.
Gently add 1 mL/well, media without serum and incubate cells for 2 hours at 37°C to clear cell surface receptors (referred to as “clearing the receptors”).
- Approximately 15 minutes before the end of the clearing step, prepare the phage panning solution as follows:
- 10 µl Chloroquine (100 × Stock)
- 40 µl protease inhibitor without EDTA (25 × Stock)
- 10 – 100 library equivalents of the phage library. The phage library used for much of our work has a diversity of 1×108 members(5, 19). Therefore, we add 1×109 – 1×1010 phage to the input sample for round one. Thus, each library member should be present in 10 to 100 copies in the input mixture. For each successive round of biopanning we input ~1.5×109 phage.
- Bring mixture to 1 mL final volume by addition of PBS+ with 0.1% BSA.
Remove RPMI from the cells. Wash cells once with 1mL PBS+ with 0.1% BSA that was pre-warmed to 37°C. Remove the wash solution from the cells.
Save 50 µl of the input phage solution for titer determination. Add the remainder of the phage solution to the cells and incubate for 1 hour at 37°C in a standard tissue culture CO2 incubator.
- After the 1 hour incubation, aspirate the supernatant. We do not save this solution containing unbound phage. Wash the cells 4 times at room temperature:
- Add 1 mL PBS+ with 0.1% BSA (room temperature)
- Incubate for 5 minutes.
- Aspirate buffer and repeat.
- Acid elute/wash 1–2 times at room temperature:
- Add 1 mL 0.1 M HCl-Glycine, pH 2.2 + 0.9% NaCl.
- Incubate for 5 minutes. Time could be reduced if the cell line is fragile and lysis is problematic. (See Note 9).
- If you are interested in phage that bind to the cell surface but are not internalized, you can keep this acid wash fraction when it is removed from the cells and amplify the recovered phage as detailed below. Adjust the pH of the acid wash material by addition of 1.5 M Tris-HCl, pH 8.8 after it is removed from cells.
- Repeat acid wash once.
Remove the second acid wash and add 1 mL of 30mM Tris –HCl, pH 8.0 to the cells and incubate on ice for 30 minutes. This hypotonic media is used to swell the cells and enhance lysis and recovery of phage.
Freeze cells in plate. This is a suitable place to stop the protocol if needed.
Thaw cells and scrape off plate. The freeze-thaw cycle disrupts the cells and releases any internalized phage. This sample is referred to as the output fraction. Examine the well under a microscope to ensure that the cells have been disrupted. If freeze-thaw does not disrupt the cells, 0.1% Triton X-100 or other detergent can be added to the hypotonic buffer.
Set up amplification of output phage as well as titration of input and output phage. Titer input, acid wash (if desired) and output. Amplify output or acid wash (if desired).
3.2 Phage panning for non-adherent cells
The panning procedure requires approximately 4 hours to complete. The cells can be removed directly from a feeder flask for the panning procedure. They do not have to be seeded into a separate flask prior to the day of the panning procedure.
Transfer cells from feeder flask to a 50 mL centrifuge tube and pellet cells (Speed and time required for forming a good pellet will vary with cell type).
Resuspend cells in 10 mL media without serum and pellet again.
Resuspend cells in 10 mL media without serum and incubate cells for 2 hours at 37°C incubator to clear the receptors.
Count cells during clearing and determine volume needed for 2 million cells.
Approximately 15 minutes before the end of the clearing step, prepare the phage solution as detailed in Step 5 of Procedure 3.1, above.
Pellet 2 million cells and wash one time with 10 mL PBS+ with 0.1% BSA pre-warmed to 37°C.
Save ~50 µl input solution for titer determination. Pellet cells and resuspend the cell pellet gently in the remainder of phage solution and incubate for 1 hour at 37°C in tissue culture incubator with 5% CO2.
At the end of the incubation, dilute the sample to 10 mL with PBS+ with 0.1% BSA (room temperature).
Wash the cells 4 times by centrifugation and resuspension. Resuspend cells in 10 mL PBS+ with 0.1% BSA and incubate for 5 minutes at room temp. Pellet cells.
- Acid wash cells at room temperature:
- Resuspend cell pellet in 1 mL 0.1 M HCl-Glycine, pH 2.2, 0.9% NaCl.
- Incubate 5 minutes (See Note 9).
- Pellet cells and remove supernatant.
- Repeat acid wash once. Remove supernatant solution.
Remove the second acid wash and add 1 mL of 30mM Tris–HCl, pH 8.0 to the cells and incubate on ice for 30 minutes.
Freeze cells and thaw. The freeze-thaw cycle disrupts the cell and releases any phage. This is referred to as the output fraction.
Set up amplification and titration of phage. Titer input, acid wash (if desired) and output. Amplify output and/or acid wash (if desired).
3.3 Amplification of phage mixtures from biopanning
Between successive rounds of phage biopanning, the output phage sample (and/or the acid wash sample, if desired) must be amplified. This procedure may be performed in parallel with the phage titering as detailed in section 3.3 above or may be performed independently.
On day one, pick a single K91 bacterial colony and inoculate 10–15 mL LB media without antibiotics for each sample that will be amplified.
Culture bacteria at 37°C with shaking until an OD600nm of 0.2 – 0.4 is obtained.
Spin down bacterial cells at 3,000 × g for 10 minutes at 4°C.
Re-suspend the pellet in 1/10 the original volume using LB media by pipeting up and down.
Add your phage sample to be amplified (the entire phage sample - 50µl aliquot removed for titration) to resuspended K91 cells and incubate for 15 minutes at 37°C.
Dispense the complete mixture of phage-infected K91 cells onto four, 150mm LB-tet plates. Plate 1/4 of the bacterial mix/plate.
Allow liquid to be absorbed into plate.
Invert plates and incubate at 37°C overnight.
On day two, harvest phage. Add 10mL LB media to each of the four LB-tet plates. Incubate for 10 minutes at room temperature.
Scrape bacteria off of the plate with a glass spreader. Collect all the material from the four inoculated plates in a single 50 mL centrifuge tube. Some of the media will not be recovered from the plate.
Add 10 mL fresh LB media to one of the four plates. Use the glass spreader to clean the plate further and transfer the wash material to the second plate. Continue until all four plates have been washed in this manner. Combine this wash material with the original harvest in the 50 mL tube.
Centrifuge the harvested material to obtain a firm pellet of bacterial cells. (Example: 3,000 × g for 10 minutes at 4°C in Beckman Coulter Allegra® 25R centrifuge.)
The infectious phage particles will be in the supernatant from this centrifugation step. Transfer the supernatant to a fresh centrifuge tube and measure the volume.
Add ¼ of the supernatant volume of 2.5 M NaCl + 20% PEG 8000 to the supernatant. Example: if volume of supernatant is 40 mL, add 10 mL of the NaCl/PEG solution. Incubate this mixture on ice for 1 hour to precipitate the phage particles.
Collect the phage precipitate by centrifugation at 11,000 × g for 30 minutes at 4°C. The phage should produce a firm pellet under these conditions.
Pour off and discard the supernatant making sure no standing liquid is left in the centrifuge tube. Tilt the centrifuge tube to drain off any residual PEG solution. Leave the tube inverted for 1 hour at 4°C. Residual PEG solution will make it more difficult to completely resuspend the phage pellet.
After draining for 1 hour, put the tube upright and add 1mL PBS to the pellet. Incubate on ice for 30 minutes. During this incubation, tilt the tube so that the pellet is completely covered with PBS.
Gently resuspend the phage pellet using a 1 mL pipet. Do not vortex. Vortexing concentrated phage solution may result in shearing of the phage. Mix the samples so that there are no visible chunks or cakes in the sample. Transfer the resuspended phage to a clean 1.5 mL microcentrifuge tube.
Pellet insoluble debris by centrifugation at 16,000×g for 2 minutes in a bench top microcentrifuge.
Transfer supernatant to a fresh microcentrifuge tube and incubate at 65°C for 15 minutes in a water bath or heating block to kill any bacteria remaining in the sample. Do not extend time of this incubation or the phage will lose infectivity.
Pellet insoluble debris by centrifugation at 16,000 × g for 2 minutes.
Transfer supernatant to a clean tube. Discard pellet. Mix and dispense aliquots of the purified phage to clean microcentrifuge tubes. Label tube with the cell type, panning round number, date and operator’s initials.
Store aliquots of the phage preparation at −80°C until use.
Before using on cells, set up bacterial titration to determine the yield of infective phage. The titration should be performed as detailed in 3.3 for the input phage sample except that more dilute samples are required to infect with bacteria. We typically dilute amplified phage preparations 10−2, 10−4, 10−6, 10−7, and10−8. The samples diluted 10−6, 10−7, and10−8 are used to infect K91 bacterial cells and aliquots of these infections are plated.
For amplification of individual phage clones (see Note 10).
3.4 Bacterial cell culture and phage titration (see Note 11)
We maintain K91 cells on minimal media minus proline supplemented with 0.5µg/mL thiamine. The bacteria grow slowly on these plates, generally requiring 2 days of culture to produce suitable colonies. Each day that a titration will be performed, start a liquid culture of the bacteria from a single colony.
Pick a single colony and inoculate 5–10 mL LB media without antibiotics.
Culture bacteria at 37°C with shaking until an OD600nm of 0.4 is obtained. If culture goes past an OD600nm of 0.6, the culture should be diluted ~ 10-fold with LB media and continue culturing until the proper optical density is obtained.
If bacterial cells are ready before samples that will be titered, place bacterial cells on ice until needed. If placed on ice, re-warm the cells to 37°C prior to mixing with phage samples.
- Prepare serial dilution of input phage sample (50 µl aliquot was saved for titering):
- Add 10 µl input phage to 990 µl LB media. (=10−2 dilution.)
- Add 10 µl of 10−2 input phage dilution to 990 µl LB media. (=10−4 dilution.)
- Add 100 µl of 10−4 input phage dilution to 900µl LB media. (=10−5 dilution.)
- Add 100 µl of 10−5 input phage dilution to 900 µl LB media. (=10−6 dilution.)
- For titration of biopanning output samples:
- Mix the freeze-thaw cell lysate well by flicking.
- Add 50 µl of cell lysate to 450 µl LB media. (=10−1 dilution).
- Add 100 µl of 10−1 dilution to 900 µl LB media. (=10−2 dilution).
- For the early rounds of panning, these first two dilutions should be adequate. After round three, an additional 10-fold dilution is suggested.
Dispense 900 µl K91 bacterial cells to sterile tubes for phage infection. For input phage samples, dispense aliquots to be infected with the 10−4, 10−5, and 10−6 dilutions respectively. For the output phage samples, dispense aliquots to be infected with the 10−1 and 10−2 dilutions respectively. Dispense one K91 aliquot that will serve as a non-infected control.
Add 100 µl of diluted phage sample to 900 µl K 91 cells. Incubate at 37°C for 15 minutes (see Note 12). Label LB-tet plates for each sample. We inoculate 2 LB-tet plates for each infected K91 sample, one plate with 100 µl and second plate with 50 µl.
Add 100 µl of each infected K91 sample onto a separate, labeled LB-tet plate and spread evenly.
Add 50 µl of each infected K91 sample onto a separate, labeled LB-tet plate and spread evenly.
Additionally, plate 100 µl of uninfected K91 cells as a contamination control. After overnight incubation, these control plates should not have any colonies.
Allow plates to dry before inverting.
Incubate at 37°C overnight to allow colonies to grow.
After overnight incubation, count and record the number of colonies present on each plate. There should not be any colonies on the uninfected K91 cell plates. If colonies are present on these plates, the source of contamination needs to be eliminated and the samples need to be titered again.
Calculate titer of each sample. For each plate, use the formula: (# of colonies × dilution factor × 10 for dilution into K91)/ mL of K91 mixture plated = colony forming units (cfu)/mL in the original sample. Calculate the independent determinations for each sample and average them to obtain sample titer.
The output titer plates from biopanning round 3 and subsequent rounds are saved for DNA sequence analysis and determination of phage displayed peptide sequences (Section 3.6).
3.5 Real-time, quantitative-PCR phage titration (see Note 13)
This protocol assumes the use of the BioRad iCycler. Adjustments to the protocol may be required using other devices. We use the generalized Sybr® green detection system that does not require the generation of independent labeled probes.
Turn on the lamp, camera and thermal cycler at least 30min before a reaction starts to stabilize per manufacturer’s suggestion.
Prepare standards and samples. Our calibration standard for titration consists of a series of 10-fold dilutions of phage. We routinely generate this calibration standard line of infectious phage that ranging from 100 phage/mL to 1×109 phage/mL. We have also run ssDNA and dsDNA preparations of phage in q-PCR reactions.
- Prepare the master mix for the number of reactions needed.
Component Volume per sample 2× IQ SYBR® Green Supermix 50ul 10uM forward primer 3ul 10uM reverse primer 3ul H2O 34ul Total 90ul Dispense 90 µl aliquots of mastermix to clean PCR tubes (1 aliquot/sample or DNA standard). Add 10ul of standard DNA or phage sample to 90ul of the master mix.
Mix the 100 µl complete reaction mixture and dispense the reaction mixture into 3 wells of a 96-well plate that is optically suitable for Q-PCR reaction at 25µl/well using an 8 channel pipet.
Spin down the plate to exclude bubbles at the bottom of the wells.
- Enter the PCR Protocol and Plate Setup files from the iCycler or appropriate software. We use a 3-step protocol for the individual amplification cycles:
Procedure Temperature Time Hot start 95°C 3min Amplification cycles: 1. denature 95°C 30sec 2. anneal 55°C 30sec 3. extension 72°C 30sec 40 cycles Denature before melt curve analysis 95°C 1min Annealing before melt curve analysis 55°C 1min Melt curve analysis 0.5°C up 10sec 80 cycles End 4°C hold The software for the iCycler (and other real-time thermocyclers) will automatically determine the parameters of the standard line and calculate the values of phage titers for each sample. Attention should be paid to the threshold parameter established by the software, the slope of the standard line (should be close to 3.2–3.3), and the melt curve analysis which indicates the specificity of the amplified product. The PCR products can be evaluated by agarose gel electrophoresis if there is a question about amplification specificity.
3.6 Colony PCR for determination of displayed peptide sequence
Dispense 50 µl water to16 PCR tubes.
Label and pick 16 well-spaced colonies on a titer plate of interest (see Note 14).
Using a plastic pipet tip or toothpick, stab each colony and mix it into a different tube prepared in step 1.
Heat the samples at 95°C for 5 minutes to lyse bacteria and denature proteins.
Cool the samples while preparing PCR master mix containing 100 µl 10× PCR buffer without MgCl2, 60 µl 25 mM MgCl2, 20 µl 10 mM dNTP mix, 20 µl 10 µM forward primer (fd-tet F1), 20 µl 10 µM reverse primer (fd-tet B1), 730 µl water and 10 µl Taq polymerase.
Dispense 48 µl PCR mastermix to 16 new PCR tubes.
Add 2 µl of lysed bacterial colony sample to each aliquot of mastermix.
- Perform PCR. We use the following protocol:
Procedure Temperature Time Hot start 95°C 2 min Amplification cycles: 1. denature 95°C 30 sec 2. anneal 55°C 30 sec 3. extension 72°C 30 sec 35 cycles Final extension 72°C 5min End 4°C hold Evaluate PCR products on 1 % agarose gel. Single products of approximately 450 bp are expected.
Remove dNTPs and oligonucleotide primers from the PCR product by combining 20 µl of PCR product with 2 µl exonuclease I and 2 µl shrimp alkaline phosphatase (SAP). Incubate at 37°C for 30 minutes followed by 15 minutes at 85°C.
After exonuclease I and SAP treatment, the PCR product can serve directly as template in a dideoxy terminator DNA sequencing reaction. We use BigDye®Terminator v3.1 in the following mixture: 5 µl treated product, 1 µl 10 µM primer (fd-tet F1), 3 µl 5× reaction buffer (supplied with BigDye® Terminator), 2 µl BigDye® Terminator Mix, and 9 µl water. BigDye® reactions are prepared in a 96 well plate.
- Perform sequencing PCR reaction using the following conditions:
Procedure Temperature Time Hot start 95°C 2 min Amplification cycles: 1. denature 95°C 30 sec 2. anneal 55°C 30 sec 3. extension 72°C 30 sec 30 cycles Final extension 72°C 5min End 4°C hold Purify BigDye reaction by adding 80 µl sequencing stop/precipitation reagent.
Collect precipitates by centrifugation at 3,000 × g for 30 minutes.
Invert plate onto a paper towel to collect liquid.
Centrifuge inverted plate at 100 × g for 1 minute to remove liquid from plate.
Wash precipitates with 150 µl 70% ethanol. Centrifuge at 3,000×g for 10 minutes.
Invert plate onto a paper towel and centrifuge at 100 × g for 1 minute to remove liquid from plate.
Add 26 µl Hi-Di formamide™ to each well.
Heat to 95°C for 5 minutes.
Cool plate and load onto ABI 3100 automated sequencer or other suitable sequencer.
Translate DNA sequence to peptide sequence for the segment encoding pIII protein at the point of the library insertion.
3.7 Selectivity and specificity determinations (see Note 15)
The selectivity determination is performed in a manner similar to the phage biopanning protocols for adherent and non-adherent cells detailed in sections 3.1 and 3.2 given above, with some important modifications.
Selectivity determinations require 2 matched wells of adherent cells or 2 aliquots of non-adherent cells. The adherent cells should be seeded onto wells 24–48 hours before the experiment. For non-adherent cells, use 2 million cells/phage.
On the day of the experiment, the receptors are cleared by incubating cells in serum-free media for 2 hours.
- Approximately 15 minutes before the end of the clearing step, prepare the specific phage and control phage as separate solutions, each as follows:
- 10 µl Chloroquine (100 × Stock)
- 40 µl protease inhibitor without EDTA (25 × Stock)
- 1×108 phage as input (see Note 16).
- Bring each mixture to 1 mL final volume by addition of PBS+ with 0.1% BSA.
Wash cells once with 1mL PBS+ with 0.1% BSA that was pre-warmed to 37°C. Remove the wash solution from the cells.
Save 50 µl of the input phage solution for titer determination. Add the remainder of the phage solution to the cells and incubate for 10 minutes at 37°C in a standard tissue culture CO2 incubator.
After the 10 minutes incubation, aspirate the supernatant directly from adherent cells. Pellet non-adherent cells for each wash cycle.
Add 1 mL PBS+ with 0.1% BSA (room temperature)
Incubate for 5 minutes.
Aspirate buffer and repeat step 8–10 4 times to remove all weakly bound phage.
Acid elute/wash 1–2 times at room temperature: add 1 mL 0.1 M HCl-Glycine, pH 2.2 + 0.9% NaCl.
Incubate for 5 minutes. Time could be reduced if the cell line is fragile and lysis is problematic. (see Note 9).
Repeat acid wash once.
Remove the second acid wash and add 1 mL of 30mM Tris –HCl, pH 8.0 to the cells and incubate on ice for 30 minutes.
Freeze cells in plate or centrifuge tube. This is a suitable place to stop the protocol if needed.
Thaw cells and scrape off plate, if needed. This sample is referred to as the output fraction.
Set up titration of input and output phage for the control and specific phage as detailed in section 3.3.
- The following calculations are used to compare phage binding to cells:
- Output/Input ratio (O/I) = output titer of phage/ input titer of phage applied to cells.
- Selectivity index = (O/I for specific phage clone)/(O/I for control phage).
- Specificity is determined as the selectivity index for a specific phage clone tested on a panel of cell lines and primary cells.
3.8 Monomeric peptide synthesis
Monomeric peptide syntheses are performed on a Symphony® peptide synthesizer by Fmoc solid-phase peptide synthesis but all methods reported here can be adapted to other solid phase synthesizers.
For a 0.1 mmol synthesis, place 141 mg Rink Amide AM resin (substitution level 0.71 mmol/g) in the peptide synthesis reaction vessel. Place the reaction vessel in one of the positions of the synthesizer. Add 2.5 mL DMF to swell the resin. Flush with nitrogen to form a suspension of resin for 30 minutes (see Note 17). Drain off DMF.
Repeat step 2 three times.
Add 2.5 mL Piperidine in DMF (20%) to deprotect the resin by removing the Fmoc moieties. Flush with nitrogen for 10 minutes. Drain off reagent. Repeat twice.
Add 2.5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Add 2.5 mL Fmoc-protected amino acids in DMF (200 mM) to the deblocked peptidyl resin. Add 2.5 mL HBTU, HOBt and NMM in DMF (200 mM) to the resin. Flush with nitrogen for 45 minutes. Drain off reagent.
Add 2.5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Repeat the cycle from step 4 to step 7 for the next amino acid coupling until the completion of the peptide synthesis. (see Notes 18,19)
Add 2.5 mL of 20% piperidine in DMF to the resin. Flush with nitrogen for 10 minutes. Drain off reagent. Repeat twice.
Add 2.5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Add 2.5 mL DCM to the resin. Flush with nitrogen for 30 sec. Drain off DCM. Repeat nine times.
Dry the resin for 10 minutes.
Place the dry resin in a 50 mL round bottom flask. Add 5 mL cleavage cocktail TFA: EDT:H2O:TIS (94%:2.5%:2.5%:1%) to the resin. For cysteine containing peptides use TFA: EDT:H2O:TIS (94%:2.5%:2.5%:1%) (see Notes 7 and 20). Flush flask with nitrogen, stopper and leave to stand at room temperature with occasional shaking for 3 hours.
Remove the resin by filtration under reduced pressure through a sintered glass funnel. Wash the resin twice with 3 mL cleavage cocktail (see Note 21).
Combine filtrates and transfer to an appropriate sized round-bottomed flask. Concentrate the TFA and scavenger mixture quickly to a volume of approx 3 mL on a rotatory evaporator (see Note 22).
Fill a 50 mL conical tube about two-thirds full with cold diethyl ether. Add the concentrated peptide / TFA solution into cold ether using a Pasteur pipette. Place the cold ether with the peptide precipitate at −80° C freezer overnight.
Centrifuge the cold ether with the peptide precipitate solution at 4°C for 10 minutes at 2800–3000 × g. Carefully decant the ether from the tube (see Note 23).
Add 50 mL fresh diethyl ether, seal and shake the tube to resuspend the peptide. Centrifuge for 10 minutes under the same conditions. Repeat three times.
Decant the ether from the tube. Put the tube in the hood for aproximatley15 minutes to evaporate trace amounts of residual ether. Put the tube in the vacuum desiccator and dry the peptide under vacuum for 3 hours. Weigh the peptide.
3.9 Linear tetrameric peptide synthesis (see Note 24)
Tetrameric peptides syntheses are performed on a Symphony® synthesizer (Rainin Instruments, Protein Technologies, Inc. Woburn, MA) by Fmoc solid-phase stepwise peptide synthesis on trilysine core. These instructions are easily adaptable to other automated peptide synthesis instruments.
For a 0.1 mmol synthesis, Place 476 mg Fmoc4-Lys2-Lys-β-Ala-CLEAR™ Acid Resin (substitution level 0.21 mmol/g) in the peptide synthesis reaction vessel (see Note 25). Place the reaction vessel in one of the positions of the synthesizer. Add 5 mL DMF to swell the resin. Flush with nitrogen to form a suspension of resin for 30 min (see Note 17). Drain off DMF.
Repeat step 2 three times.
Add 5 mL of 20% piperidine in DMF to remove the Fmoc protecting groups on the resin. Flush with nitrogen for 10 minutes. Drain off reagent. Repeat twice.
Add 5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Add 5 mL Fmoc-protected amino acids in DMF (200 mM) to the deblocked peptidyl resin. Add 5 mL HBTU, HOBt and NMM in DMF (200 mM) to the resin. Flush with nitrogen for 45 minutes. Drain off reagent.
Repeat step 5–6. (see Note 26)
Add 5 mL DMF to the resin. Flush with nitrogen for 30 seconds. Drain off DMF. Repeat six times.
Repeat the cycle from step 4 to step 8 for the next amino acid coupling until the completion of the peptide synthesis.
Add 5 mL Piperidine in DMF (20%) to the resin. Flush with nitrogen for 10 minutes. Drain off reagent. Repeat twice.
Add 5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Add 5 mL DCM to the resin. Flush with nitrogen for 30 sec. Drain off DCM. Repeat nine times.
Continue from step 12, section 3.8
3.10 Core synthesis for convergent tetrameric synthesis
Maleimido activated core syntheses are performed on a Symphony® synthesizer by Fmoc solid-phase peptide synthesis. These methods can easily be adapted for other solid phase peptide synthesizers.
For a 0.1 mmol synthesis, place 476 mg Fmoc4-Lys2-Lys-β-Ala-CLEAR™ Acid Resin, Fmoc4-Lys2-Lys-Lys(Biotin-PEG)-β-Ala-CLEAR™ Acid Resin and Fmoc4-Lys2-Lys-Cys(Acm)-β-Ala-CLEAR™ Acid Resin respectively (substitution level 0.21 mmol/g) in the peptide synthesis reaction vessel (see Note 27,28). Place the reaction vessel in one of the positions of the synthesizer. Add 5 mL DMF to swell the resin. Flush with nitrogen to form a suspension of resin for 30 minutes. Drain off DMF.
Repeat step 2 three times.
Add 5 mL of 20% piperidine in DMF as a deprotection reagent to remove Fmoc protecting groups from the resin. Flush with nitrogen for 10 minutes. Drain off reagent. Repeat twice.
Add 5 mL DMF to the resin. Flush with nitrogen for 30 sec. Drain off DMF. Repeat six times.
Add 2.5 mL 3-Maleimidopropionic acid in DMF (200 mM) to the deblocked peptidyl resin. Add 2.5 mL HBTU, HOBt and NMM in DMF (200 mM) to the resin. Flush with nitrogen for 24 h. Drain off reagent.
Continue from step 10, section 3.98
3.11 Tetrameric peptide synthesis by convergent strategy
3.12 Removal of an acetamidomethyl group (Acm) from a uniquely placed cysteine residue
Dissolve 1 µmol tetrameric peptide possessing an Acm protecting group in 1 mL of TFA: anisole (99:1).
Add 28 mg of silver acetate. Stir the solution at 4°C for 2 h.
Concentrate under Argon to 0.5 mL.
Add 5 mL cold diethyl ether. Centrifuge the cold ether with the peptide precipitate solution at 4°C for 10 minutes.
Decant the ether from the tube.
Add 1 mL of 0.2 M dithiothreitol prepared in 1 M acetic acid. Vortex solution at room temperature for 3 hours.
Add 0.5 mL of 8M guanidine hydrochloride. Filter the solution by syringe driven filter unit (0.22 µm porosity). Purify the peptide by HPLC.
3.13 Peptide purification and characterization
Filter eluents through a 0.45 µm Teflon® filter before use.
Dissolve 15 mg of crude peptide in 2 mL of Buffer A. Filter the sample through a 0.22 µm filter.
Equilibrate the HPLC preparative column under the following initial conditions. Solvent: buffer A. Flow rate: 10 mL /minute. Detection wavelength: 220 nm.
Once a stable baseline is obtained, inject 2 mL of the sample and use the elution profile in a linear gradient (referred to as Method A): 0–1 minute, 90%A, 10%B; 1–61 minutes, eluent B was increased from 10–40% at a flow rate of 10 mL /minute.
Collect the target peptide peak which is generally the major peak (see Note 30).
Lyophilize fractions containing the peptide product. If available, the fractions can be analyzed by MALDI MS to determine which fractions to collect.
For analytical HPLC, dissolve 1 mg of purified peptide in 1 mL of Buffer A. Filter the sample through a 0.22 µm filter.
Equilibrate the HPLC analytical column under the following initial conditions. Solvent: buffer A. Flow rate: 1 mL /minute. Detection wavelength: 220 nm.
Once a stable baseline is obtained, inject 100 µL of the sample and use the elution profile in a linear gradient (referred to as Method B): 0–1 minute, 90%A, 10%B; 1–51 minutes, eluent B was increased from 10–60% at a flow rate of 1 mL /minute.
Confirm the peptide mass by mass spectrometry. For ease we typically perform matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI MS) (see Note 31).
Edman N-terminal sequence analysis can be performed to further confirm the sequence of the peptide.
3.14 Inhibition of phage uptake by free synthetic peptides (see Note 32)
Perform phage uptake experiments in the same manner as outlined in section 3.7 except that the free peptide is added to the phage solution before addition to the cells. No prior addition of the peptide to the cells is required. We typically cover a peptide concentration range from 1 nM to 10 µM. (see Note 33)
Titer the common input sample and the individual output samples as detailed in section 3.3.
Calculate the output phage to input phage ratio in the presence of the peptide compared to the same ratio without added peptide.
3.15 Microscopy/Qdot delivery (see Note 34)
In a final volume of 100 µl PBS, streptavidin-Qdots (200 nM) are mixed with 600 nM biotinylated peptide and incubated for 2 hours at room temperature. This incubation is performed on the day the Qdots are to be used. Control SA-Qdots can be prepared using no peptide, an irrelevant sequence peptide or a scrambled version of the specific peptide.
At the end of the SA-Qdot-peptide incubation, unoccupied streptavidin sites on the Qdots are quenched by the addition of excess biotin (25 µl of a 20 µM stock) and incubated for 15 minutes at room temperature.
The mixture is diluted to 1 ml with PBS+ with 0.1% BSA to obtain a 20 nM Qdot solution for cell uptake.
Cells are incubated with Qdots on chamber slides or in polypropylene tubes for 10 minutes to 2 hours.
At the end of the incubation, the solution containing Qdots is removed and cells are washed briefly 4 times in PBS+ with 0.1% BSA. The wash solution is added and then is removed without further incubation.
Cells are briefly washed with 0.1 M HCl-glycine, pH 2.2. + 0.9% NaCl. Acid wash is removed after addition without further incubation.
Cells are washed with PBS and excess liquid is removed from the slide.
Cells can be viewed without fixation or they can be fixed using 0.4% formalin solution, 70% ethanol or cold acetone solution if desired. In this case, a 5 minute incubation in fixative is followed by PBS washes to remove fixative solution.
Incubation chamber is removed from chamber slide and Prolong® Gold antifade reagent with DAPI stain is added to the samples. If suspension grown cells were used, samples can be spotted by hand onto slide or by using a CytoSpin centrifuge, if available.
Add cover slip to slide and seal with fingernail polish.
Observe samples under microscope.
3.16 Capture of cells with phage-coated tissue culture wells
Phage coated plates can be prepared by incubating phage solution (106 phage/mL) in wells at 4° C overnight. For 12 well plates, use 1 mL phage solution per well. For 96 well plates, use 0.1 mL per well.
Phage solution is flicked from plate and residual binding sites are masked by incubation of the wells (filled to capacity) in 0.1% BSA in PBS (1 hour to overnight).
Non-adherent cells are removed from their culture flask and washed by centrifugation in PBS+ with 0.1% BSA. Cells are dispensed to wells containing specific phage or control phage or no phage and incubated for 10 minutes to 1 hour.
Wells are washed 4 times with PBS+ with 0.1% BSA to remove unbound cells.
The number of captured cells can be determined by direct cell counting – in the well or after release using non-enzymatic cell release solution or trypsinization. Alternatively, cell numbers can be determined by lysis and assay for a specific cellular component such as ATP. ATP content can be assayed using the commercially available reagent that requires only a single reagent addition to the wells (CellTiterGlo™, Promega).
The captured cells can be further characterized by downstream processes for gene or protein expression.
3.17 Capture of cells with peptide coated magnetic beads
Cells are cleared by incubation for 2hours in RPMI without serum as detailed in procedures 3.1 and 3.2.
50 µl Streptavidin-coated Dynabeads® are washed twice by suspension in 1 mL PBS and capture with a magnet for 5 minutes and removal of the wash media by aspiration.
The magnetic beads are suspended in 1 mL PBS and allowed to react with biotin-modified synthetic peptide (50 nM) for 30 minutes at room temperature.
The ligand-coated beads are washed twice in RPMI without serum to block any remaining streptavidin sites (RPMI contains 200 mg biotin/l).
Magnetic beads are washed once in PBS+ with 0.1% BSA, then resuspended in 1 mL PBS+ with 0.1% BSA and mixed with cells.
Cells are incubated with the ligand coated magnetic beads for 15 minutes at 37°C.
Non-adherent cells that take up magnetic beads are captured on the magnet (5 minutes at room temperature).
Adherent cells are released from wells using Enzyme-Free Cell Dissociation Buffer (GIBCO) before capture on the magnet. Time of dissociation will vary with the cell type. Some cells are easily released after a 5 minute incubation on ice in dissociation buffer while other are released more effectively at room temperature or even 37°C.
Cells are washed by suspension in PBS+ with 0.1% BSA by release from the magnet and recapture.
Captured cells are then suitable for additional analysis.
3.18 Flow cytometry
Cell samples are cleared by incubation in RPMI without serum for 2 hours.
- During last 15 minutes of clearing peptide solution is prepared containing:
- 10 µl Chloroquine (100 × Stock)
- 40 µl protease inhibitor without EDTA (25 × Stock)
- Synthetic peptide construct with biotin - concentration required varies with cell and peptide combination. No peptide addition and control peptide solutions are prepared separately.
- PBS+ with 0.1% BSA to volume of 1 mL/sample.
Incubate cells with peptide for 15 minutes at 37°C.
Wash the cells in PBS+ with 0.1% BSA 4 times at room temperature.
Cells are diluted in PBS+ with 0.1% BSA containing streptavidin-phycoerythrin (SA-PE) at 1:100 dilution in the mix.
SA-PE conjugate is added and cells are incubated 15 minutes at room temperature.
Stained cells are washed once and resuspended in fresh PBS+ with 0.1% BSA.
Adherent cells can be released from the wells by incubation in Enzyme-Free Cell Dissociation Buffer as detailed in procedure 3.17.
Cell staining is evaluated by flow cytometry. Cells can be counterstained with viability marker such as Alexafluor 488 -Annexin V added to resuspension buffer and incubating 5 minutes at room temperature prior to loading of sample.
Figure 1. A tetrameric lung cancer targeting peptide can selectively deliver a fluorescent nanoparticle to its target cells.
The peptide TP H1299.2 was isolated by biopanning on the large cell lung cancer cell line H1299. H1299 cells were incubated with 20nM of the tetrameric TP H1299.2 peptide conjugated to SAQDot605 (panels D–F) or 20nM tetrameric control peptide conjugated to SAQDot605 (panel A–C). Bright field images (Panels A, D) and nuclear staining images (Panels B, E) of the corresponding fields are shown. The fluorescence of the Qdot was visualized at 200-fold magnification on a Nikon TE2000 fluorescent scope. Higher magnification (1000×) shows cell surface binding of the tetrameric TP H1299.2 peptide-SAQDot605 conjugate as well internalized particles (Panels G–I). (Reproduced from ref. 5 with permission from Elsevier Science.)
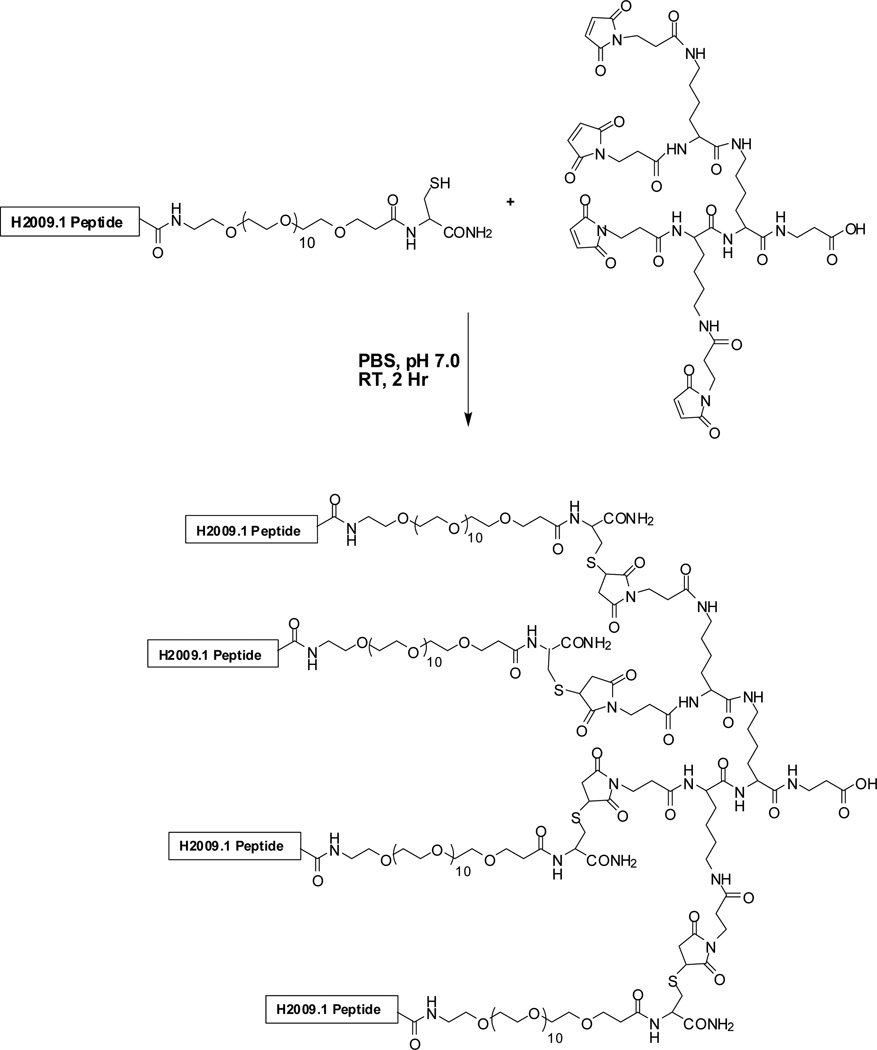
Scheme I. Convergent Synthesis of Tetrameric Cell-Binding Peptides.
A biotin or a unique cysteine can be incorporated into the peptide for use in other applications. A PEG moiety is placed between the trilysine branch and the selected peptide to increase aqueous solubility.
Acknowledgements
This work was supported by the National Cancer Institute of the NIH (1RO1CA106646 and R211R21CA114157-01).
Footnotes
The phage library used in most of our selections for cell ligands displays a 20mer peptide at the amino-terminus of the minor coat protein pIII (19). We have selected peptides from other phage display libraries (5), including the Ph.D. 12-mer library available from New England Biolabs(MJM and KCB unpublished results).
20% PEG-8000 in 0.9% NaCl solution should be prepared in advance. We routinely prepare 500 –1000 mL of this solution. PEG-8000 dissolves slowly even with constant stirring. Preparation of the solution can take several hours. After all of the PEG-8000 is in solution, filter-sterilize the solution using 0.22 µm membranes (Millipore Corp., catalog # SCGP05RE). This process is also relatively slow due to the viscous nature of the solution.
2× Sybr® green mixes for q-PCR are available from a number of companies. We have found the DyNAmo™-Sybr® Green qPCR kit (Finnzymes Inc., Distributed in USA through New England Biolabs) to produce the most consistent results in amplification of phage DNA recovered from mammalian cells and tissues. Some mammalian tissues appear to have a factor that inhibits the quantitative amplification of phage DNA from crude extracts. In this case we have purified total DNA from the tissue, using genomic DNA isolation kits from Qiagen, prior to qPCR. Even using purified DNA, inhibition was observed in some tissues. The recommended Sybr® green mix uses a polymerase that appears to be less subject to inhibition by these tissues.
While we amplify a region of the Tet resistance gene, other constant regions of the phage genome can be amplified.
Fmoc-NH-(PEG)11-COOH is incorporated to increase the water solubility of the peptide if necessary. We routinely place this PEG linker between the trilysine core and the targeting peptide in the tetrameric constructs.
In contrast to Fmoc-Lys(biotin)-OH, Fmoc-Glu(biotinyl-PEG)-OH has excellent solubility in DMF and other solvents used in solid phase peptide synthesis. The PEG-spacer restricts hindrance between the peptide and avidin, leading to better biotin binding.
Cleavage cocktails should always be prepared fresh. The cleavage procedure generally takes 2–4h to perform. However, some protecting groups are quite stable to TFA depending on the location and number in a sequence, requiring up to 12h of treatment for complete removal.
Amine impurities that could possibly remove the Fmoc group include dimethylamine found in DMF. It is recommended that DMF is protected under nitrogen or freshly purified before use.
While most cells we have tested have been stable during the acid wash step of the protocol, some cells are lysed by this treatment (primary cardiac myocytes and A20 B cell lymphoma cells as examples). In these cases, we have deleted this step from the protocol, reduced the incubation time in the acid, or only performed a single acid wash. If necessary to isolate only internalized phage, others have treated the cells with subtilisin or other proteases to inactivate surface bound phage(16).
Since the output phage sample is a mixture of individual library members, we prefer to amplify the phage mixtures as colonies on large LB-tet plates. This will allow the individual members to grow without interference from competing phage. For single phage clones, we grow infected E. coli in liquid media (LB + 12 µg/mL tetracycline). The preparation of phage from liquid cultures is the same as from plates from step 13 of section 3.4.
Titration is used to determine the number of infectious phage particle per milliliter of solution. It is used in determining the volume of a phage stock that will be added to a panning solution as well as the actual phage number in the input and output samples from a round of panning or comparative binding. Phage infection of E. coli requires that the bacteria express pili. With fd-tet phage, the phage confer tetracycline resistance on the bacteria. Bacteria without phage will not grow on the LB-tet plates. The fd-tet phage do not cause cell lysis. In fact, only phage-infected bacteria will produce isolated colonies on LB-tet plates.
During this step, phage are allowed to infect bacteria. Do not incubate the samples for longer than 15 minutes. Place samples on ice immediately after removal from the incubator. Bacteria should be plated before cells have time produce progeny phage.
We use real-time quantitative PCR for 3 distinct purposes. The first is to determine phage copy number after the phage have lost the ability to infect bacteria. For example, phage infectivity decreases rapidly after injection into a mammalian host. Secondly, we have used q-PCR to determine the presence of a specific phage clone in a mixture of phage. This has allowed us to add mixtures of phage in an experiment and have one phage serve as an internal control. Third we have used q-PCR to determine phage levels in a large number of samples simultaneously. We can determine total phage copy number using sets of primers directed at a nucleotide sequence in the backbone of the phage DNA. Additionally, we can determine the copy number of a specific phage clone using one primer directed at the nucleotide sequence encoding the displayed peptide and a second primer for a sequence from the phage backbone. Not all nucleotide sequences for the displayed peptides have been suitable for generating useful clone-specific primer sequences.
Titration plates with well-spaced and defined colonies can be used for determination of displayed peptide sequence. We initiate sequencing with the output of the third round of biopanning. DNA sequencing is also performed to verify the identity of an amplified phage clone. Since we use an ABI 3100 DNA sequencer that has a 16 capillary array, we routinely sequence samples in groups of 16.
To determine the initial success of a phage display peptide selection, we compare the binding and uptake of a specific phage with the binding and uptake of a randomly chosen, control phage using the same cells employed for the selection. The randomly chosen, control phage mimics the binding characteristics of the whole phage display library in these assays. The selectivity value is the ratio of specific phage output/input and the control phage output/input. The evaluation of specificity compares the selectivity values of an individual phage across a battery of cell lines or primary cells.
Determinations of selectivity and specificity are comparative binding assays based on the ratio of the number of phage taken up by a cell divided by the number of phage to which the cells were exposed. A specific phage clone is being compared to a control phage that represents a non-specific component of phage uptake by a cell sampling its environment. Therefore, it is important to match the amount of specific and control phage used as input. For these assays, the input phage should be added at 1 × 108 phage/sample. Deviations from the input phage number will distort the ratios used for comparison and produce artificially high or low selectivity indices.
We recommend adjusting the N2 flow to 10psi. Higher flow rates flush the resin to the top of the reaction vessel resulting in incomplete coupling.
Sometimes the coupling reaction of an activated carboxy group and a deprotected amino group is difficult to accomplish. These difficult couplings are usually sequence-dependent and not residue-specific. In these cases, a double coupling is required (i.e. repeat step 5–6 before step 7).
Fmoc-NH-(PEG)11-COOH, Fmoc-Glu(biotinyl-PEG)-OH and Fmoc-Lys(biotin)-OH are coupled in the same fashion as the natural Fmoc-protected amino acids.
Always handle thiol-containing substances in proper ventilation. These compounds have an offensive odor that can be neutralized with bleach. After the final cleavage operation, rinse all glassware, pipettes, and tubes that came into contact with scavengers with bleach before taking them out of the hood.
The expended resin should not be discarded but retained, in case it should prove necessary to repeat the cleavage reaction. Many times during a poor extraction step, peptide remains adhered to the resin beads and must extracted with an alternative solvent.
Temperature of the water bath should below 37°C.
The diethyl ether washes should be retained until the yield of product has been established. If a poor yield is obtained, the washings should be evaporated under vacuum to dryness.
Linear synthesis of the tetrameric peptides is only recommended for shorter peptides, on the order of 10 amino acids or less in length. We strongly prefer the synthetic route outlined section 3.11.
We strongly encourage reducing the substitution of the resin for tetrameric peptide synthesized linearly, longer molecules (>30 residues) or for peptides rich in β–structural elements to a substitution value lower than 0.25 mmol/g of resin.
A double coupling is usually preformed to increase the step-wise coupling yield and avoid deletion contaminants.
For the maleimido activated core synthesis, the substitution of the resin does not have to be lower than 0.25 mmol/g. A normal substitution between 0.5 – 0.8 mmol/g will suffice the synthesis.
The resin of choice depends on the down-stream application of the peptide. We frequently attach the peptides to a desired support, bead, or molecule via a unique cysteine located before the trilysine branch point. The Acm protecting group can be removed as described in section 3.12 without loss of the peptide branches. If using streptavidin conjugates, the biotin containing resin is used instead.
After 2h reaction time, the reaction solution must be purified by HPLC immediately. Otherwise, self-oxidized peptide dimer side products occur as a result of disulfide formation.
For the tetrameric peptide synthesized by linear strategy, the RP-HPLC trace typically shows one broad main peak. For monomeric peptides, the RP-HPLC trace usually shows one clear main peak. The occurrence of peaks with longer retention times than the main peak is suggestive of incomplete removal of protecting groups. For the tetrameric peptide synthesized by convergent strategy, the RP-HPLC trace typically shows two major peaks. The peak with the lower retention time is excess amount of monomeric peptide. The other with the higher retention time is the product tetrameric peptide.
For monomeric peptides, the mass is confirmed by MALDI MS using α–cyano-4-hydroxycinnamic acid as the matrix using a Voyager DE™ Pro instrument in reflector mode. MALDI Mass Spectra of tetrameric peptide is obtained in linear mode using sinapinic acid as matrix.
To determine if the peptide is functional outside of the phage particle, we determine if the peptide can block cell binding of its cognate phage. This assay is particularly useful when the cellular target of the peptide is unknown. Half maximal phage blocking can be determined as a measure of peptide affinity.
Do not use DMSO or DMF for preparation of peptide stock solutions. These compounds appear to produce a higher level of cell uptake of phage when added to cell-phage mixtures. Incorporation of a PEG moiety improves the peptide’s water solubility and often alleviates the need for organic co-solvents.
References
- 1.Brown KC. New approaches for cell-specific targeting: identification of cell-selective peptides from combinatorial libraries. Curr. Opin. Chem. Biol. 2000;4:16–21. doi: 10.1016/s1367-5931(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.McGuire MJ, Sykes KF, Samli KN, Barry MA, Stemke-Hale KA, Tagliaferri F, Logan M, Jansa K, Takashima A, Brown KC, Johnston SA. A library selected Langerhans cell-targeting peptide enhances an immune response. DNA Cell Biol. 2004;23:742–752. doi: 10.1089/dna.2004.23.742. [DOI] [PubMed] [Google Scholar]
- 3.McGuire MJ, Samli KN, Johnston SA, Brown KC. In vitro selection of a peptide with high selectivity for cardiomyocytes in vivo. J. Mol. Biol. 2004;342:171–182. doi: 10.1016/j.jmb.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 4.McGuire MJ, Samli KN, Chang Y, Brown KC. Novel Ligands for Cancer Diagnosis: Selection of Peptide Ligands for Identification and Isolation of B-Cell Lymphomas. Exp. Hem. 2006;34:443–452. doi: 10.1016/j.exphem.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Oyama T, Rombel IT, Samli KN, Zhou X, Brown KC. Isolation of multiple cell-binding ligands from different phage displayed-peptide libraries. Biosensors Bioelect. 2006;21:1867–1875. doi: 10.1016/j.bios.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett. 2003;202:219–230. doi: 10.1016/j.canlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Samli KN, McGuire MJ, Newgard CB, Johnston SA, Brown KC. Peptide-mediated targeting of the islets of Langerhans. Diabetes. 2005;54:2103–2108. doi: 10.2337/diabetes.54.7.2103. [DOI] [PubMed] [Google Scholar]
- 8.De J, Chang Y, Samli KN, Schisler JC, Newgard CB, Johnston SA, Brown KC. Isolation of a Mycoplasma-specific binding peptide from an unbiased phage-displayed peptide library. Mol. BioSyst. 2005;1:149–157. doi: 10.1039/b504572j. [DOI] [PubMed] [Google Scholar]
- 9.Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB Journal. 2003;17:256–258. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- 10.Shadidi M, Sioud M. Selection of peptides for specific delivery of oligonucleotides into cancer cells. Methods Mol. Biol. 2004;252:569–580. doi: 10.1385/1-59259-746-7:569. [DOI] [PubMed] [Google Scholar]
- 11.Hong FD, Clayman GL. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Research. 2000;60:6551–6556. [PubMed] [Google Scholar]
- 12.Zhang J, Spring H, Schwab M. Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Letters. 2001;171:153–164. doi: 10.1016/s0304-3835(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 13.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: Selection of cell-binding peptides from random peptide-presenting phage libraries. Nature Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Lillo AM, Steiniger SCJ, Liu Y, Ballatore C, Anichini A, Mortarini R, Kaufmann GF, Zhou B, Felding-Habermann B, Janda KD. Targeting heat shock proteins on cancer cells: Selection, characterization, and cell-penetrating propoerties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–9444. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 15.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, Cardo-Vila M, Giordano RJ, Jaalouk DE, Ozawa MG, Moya CA, Souza GR, Staquicini FI, Kunyiasu A, Scudiero DA, Holbeck SL, Sausville EA, Arap W, Pasqualini R. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006;66:34–40. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 16.Robinson P, Stuber D, Deryckere F, Tedbury P, Lagrange M, Orfanoudakis G. Identification using phage display of pepitdes promoting targeting and internalization into HPV-transformed cell lines. J. Mol. Recognit. 2005;18:175–182. doi: 10.1002/jmr.723. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Liu Y-h, McGuire MJ, Brown KC. Facile Synthesis of Multimeric Peptides: Development of Cell-Specific Delivery Systems. (submitted) [Google Scholar]
- 18.Zhou X, Chang Y, Oyama T, McGuire MJ, Brown KC. Cell-Specific Delivery of a Chemotherapeutic to Lung Cancer Cells. J. Am. Chem. Soc. 2004;129:15656–15657. doi: 10.1021/ja0446496. [DOI] [PubMed] [Google Scholar]
- 19.Cwirla SE, Peters EA, Barrett RW, Dower WJ. Peptides on phage: A vast library of peptides for identifying ligands. Proc. Natl. Acad. Sci. USA. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]