1. INTRODUCTION
The surface of a cell represents a collection of macromolecules, which provides the cell with a unique cellular landscape specific to the type and state of the cell. Ligands that discriminate between subtle differences in cell surface phenotypes have utility in a wide variety of research and clinical applications. In particular, cell-binding ligands that can deliver biologically active cargo to a specific cell type or a diseased cell are highly sought. While the concept of the magic bullet drug was introduced by Paul Erlich over a century ago, the scientific community has yet to fully realize this goal.1 This stems primarily from hurdles in obtaining high-affinity cell-binding ligands with the necessary discriminating power. The difficulty of the problem is realized by considering that the human body contains 210 distinct cell types, not including diseased cells, and is composed of ~1014 cells. Furthermore, once isolated, the ligand must be able to be prepared in large quantities, must be amenable to chemical modification for optimal in vivo biodistribution, and must be able to be tailored to suit a variety of clinical applications.
As antibodies typically have high affinity and specificity for their targets, they have garnered attention as cell-targeting agents. Monoclonal antibodies (mAbs) can be generated against differentially expressed cell surface features, and the number of FDA-approved mAbs that bind to cell surface antigens continues to grow.2 mAb therapies are used to treat a variety of diseases. However, most of the clinically approved therapeutic mAbs are not conjugated to drugs or toxins and therefore fall into the category of molecularly targeted therapies. Such antibodies function passively by either blocking the activity of receptors or activating the immune system to destroy the antibody target.3 Only a few clinically approved mAbs carry a deliverable. For example, two radiolabeled antibodies, Zevalin (ibritumomab tiuxetan) and Bexxar (iodine-131 tositumomab), are approved in the United States; both are anti-CD20 antibodies used for select patients with non-Hodgkin’s lymphoma. The only clinically approved antibody–drug conjugate in the United States is Adcetris (brentuximab vedotin). Approved in 2011, Adcetris is an anti-CD30 antibody conjugated to the highly toxic microtubule-disrupting agent monomethyl auristatin E and is utilized for the treatment of Hodgkin’s lymphoma (HL) or systemic anaplastic large-cell lymphoma (sALCL). Mylotarg, a calicheamicin anti-CD33 antibody conjugate, was recently removed from the market after 10 years in the clinic for failing to show efficacy.
Despite their successes, mAbs have limitations, especially in their ability to serve as delivery vehicles. Significantly, chemically modifying antibodies is challenging, and production costs are substantial. Additionally, nonspecific clearance of antibodies by the reticuloendothelial system can lead to accumulation of conjugated drugs or toxins in unwanted sites such as the liver and bone, damaging these organs.4,5 Recently, concerns have risen over post-translational modifications on mAbs, especially glycosylation, which can trigger severe hypersensitivity reactions. Due to their long in vivo half-lives, intact mAbs are not well suited for molecular imaging techniques, requiring the use of antibody fragments (Fab’s). Of the approved mAb therapies, only 11 different cell surface biomarkers are targeted. This is a minute fraction of the cell surface repertoire.
Peptides are an attractive alternative to antibody-targeting therapies. Unlike antibodies, peptides are easy to synthesize in large quantities,6 and their smaller size improves tissue penetration while preventing nonspecific uptake by the reticuloendothelial system. Additionally, peptides can be chemically modified to alter affinity, charge, hydrophobicity, stability, and solubility and can be optimized for in vivo use through reiterative modifications. Importantly, peptides can display antibody-like affinities for their receptors. The biological half-life of peptides is well matched with that of many clinically used radionuclides, making them attractive probes for molecular imaging. Several naturally occurring peptides have been used as delivery agents. For example, reproductive hormone peptides and their derivatives are useful for tumor targeting, due to overexpression of their receptors on many cancer cells.7,8 However, relying on known peptidic ligands limits the types of cells that can be targeted. For this reason, chemists and biologists have turned to diverse peptide libraries to select additional peptides that bind to specific cell types.
2. SCOPE OF THIS REVIEW
This review focuses on methods of selecting cell-targeting ligands from peptide libraries and the downstream use of these peptides. It includes the use of different types of peptide libraries and different selection methods. To highlight the utility of the selected ligands, we have not limited our discussion to a single cell type or disease state. Additionally, we have not merely concentrated on a single application in which these peptides can be used but have presented a broad overview of different applications. We focused on peptides isolated within the past five years but have also included peptides that have been widely used and merit discussion. It is our intention to present a complete compilation of cell-targeting peptides, but due to the scope of the field, we apologize if a peptide has been inadvertently missed. We have not included peptides that bind to nonmammalian cells, the use of naturally occurring peptide-targeting ligands, or studies using directed libraries based on known peptide sequences. Cell-penetrating peptides are not discussed as these peptides do not deliver cargo in a cell-specific fashion. These topics have been reviewed elsewhere.7–10
3. PEPTIDE LIBRARIES USED TO SELECT CELL-BINDING PEPTIDES
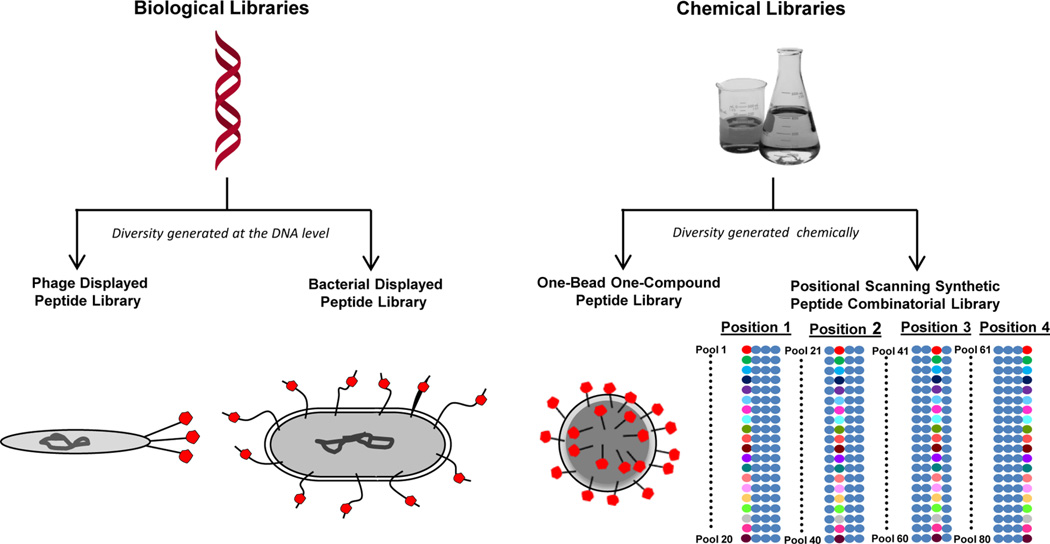
Peptide libraries used to select cell-binding peptides can be divided into two main categories: biological libraries and chemical libraries (Figure 1, Table 1). Biological libraries have a genotype, or DNA sequence encoding the peptide sequence, that is linked to the phenotype, or expression of the peptide, as part of the library’s normal structure. This genotype–phenotype link was first demonstrated for bacteriophage display,11 which still remains the major type of combinatorial library in use for the isolation of cell-binding peptides. Other types of biological libraries include bacterial, ribosome, mRNA, yeast, cDNA, retrovirus, baculovirus, and mammalian cell display. While all of these library types are promising, only phage and bacterial display have been used to isolate mammalian-cell-binding peptides. Among the numerous types of nonbiological combinatorial libraries, one-bead one-compound (OBOC) libraries and positional scanning synthetic peptide combinatorial libraries (PS-SPCLs) are the two types that have been used to isolate peptides that bind to cells. The generation of each type of library is a review within itself, and others have reviewed this topic for each type of library.12–16 However, a brief description of the different peptide libraries is included below as the library type affects both the method of selection that can be used and the characteristics of the isolated peptides.
Figure 1.
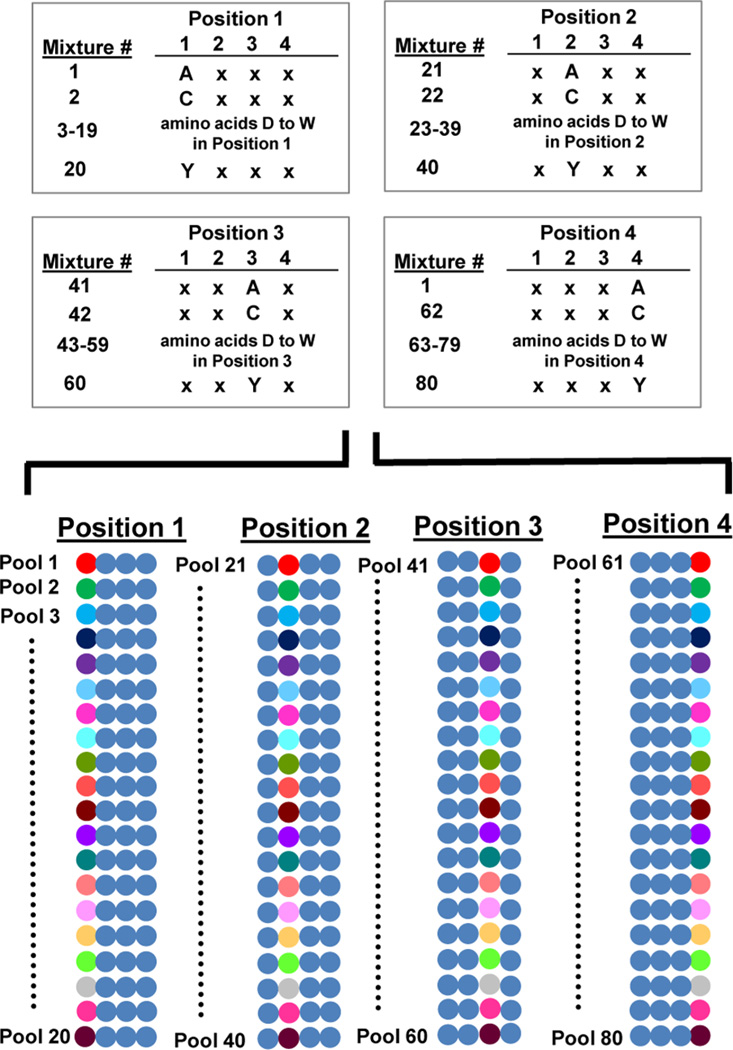
Peptide libraries used for the selection of cell-binding peptides. Biological and chemical peptide libraries have been used to isolate cell-specific peptides. For phage and bacterial display, the diversity is generated at the DNA level and there is an inherent genotype–phenotype connection. For one-bead one-compound and positional scanning synthetic peptide libraries, the diversity is generated chemically and is based on the use of a collection of monomers. The resultant peptides are displayed in red for clarity. The PS-SPCL schematic illustrates the pools of peptide libraries generated for a tetrameric peptide where each of the 20 amino acids is a unique colored circle and the mixture of 20 amino acids is shown in blue.
Table 1.
Comparison of Different Peptide Libraries
| Library Type |
Format | Strengths | Weaknesses |
|---|---|---|---|
| Biological Libraries | Phage display |
|
|
| Bacterial display |
|
|
|
| Chemical Libraries | Positional Scanning Synthetic Peptide Combinatorial Library (PS-SPCL) |
|
|
| One-bead one-compound (OBOC) |
|
|
3.1. Phage Display Libraries
Bacteriophage (phage) is a single-stranded DNA virus that infects bacteria and is widely used to generate biological ligand libraries, known as phage display. The field of phage display began with George Smith’s discovery in 1985 that foreign peptide sequences can be inserted into coat proteins of filamentous phage without altering phage function.11 DNA sequences encoding a unique peptide are inserted into the DNA for a phage coat protein such that, as the phage assembles, it expresses the protein–peptide fusions and incorporates them into the normal phage structure. The result is a phage that displays a unique peptide on the surface of one of its coat proteins, allowing this peptide to direct phage binding to a target of interest. Phage manipulation has allowed for display of numerous ligand types, including peptides, antibodies, and receptors.14,17,18 Moreover, phage coat proteins are accommodating and allow display of both linear and cyclic, cysteine disulfide-linked peptides. Peptide phage libraries usually have a diversity of 108–1010 different phage displaying different peptide sequences. Importantly, phage manipulation is relatively straightforward; phage are easy to grow and amplify by infecting the bacteria Escherichia coli. Additionally, the unique peptide encoded by a phage is easily determined using DNA sequencing.
3.1.1. Types of Phage Display Libraries
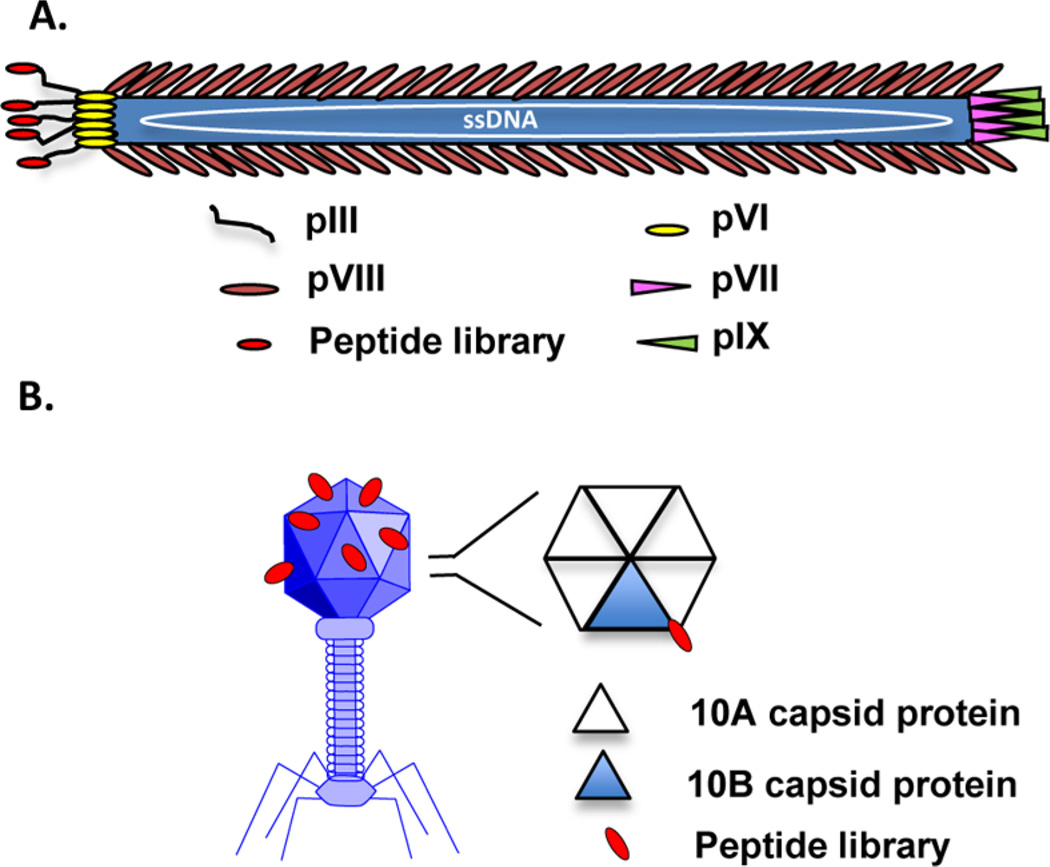
Nonlytic filamentous phages, which assemble in and secrete from their bacterial hosts without bacterial cell lysis, are commonly used for library construction. The filamentous phage is a flexible rod composed of five capsid protein types encasing a large, circular single strand of DNA (Figure 2A).14 The majority of the phage body is comprised of ~2700 copies of the major coat protein pVIII, encoded by a single pVIII gene. Both ends of the phage body are composed of minor coat proteins; one end displays five copies of the minor coat proteins pIII and pVI, while the other end displays five copies of the minor coat proteins pVII and pIX. While all of the phage proteins can accommodate a foreign peptide sequence, peptides are generally displayed at the N-terminus of the pIII or pVIII proteins.12 If display of the peptide must present a free carboxy terminus, inverted pVIII proteins have been developed.19,20 The filamentous phage M13 and the closely related fd filamentous phage are most commonly used for polypeptide display11 due to their ease of replication and their ability to accommodate large pieces of foreign DNA. M13 phage displaying peptides from the N-terminus of their pIII protein are widely used due to their commercial availability. The fd filamentous phage is also commonly used for pIII and pVIII display, largely due to vectors generated by George Smith’s laboratory.21–23
Figure 2.
Filamentous and lytic phage structures. (A) Schematic representation of fd filamentous phage. The random peptide is shown fused to the amino terminus of the pIII coat protein. (B) Representative T7 lytic phage structure. The T7 phage head is comprised of the 10A and 10B capsid proteins arranged as hexamer or pentamer units at a total of 415 proteins per head. A graphical representation of the hexamer capsid unit is shown with a random peptide (red) fused to the 10B protein (blue triangle). T7 phage can be modified to express varying ratios of 10B to 10A protein, displaying peptide sequences in 1–415 copies.
Peptides can be displayed such that every copy of the coat protein displays a peptide. Alternatively, the peptide-displaying coat protein can exist as a hybrid with the normal, wild-type coat protein.14 pIII peptide libraries typically express the peptide on every copy of the pIII protein, resulting in a multivalent presentation of the peptide on the tip of the phage particle.21,24–26 However, pIII mediates phage binding to the F pilus of an E. coli and initiates infection of the bacterium, which is required for library amplification. Large fusion proteins can disrupt this process, requiring the use of a pIII hybrid phage; the wild-type pIII initiates phage binding to E. coli, while the pIII–peptide fusion presents the randomized library. As the pVIII coat protein exists in many copies, pVIII display libraries are frequently used in the hybrid form in which only a fraction of the pVIII protein expresses peptides.23 However, some groups have used a “landscape” display in which all of the pVIII proteins express peptides.22
Although less common, lytic phage that lyse their bacterial hosts as they exit are also used for phage display of peptides. The lytic phage structure is very different from the filamentous phage structure; lytic phage have an icosahedral head and a short tail (Figure 2B). The T7 lytic phage species is typically used for phage display. The outer shell of the T7 phage head is comprised of the 10A and 10B capsid proteins, at a total of 415 proteins per head.27 Peptide sequences are typically displayed as C-terminal fusions of the 10B capsid protein. In the wild-type phage, approximately 10% of the total capsid protein is the 10B form.28 However, the T7 phage can be modified to express different amounts of 10B versus 10A protein, displaying peptide sequences in 1–415 copies.29
The diversity of phage-displayed peptide libraries is generated at the DNA level.30 Most libraries use an NNK construction in which N = A, C, G, or T and K = G or T. This construction produces all 20 amino acids and minimizes stop codons. The one possible stop codon, TAG, is suppressed in certain E. coli strains. However, this construction does not result in all amino acids being represented equally. For example, the amino acids leucine, arginine, and serine are represented three times, whereas most amino acids are represented only one time. Therefore, these amino acids can be over-represented in peptides isolated from these libraries. To overcome this problem, the random DNA insert can be synthesized using trinucleotide blocks.31 This eliminates stop codons, amino acid bias, and rare codons.
Important consequences arise from the choice of phage library. As multiple copies of a peptide are displayed on the phage particle, the selection process can rely on multivalent binding. This is especially true for pIII libraries in which the peptides are displayed on the tip of the phage. Peptides isolated from these libraries often have poor affinities when synthesized as monomeric peptides. However, multimerizing these peptides on a scaffold often rescues their affinity. The use of hybrid libraries or low-copy-number T7 phage can bias the selection toward peptides that have higher affinities as monomers. In addition, the “completeness” of diversity must be considered. As the length of the peptide increases, the representation of all possible sequences in the library decreases (20n). For cell-binding peptides, most of the focus has been on the longer peptide libraries (12–20-mers) at the sacrifice of library completeness. Surprisingly, this has not significantly impacted the isolation of cell-binding peptides; there is almost always a cell-binding peptide in the library. However, the isolated peptide may not be the optimal sequence and may need to undergo further optimization. Additionally, identification of consensus sequences among phage clones is unlikely as nearest neighbors may simply not exist.
3.1.2. Advantages and Disadvantages of Phage Display
There are a number of advantages to using a biological peptide library such as phage display (Table 1). Phage display libraries are inexpensive, commercially available, and easy to amplify and reuse by simply allowing the phage to replicate in bacteria. In addition, they can be aliquoted and stored at −80 °C for years. Importantly, phage can accommodate different peptide sequences, including cyclic peptides, and typical libraries display from 108 to 109 different peptide sequences. Peptide selection can occur in vitro, ex vivo, or in vivo, as described in more detail in section 4. Phage libraries are tolerant of a variety of selection conditions and can endure harsh washing conditions. Despite these many advantages, phage display is not without disadvantages. First, as for any biological library, amino acid and sequence biases will exist in the library, resulting in a decrease in library diversity.16,32 This results from differences in the synthesis and packaging of different peptide sequences that are fused to the phage coat protein as well as differences in the efficiency of E. coli infection of different clones. Small differences in growth rates during the amplification of the phage pool dramatically affect diversity, although approaches such as amplification on agarose plates or in monodispersed droplets can minimize these problems. T7 libraries exhibit less bias than filamentous phage libraries but are not completely free of this problem.33 Second, while phage are ideal for displaying linear peptides and simple cyclic peptides, they cannot accommodate more complicated chemical structures, such as branched or bicyclic compounds, and they are typically limited to naturally occurring amino acids.18 d-Amino acids are traditionally difficult to incorporate, 18 although a recent study demonstrated the ability to incorporate d-amino acids into phage libraries.34
3.2. Bacterial Display Libraries
3.2.1. Types of Bacterial Display Libraries
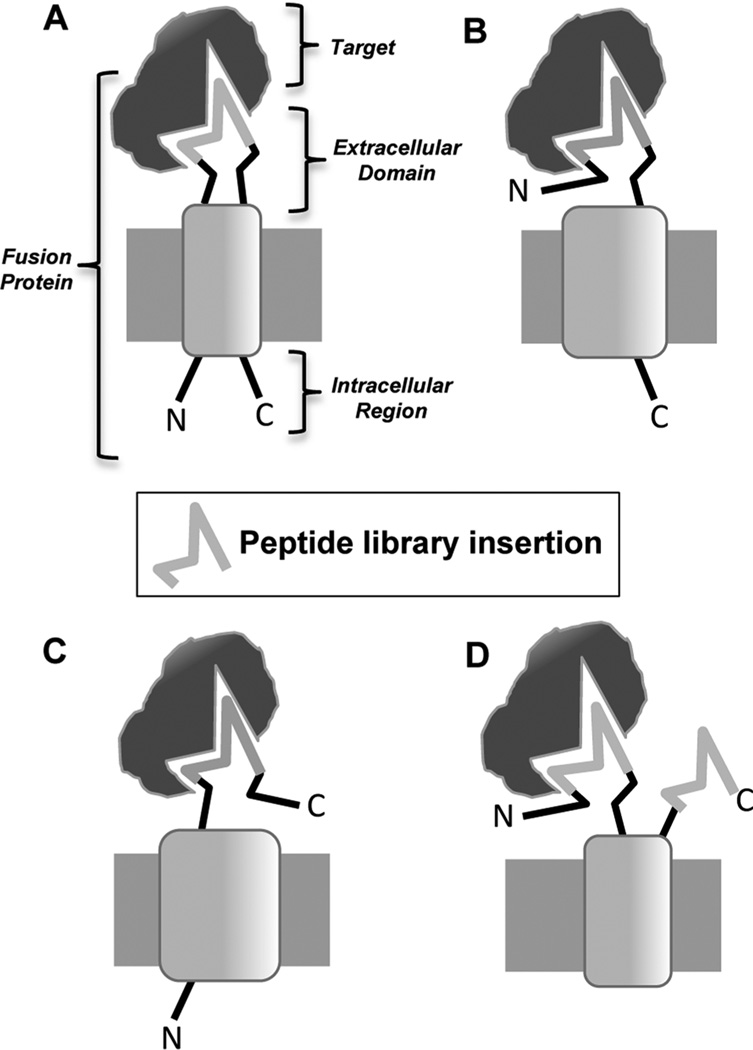
Peptide libraries can also be displayed on the surface of bacteria. As E. coli is easy to manipulate and grows quickly, it is ideal for display libraries.35 Typical libraries can incorporate up to 1011 different peptides.35 Once libraries are made, they are amplified by growth in typical bacterial liquid culture, and specific clones can be isolated by plating the bacteria on agar.36 E. coli libraries are made by genetically incorporating peptides into the membrane flagella and fimbriae proteins. While a variety of different bacterial proteins can be used for these libraries,35 peptide libraries have been incorporated into FliTrx, OmpA, CPX, and invasin proteins for creation of cell-binding peptide libraries. For all of these display formats, the peptide can be fused to the N- or C-terminus of the bacterial protein or inserted into the middle of the protein (Figure 3).35 Several reviews describe the use of bacterial display.35–40
Figure 3.
Different types of bacterial display peptide libraries. Peptide libraries, including FliTrx, OmpA, CPX, and invasin libraries, have been incorporated into bacterial membrane proteins at different locations as shown in the four panels. (A) Peptide library insertion into the middle of the membrane protein. (B) Display of the peptide library at the N-terminus of the membrane protein. (C) Display of the peptide library at the C-terminus of the membrane protein. (D) Display of the peptide library through a combination of N- and C-terminal display.
Insertional libraries, where the peptide library is inserted into the middle of the bacterial membrane protein such that it forms a loop that sticks out of the membrane with the N- and C-termini of the membrane protein inside the bacterium, include the FliTrx and OmpA libraries. The FliTrx library is particularly convenient as it is commercially available. In this type of library, developed by McCoy and colleagues, peptides are inserted into the active site of the E. coli thioredoxin protein and the entire peptide–thioredoxin protein fusion is subsequently inserted into the E. coli flagellin protein.41 Thioredoxin is used because the active site forms a disulfide bond constrained loop that can accommodate foreign sequences and still fold properly. As the flagellin protein is the major component of the bacterium’s flagella, the peptide–thioredoxin–flagellin fusion protein is displayed on the cell surface as a partially functional flagellum.41 The unique peptide is displayed in a disulfide bond constrained loop that extends out of the body of the thioredoxin protein, allowing it to bind selectively to the target protein or cell type.41,42 An advantage of this form of peptide display is that the isolated cyclic peptides are structurally constrained and are active outside the context of the thioredoxin protein.
N-terminal libraries, such as the CPX library, involve fusion of the peptide to the N-terminus of an outer membrane protein. The CPX library involves a rearrangement of the OmpX outer membrane protein such that its N- and C-termini stick outside the cell membrane.43 This allows for peptide fusion at the OmpX N-terminus, C-terminus, or both,43 although most applications have used the N-terminus of the protein for peptide display. C-terminal libraries have also been used, including the invasin library. This library was created by expressing a modified form of the invasin protein from the pathogenic bacteria Yersinia pseudotuberculosis in nonpathogenic E. coli.44 Invasin is a bacterial membrane protein that binds to integrins, allowing the Y. pseudotuberculosis to penetrate mammalian cells. Replacing its integrin-binding C-terminus with a random peptide library and subsequent transformation into E. coli results in E. coli displaying invasin–peptide fusions at the outer membrane.
3.2.2. Advantages and Disadvantages of Bacterial Display
Bacterial display libraries have many positive characteristics (Table 1). E. coli grows quickly and is easy to manipulate both genetically and physically.35 Significantly, unlike phage libraries, which require both phage and bacteria, bacterial libraries only have one component: the bacteria. This allows for easy library growth and amplification using typical bacterial liquid culture and for the selection of specific clones by plating the bacteria on agar.36 Another major advantage of bacterial libraries is the ability to use fluorescence-activated cell sorting (FACS) for library screening, allowing for quantification of clone binding.37 This screening is relatively straightforward as the bacteria can be modified to incorporate a fluorescent label such as green fluorescent protein (GFP).37 However, the screening process is then limited to the rate of the flow cytometer, which can significantly slow the selection process.36 Other disadvantges include the complexity of the bacterial surface, which may interfere with binding of the displayed peptide.36 Additionally, while typical E. coli libraries can incorporate up to 1011 different peptides,35 other bacteria can only incorporate a library size of approximately 105.36 Significantly, bacterial display libraries can only be screened in vitro or ex vivo; they cannot be used for in vivo screening due to bacterial sepsis that would occur in the animal.
3.3. OBOC Libraries
3.3.1. Synthesis of OBOC Libraries
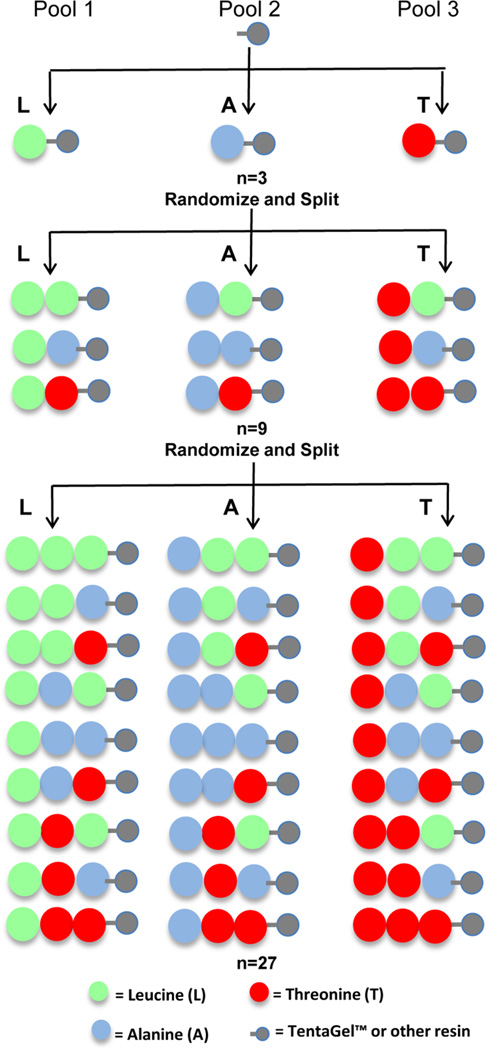
OBOC libraries are combinatorial peptide libraries synthesized on 80–100 µm beads such that each bead displays approximately 1013 copies of a single peptide.18,45 The OBOC approach using the “split-mix” synthesis method (Figure 4) was first described by Lam et al. in 199146 and has previously been reviewed in detail.18,45,47 Libraries containing α-amino acids are both easy to synthesize using standard solid-phase peptide chemistry and easy to sequence by Edman degradation using automated protein sequencing.18 However, Edman degradation requires a free N-terminus, so libraries with more complex peptide structures, such as cyclic or branched peptides or peptides containing β- or γ-amino acids, necessitate inclusion of a chemical tag in the bead structure.18 These chemical tags can be incorporated into the interior of the bead so as to not interfere with binding of the library peptides to targets of interest and then subsequently sequenced using either Edman microsequencing or mass spectrometry.18 Lam and co-workers recently described two novel methods for generating beads with interior tags, termed bilayer beads,48,49 and have recently reviewed these methods.18 OBOC libraries have also been used to select multimeric ligands; Denmeade and co-workers synthesized OBOC dimer libraries for the selection of dimeric peptides.50,51 It should be noted that the surface density of the displayed peptide results in a high local concentration of the ligand (~100 mM on a TentaGel bead), which can lead to the selection of low-affinity peptides.52 To avoid this problem, the selection can be performed in the presence of competing proteins or the stringency of the washes can be increased. Alternatively, the peptide density on the bead can be reduced, but this requires bilayer bead encoding as there is insufficient peptide on the bead for sequencing.53 Alternatively, the beads can be spatially separated.
Figure 4.
OBOC peptide library generation using “split-mix” synthesis. An example of split-mix synthesis for tripeptides composed of leucine (L), alanine (A), and threonine (T) is shown. The beads are divided into three different pools, one pool for conjugation to each of the amino acids using standard solid-phase synthesis. Pool 1 is coupled to L, pool 2 to A, and pool 3 to T. The beads from all pools are combined and randomly split into three new pools before a second round of amino acid conjugation. As before, pool 1 beads are coupled to L, pool 2 beads to A, and pool 3 beads to T. Finally, the pools are mixed and randomly sorted again for another round of amino acid conjugation. This results in a library of bead-bound peptides composed of every combination of the 3 amino acids, totaling 27 different peptide sequences (33).
3.3.2. Advantages and Disadvantages of OBOC Libraries
Similar to phage display, synthetic chemistry OBOC libraries are relatively inexpensive and easy to generate. OBOC libraries can display up to 108 different peptides, although most OBOC libraries are smaller in size (Table 1).18 However, unlike phage display, OBOC libraries are not constrained to natural amino acids and can include both unnatural and d-amino acids, in addition to secondary structures not tolerated by the phage.18 In fact, completely unnatural peptoid libraries have been used to select cell-binding ligands.54 As unnatural and d-amino acids are less susceptible to proteases and peptidases than natural l-amino acids, OBOC libraries have the potential to rapidly identify stable peptide sequences. Incorporation of post-translational modifications, such as glycosylation and phosphorylation, can be incorporated into the library design as well.55,56 The design of OBOC libraries also makes them ideal for use in optimization of known ligands. Peptides previously isolated by phage display or other methods can be used as lead compounds for OBOC library construction, allowing for rapid generation of optimized peptides with higher affinity or specificity.18 Additionally, these libraries are ideally suited for screening for peptides that induce particular cellular phenotypes, such as the induction of apoptosis.57 Finally, the process from library synthesis to sequencing positive hits can potentially be automated.58 Despite these advantages, OBOC libraries are not as widely used as biological peptide libraries. Although the recent advances in creating bilayer beads has made it easier to identify the peptides displayed by the beads, the required techniques are more involved than the DNA sequencing used to identify phage or bacterial library peptide sequences. Importantly, OBOC libraries cannot be used to select specifically for peptides that internalize into cells in vitro or for in vivo selection due to the large size of the beads.
3.4. PS-SPCLs
3.4.1. Synthesis of PS-SPCLs
Positional scanning synthetic peptide combinatorial libraries are generated by making individual synthetic peptide combinatorial libraries with one amino acid held constant while the remaining amino acids are varied.59–61 The peptide sequence is then scanned by creating additional unique combinatorial libraries, each holding a different amino acid constant. For a tetrapeptide positional scanning library, this results in four distinct library subsets such that each library subset holds one of the amino acid positions constant. These library mixtures are represented by the designations O1XXX, XO2X, XXO3X, and XXO4. For each library, the O denotes the position that is held constant with 1 of the 20 amino acids, while the X represents any amino acid (Figure 5).62 Mixture 1 consists of all peptides with a first amino acid of “A”, while mixture 2 is all peptides with a first amino acid of “C”. Each of mixtures 3–20 displays 1 of the remaining 18 amino acids in the first amino acid position. The next library subset, mixtures 21–40, contains 1 of the 20 amino acids held constant in the second library position. This scanning is continued until each of the tetrapeptide positions has its own pool of libraries.
Figure 5.
Design of a PS-SPCL library. Mixture 1 consists of all peptides with a first amino acid of “A”, while mixture 2 is all peptides with a first amino acid of “C”. Each of mixtures 3–20 displays 1 of the remaining 18 amino acids in the first amino acid position. The next library subset, mixtures 21–40, contains 1 of the 20 amino acids held constant in the second library position. This scanning is continued until each of the tetrapeptide positions has its own pool of libraries. This is represented graphically with each amino acid being represented by a unique colored circle and a mixture of the 20 amino acids being represented as a blue circle.
3.4.2. Advantages and Disadvantages of PS-SPCLs
Advantages and disadvantages of PS-SPCLs are listed in Table 1. As the PS-SPCLs can be used in solution, they are adaptable to almost any selection technique.59 They can be incubated with cells or receptors, typically in a high-throughput fashion such as in a 96-well plate or a microarray, and numerous readouts for binding exist. For example, screens can be made for binding versus competitor fluorescently tagged natural ligands (looking for loss of fluorescence)63,64 or using biotinylated peptide and streptavidin–horseradish peroxidase (HRP) as a detection reagent.65 PS-SPCLs are relatively easy and inexpensive to synthesize in large numbers. However, this approach depends upon the idea that each amino acid contributes individually to binding to the target of interest, which may make it difficult to determine ideal peptide sequences when multiple peptide motifs exist for the given target.66 Additionally, PS-SPCL selections typically require multiple rounds of peptide generation and testing for binding. After the ideal amino acids at each peptide position are determined, all possible combinations of peptides using these ideal amino acids must be generated and further tested for binding. Finally, unlike the other peptide libraries discussed previously, PS-SPCLs require spatial resolution of each minilibrary. While PS-SPCLs are not used as frequently for the initial isolation of cell-targeting peptides, they are an excellent way to optimize lead peptides isolated from a phage-displayed or bacterial peptide library.
4. USING PEPTIDE LIBRARIES TO ISOLATE CELL-BINDING PEPTIDES
4.1. Peptide Isolation by Phage Display Libraries
Peptide phage libraries were initially used to isolate ligands against known target proteins.11,12,14 This in vitro approach met with great success and allowed selection of peptide ligands for receptors without known naturally occurring ligands. Significantly, two papers published in 1996 expanded the field of phage display to include unbiased selection methods. For the first time, phage libraries were used to isolate peptides specific for given cell types without prior knowledge of the cellular receptor. Pasqualini and Rhouslahti pioneered in vivo phage display by intravenously injecting phage libraries into mice.67 After isolating organs of interest and recovering bound phage, they obtained peptides specific for the vasculature of the organs. In the second paper, Johnston and co-workers pioneered in vitro phage display against whole cells, by using cultured cells as the target and specifically selecting for peptides that could bind and internalize into the cells.68 The ligands isolated in both studies preferentially bound their target cell types over other nontarget cells. Importantly, these studies also demonstrated the feasibility of selecting peptides against receptors present in their native cellular conformations. Since these seminal papers, both in vitro selection against known target proteins and in vitro or in vivo unbiased selection against cells or tissues have been used extensively to isolate cell-binding peptides.
4.1.1. Panning against Known Targets
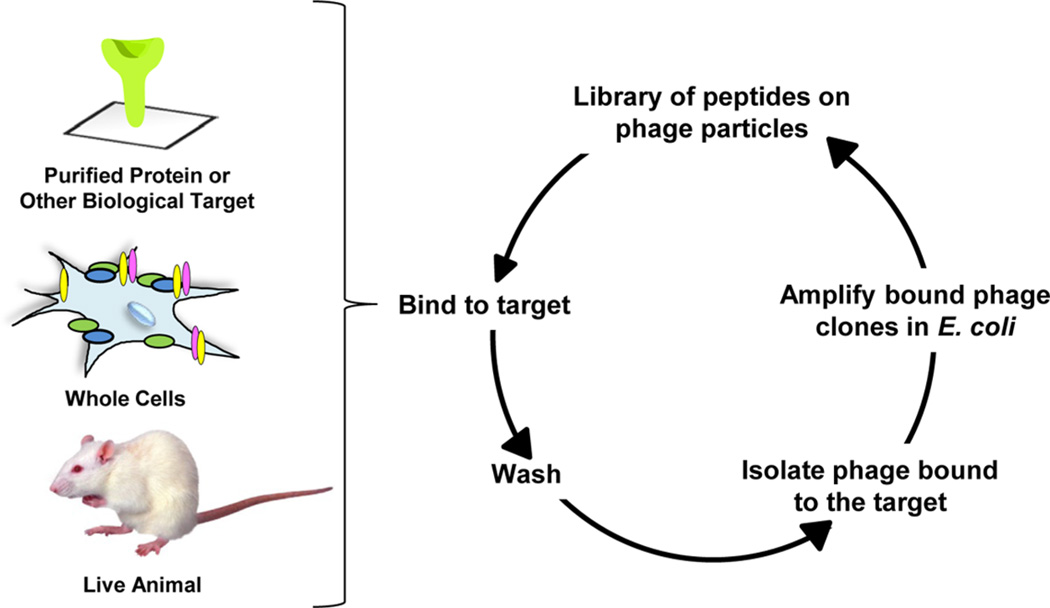
During the peptide selection process, commonly known as biopanning (panning), random phage-displayed peptide libraries are incubated with a target protein of interest to select for phage displaying peptides that specifically bind the protein (Figure 6). Typically, the target receptor is immobilized on a solid support before addition of the phage library.14 After the receptor binds and captures specific phage, the unbound phage are washed away and discarded before elution of the bound phage. The resultant phage population is enriched for binding phage and is amplified by infection in E. coli. Subsequent panning rounds are then repeated approximately 3–5 times, until specific phage clones emerge. After the final round of selection, the DNA of the resulting phage clones is sequenced to determine their peptide content and reveal the candidate receptor-specific peptides. If desired, the panning process can also include negative selections in which the phage library is incubated with a control protein or cellular lysate. Performing this selection prior to the selection against the target protein allows for narrowing of the phage library to exclude peptides that bind nonspecifically to other, nontarget proteins.
Figure 6.
Panning of phage-displayed peptide libraries. In each case, the phage library is bound to the target, which can be a purified protein, viable cells, or an animal. Nonbinding clones are removed by stringent washes, and phage associated with the target are amplified in E. coli. The process is repeated, enriching for binding peptides at each round.
While this process has been extremely successful for isolating protein-binding peptides, there are challenges in performing the selection on purified cell surface receptors. First, the primary challenge is simply isolating soluble and active membrane protein to use as bait. Membrane-bound proteins are notoriously difficult to work with due to their hydrophobic transmembrane domains and instability when removed from the lipid bilayer. Second, there is no guarantee that the isolated peptide will bind to the extracellular domain of the bait protein; ligands that bind to the transmembrane or intracellular domain of the protein are useless for cell targeting. The extracellular domain of the protein can be used as bait if the protein is modular, but this is not always possible. Third, by panning on purified protein, the other biological information contained within the cellular membrane is lost. Cell surface proteins interact with other proteins, undergo multiple post-translational modifications, and can be contained in microdomains on the cell surface, affecting the cell surface density and activity of the receptor; the purified protein is not an accurate portrayal of the receptor in its biological context. In summary, isolation of a peptide that binds to a cell surface protein by in vitro panning does not guarantee that the peptide will be a functional cell-targeting ligand.
To overcome the problems of using purified protein, the target protein can be overexpressed in a cell line.69,70 The receptor-overexpressing cells are employed for the selection process using the parental cell line for a negative selection. This approach has the advantage that the receptor is kept in a more relevant cellular context and overcomes the need for purification of the bait protein. However, it is dependent on a stringent negative selection and has not been used widely.
4.1.2. Unbiased Panning against Cells or Tissues in Vitro
Unbiased panning of phage display peptide libraries can be performed in vitro against specific cells, ex vivo against cells isolated from animal models or human patients, or in vivo against animal or human tissues. Johnston and co-workers first described phage display for the isolation of peptides binding to whole cells in vitro.68 Although whole cells are heterogeneous targets, isolated peptides typically have high cellular specificity, binding selectively to the cells they were isolated against and not to other related cell types. To help ensure cell specificity, negative selections against related cell types or against other normal cells can be used to exclude peptides that bind to all cell surfaces nonspecifically. However, such selections are often unnecessary as selection against the target cell type alone is generally sufficient to yield highly specific ligands.
In vitro or ex vivo panning against cells of interest involves a protocol similar to that used for in vitro panning against known target proteins except that the bait is now a viable cell. Both cultured cell lines and primary cells used ex vivo are amenable to the process. Random peptide phage libraries are incubated with the cell type of interest for a defined period of time before the cells are washed to remove both extracellular and weakly bound phage (Figure 6). At this point, either surface-bound or internalized phage can be chosen for further amplification. If the goal is to isolate surface-bound phage, the phage are eluted and allowed to infect E. coli for phage amplification for further rounds of panning. If the goal is to isolate internalized phage, surface-bound phage are removed by low-pH washes or treatment with a protease. Alternatively, the mixture can be centrifuged through a nonmiscible organic phage to separate unbound phage from the cell-associated phage. This separation process has been termed BASIL (biopanning and rapid analysis of selective interactive ligands).71 The cells are then lysed, and associated phage are used to infect E. coli. This panning process is then repeated approximately 4–6 times, until the ratio of input phage (total amount of phage originally incubated with the cells) to output phage (amount of bound or internalized phage) stagnates. As phage do not have tropism for mammalian cells and the only modified portion of the phage library is the unique peptide motif, the identified peptide should be responsible for mediating binding to the cell type of interest.
There are several advantages to unbiased phage panning on whole cells as opposed to panning on target proteins alone.72 First, cell receptors remain in their native membrane states—at their normal expression level, in their normal location, and with their normal membrane neighbors. As discussed above, it is impossible to recreate these same conditions for purified receptors. Second, selection can be tailored to isolate either surface-bound or internalized peptides. Selection against purified proteins only allows isolation of peptides that bind to the protein. Third, the selection is completely unbiased and can be performed without prior knowledge of cellular receptors, making it ideal for cells about which little is known. By contrast, panning on isolated proteins obviously requires prior knowledge of which receptors make good targets. Finally, due to the unbiased nature of the cell-panning approach, peptide identification and subsequent receptor identification can lead to the discovery of important cellular targets that were previously unknown.73,74
Cell-based biopanning is highly versatile. It has been used for cells from multiple species and can be performed on adherent and nonadherent cells. The process does not require extensive instrumentation and relies on common laboratory techniques. However, cell-based biopanning requires approximately 106 cells, limiting the types of cells that can be panned on ex vivo. For example, fine-needle biopsy yields only ~10 000 cells, and biopanning on β-cells of the pancreas required the sacrifice of six rats per round of panning simply to obtain enough cells.75 Recently, a phage-displayed peptide has been selected on live cells in a microfluidic device.76 This approach uses fewer cells (102–104), and the flow of fluids through the chamber is more efficient in removing nonbinding phage. Additionally, fewer cells are lost in the wash process, thus minimizing the risk of losing phage clones. This may represent a more efficient manner for selection of peptides for cell binding.
4.1.3. In Vivo Panning
In vivo phage display was first described by Pasqualini and Rhouslahti as a means to select vasculature-specific peptides.67 Typically, a random peptide phage library is injected into the tail vein of mice or rats and allowed a brief (5–15 min) circulation time. Phage recovery must occur relatively quickly after injection to maintain phage infectivity. The animals are then sacrificed and the desired tissues collected and homogenized. Phage isolated from these homogenates are then infected into E. coli for library amplification so that the panning process can be repeated. Typically, 3–5 pannings are sufficient to isolate target-specific peptides. As vasculature targets are more readily accessible and do not require tissue penetration, the vast majority of peptides selected in this manner target vasculature in the organ of interest and not the organ itself. While a longer phage incubation time in vivo aids in tissue penetration, phage infectivity also decreases during circulation. Several groups have circumvented this problem by first performing in vitro panning to narrow the phage library followed by subsequent in vivo panning. Other groups have performed in vivo panning by injection of the phage at sites closer to the organ of interest.
There are several advantages to in vivo phage panning. Just as with in vitro panning on cells, the approach keeps receptors in their native context, is entirely unbiased, requires no prior knowledge of a cellular receptor, and has the potential to identify new cellular targets. However, unlike any of the other selection methods, phage isolated by in vivo panning are inherently able to reach their target in vivo. They come with the assurance that the receptor they target is both accessible from the bloodstream and able to bind its ligand in sufficient quantity for detection. By contrast, peptides isolated from whole cells do not come with this guarantee as the receptor they target may not be readily accessible from the bloodstream or may have a different cellular localization in vivo compared to in vitro.
A potential disadvantage of in vivo panning in mice or rats is that any peptides identified as homing to organs or vasculature are binding to mouse or rat tissue. Even in human xenograft tumor models, the tumor vasculature is derived from the mouse. Thus, isolated peptides may not bind the corresponding human vasculature. To translate these peptides to clinically relevant ligands, the target receptors will have to be examined in human tissue and the peptide sequences may have to be further optimized to bind their human counterparts. To bypass these problems, a few groups have turned to panning in humans. Arap and Pasqualini were the first to perform in vivo phage display in human patients.77 A patient with the B-cell cancer Waldenström macroglobulinemia was injected intravenously with phage and tissue collected from five areas of the body—bone marrow, fat, skeletal muscle, prostate, and skin—for identification of specific tripeptide motifs.
4.1.4. Next-Generation Sequencing in Phage Display Selections
Regardless of the method of phage library panning, amplification is used between each round to enrich for bound clones. At each round, there is the risk of introducing more biological bias and a collapse of library diversity. This can result in the loss of binders and increases the risk of identifying false positives. There has recently been a push to use next-generation sequencing to identify positive clones in earlier rounds of panning.19,78–83 This approach has the advantage that greater than 106 reads can be obtained in a single run so that repeat sequences or consensus sequences can be identified after 1–2 rounds of panning. Deep sequencing can also be used to follow the diversity of libraries before the start of panning and at each round. This is particularly useful in detecting clones that have a competitive growth advantage that are amplified throughout the panning process but do not necessarily bind the target.82 Standard sequencing, in which a small sampling of clones is selected for characterization, cannot detect sequence repeats until the library diversity collapses onto a few sequences. For example, the commercially available Ph.D-7 phage-displayed peptide library was panned against KS483 cells differentiated into osteoblasts.78 Using the Illumina platform, phage were sequenced prior to the start of the experiment and after each round of biopanning. As expected, the library converges at each round of panning. However, of the 10 most abundant peptide sequences seen in round 4, 8 of these peptides are also in the top 10 after round 1. This suggests that binding ligands can be identified after 1–2 rounds of panning as opposed to 4–6 rounds. Additionally, false-positive clones were easily ruled out by sequencing the starting library after a single amplification in which the library is not panned on the target. As this is a relatively new technique, it is not obvious if deep sequencing will improve the quality of peptides isolated from phage-displayed libraries or will simply expedite the process.
4.2. Peptide Isolation by Bacterial Display Libraries
To date, all cell-binding peptides identified by bacterial display have been isolated using unbiased selection approaches. Targets include both cultured cells and freshly isolated murine or human cells. There are two methods of bacterial display: panning,41 similar to the method used with phage libraries, and a quantitative method employing FACS.84 Bacterial library panning involves incubating the cells with the bacterial library followed by extensive washing to remove unbound bacteria. Bound bacteria are then recovered by vortexing or centrifugation and regrown for repeated panning rounds. After the final round of selection, the DNA of the isolated bacterial clones is sequenced to reveal the identity of the cell-binding peptide. FACS screening for peptide selection is performed by first expressing the bacterial peptide libraries in E. coli that also express a GFP variant.84 After incubation of the fluorescent bacterial library with target cells and extensive washing to remove unbound bacteria, the cells are sorted by FACS according to GFP fluorescence. Cells with bacteria bound to them should carry a GFP signal that specifically allows for their isolation. As FACS quantifies the fluorescent signal, target cells can be sorted on the basis of the number of bacterial clones they bind. The consequence of this sorting is the isolated bacterial clones have high affinity and/or bind an abundant cell surface marker. The specific peptides displayed by the isolated bacterial clones are then determined using DNA sequencing.
4.3. Peptide Isolation by OBOC Libraries
Much like phage libraries, OBOC libraries have been used to select for peptides against both purified targets and intact cells. OBOC libraries were originally used to select ligands against target proteins.46 Then, in 1996, the same year that Pasqualini and Rhouslahti first described in vivo phage display67 and that Johnston and co-workers pioneered unbiased in vitro phage display against cultured cells,68 Pennington, Lam, and Cress first described the use of OBOC libraries for unbiased screening against live cells.85
4.3.1. Selection against Known Targets
Soluble target proteins can be screened for binding to OBOC libraries using several different approaches, all of which rely on the ability to selectively distinguish protein-bound beads. The protein of interest can be labeled with a tag (i.e., fluorescent or colorimetric dye, biotin, enzyme, radionuclide, epitope tag, etc.) that allows for detecting the protein-bound beads. Most commonly, an enzyme-linked colorimetric assay is employed. 45,46,86 This selection is relatively simple and involves the reaction of alkaline phosphatase and the colorimetric substrate bromochloroindolyl phosphate (BCIP). The protein target can be directly labeled with alkaline phosphatase, or if a primary antibody against the protein is available, an alkaline phosphatase (AP) secondary antibody can be employed. After incubation of the protein of interest with the OBOC library and unbound protein has been washed away, treatment with BCIP turns beads that have captured the AP-labeled target turquoise.86 The peptide content of isolated positive beads is then determined using Edman’s sequencing or mass spectrometry. The success of the screen is dependent on the stringency of the selection conditions and effective negative screens. Unlike phage display and bacterial peptide libraries, there is no amplification step. Thus, false-positive ligands are not diluted or lost during subsequent selection steps. This makes the screening conditions particularly important.
4.3.2. Unbiased Selection against Cells
Screening of OBOC libraries against cells in culture or against cells isolated for ex vivo screening is relatively straightforward.18 The OBOC peptide library is incubated with the cell type of interest. Positive “hits” are visualized by microscopy as beads covered in cells. These positive beads can then be selected and removed using a micropipet. As with phage display, negative selections can also be used to identify peptides that bind the cells nonspecifically. Lam and co-workers have employed two different negative screening methods to exclude cells that bind to the beads nonspecifically.18 The first negative screening method involves a screen against the cell type of interest. Beads which capture cells are isolated and treated with guanidium chloride so that the beads can be reused for incubation with a normal, control cell type. Beads binding both the target and control cell types are then discarded. In the second negative screening method, the target cells are fluorescently labeled and then mixed together with both control cells and the bead library. The control cells can be labeled with a different fluorophore or remain untreated. Beads bound to both target cells and control cells are then discarded, and beads that are only bound to the fluorescent target cells are considered hits. Just as with in vitro phage panning on cells, this approach keeps receptors in their native context, is entirely unbiased, requires no prior knowledge of a cellular receptor, and has the potential to identify new cellular targets.
4.4. Peptide Isolation by PS-SPCLs
PS-SPCLs have also been used to select for peptides against both known targets and against unbiased cellular targets. As the PS-SPCLs can be used in solution, they are adaptable to almost any selection technique. They are typically incubated with cells or receptors in a high-throughput fashion, such as in a 96-well plate or a microarray, and the readout for binding is varied. Screens can be made for binding versus competitor natural ligands that are fluorescently tagged (looking for loss of fluorescence)64,87 or can use biotinylated peptide and streptavidin–HRP as a detection reagent.65 Other screens have used specific cellular effects as the readout. Once all of the scanning library subsets are screened using the assay of choice to identify the “best” amino acid at each peptide position, additional peptides are synthesized using all of the possible combinations of the best amino acids. These peptides are then used in the same selection process as the original library to identify which amino acid sequences best target the protein or cell type of interest. This screening process requires multiple rounds of peptide synthesis and testing, yet optimized peptides are discovered, and no DNA or peptide sequencing is needed to determine the peptide composition.
5. ISOLATION OF DISEASE-SPECIFIC OR ORGAN-SPECIFIC PEPTIDES
Peptide libraries have been mined to isolate cell-targeting ligands for many different cell types and disease states. This has resulted in an arsenal of peptides that can be used for delivery of different functional cargos. This section of this review is broken down by cell types and highlights how the different types of peptide libraries can be used to isolate highly specific cell-binding ligands. We have focused on peptides isolated in the past five years and seminal cell-targeting peptides that have been used widely or are of high importance. Only those peptides that have subsequently been shown to bind their target receptor in the context of cells are included. Tables 2–17 list peptide sequences shown to bind different cell types and highlight their use.
Table 2.
Cancer-Targeting Peptides Selected in Vitro against Known Protein Targets
| Cellular Target |
Peptide Sequencea,b,c,d | Applications and Notes |
|---|---|---|
| HER2/ErbB2 | WTGWCLNPEESTWGFCTGSF94 (pIII) | Functional Peptide Activity: The peptide inhibits ErbB2 phosphorylation. |
| MCGVCLSAQRWT95 (pIII) SGLWWLGVDILG95 (pIII) |
Note: The peptides were selected as a β-lactamase fusion but neither was tested for activity as free peptides. | |
| KCCYSL96 (pIII) | Imaging Applications: Peptide targets HER2 positive tumors in vivo and has been employed as a molecular imaging probe.97–99 MicroSPECT/CT imaging has been performed using 111In-DOTA-peptide and 64Cu-DOTA, NOTA, and CB-TE2A-peptide conjugates. | |
| MARSGL100 (pIII) MARAKE100 (pIII) MSRTMS100 (pIII) |
Functional Peptide Activity: The peptides were fused to a pentameric protein in order to present them in a multimeric fashion. These “peptabodies” bound the extracellular domain of ErbB2 with low nanomolar affinity and bound selectively to ErbB2 expressing cells. | |
| EGFR | YHWYGYTPQNVI101 (GE11) (pIII) |
Therapeutic Applications: The GE11 peptide has been conjugated to doxorubicin and shown to kill EGFR-positive cells in vitro.102 GE11 peptide-Au nanoparticles with phthalocyanine killed cells after irradiation in vitro.103 A GE11-lytic peptide chimera killed cells in culture and inhibited tumor growth in mice.104 LPEI-PEG-PEI-PEG-GE11 conjugate delivered polyinosine/cytosine to EGFR expressing tumors resulting in a 2-fold increase in the mean survival of the tumor bearing mice.105 Imaging Applications: Cy5.5-labeled peptide and Cy5.5-labeled peptide-liposomes have been used to image EGFR positive tumors in mice.102 The synthesis of [64Cu]Cu-NOTA-Bn-GE11 has been reported but has not been evaluated in vivo as a molecular PET agent.106 Peptide polyplexed with NIS gene specifically transfected cells in vitro and homed to EGFR positive tumors in mice, allowing imaging with 123I and inhibition of tumor growth with 131I. Oligonucleotide Delivery: The peptide has been conjugated to PEI for delivery of a luciferase gene to cells in vitro and tumors in mice.101,107 GE11 modified exosomes delivered miRNA to EGFR-positive tumors in a xenograft breast cancer model.108 Delivery of let-7a by this method reduced tumor growth. Note: GE11 undergoes a clatherin mediated endocytosis without activation of EGFR signaling.109 |
| IL-6 receptor | LSLITRL110 (pIII) | Functional Peptide Activity: The peptide itself has anti-angiogenic properties. Injected i.p. into a tumor-bearing mouse, the free peptide inhibited tumor growth. |
| αvβ3 | CDCRGDCFC111,112 (RGD-4C) (pIII) | Note: See Table 5 for a full description of this widely used peptide. |
| α5β1 | GACRGDCLGA113 (pIII) | |
| α6β1 | VSWFSRHRYSPFAVS114 (pIII) HRWMPHVFAVRQGAS114 (pIII) FGRIPSPLAYTYSFR114 (pIII) |
|
| αvβ6 | RTDLDSLRTYTL115 (pIII) | Note: The peptide has been utilized as a targeting agent for a chimeric antigen receptor and used to redirect cytotoxic T cells to αvβ6-positive ovarian cancer cells in culture.116 |
| EphA2 | YSAYPDSVPMMS117 (YSA) (pIII) |
Therapeutic Applications: A modified YSA peptide has been used to deliver paclitaxel to prostate and renal tumors in vivo.118 The peptide was conjugated to superparamagentic nanoparticles and used to remove ovarian cancer cells in vitro from peritoneal fluid removed by paracentesis, reducing metastasis and increasing survival times in mouse models.119,120 Oligonucleotide Delivery: Peptide functionalized nanogels loaded with EGFR siRNA knocked down EGFR expression in vitro.121 A peptide modified adenovirus with the luciferase gene delivered the gene to cells in vitro but was unable to target a pancreatic xenograft tumor in a mouse.122 |
| EphB4 | TNYLFSPNGPIA123 (TNYL) (pIII) |
Therapeutic Applications: A cyclic version of TNYL was conjugated to a doxorubicin loaded gold nanosphere. A combination of thermal ablation and doxorubicin delivery resulted in tumor regression.124 Imaging Applications: The peptide was optimized for binding affinity to EphB4 by the addition of 3 amino acids to the C-terminal side of original peptide (TNYLFSPNGPIARAW, TNYL-RAW). DOTA-labeled TNYL-RAW was used as a molecular PET imaging probe for EphB4-expressing tumors.125 |
| MMP-9 | CTTHWGFTLC126 (CTT) (pIII) |
Therapeutic Applications: The CTT peptide conjugated to liposomal doxorubicin increased cell death,127 and a CTT derivative conjugated to doxorubicin liposomes increased survival in tumor-bearing mice.128 Conjugation of a vascular endothelial growth inhibitor (VEGI) to the peptide inhibited tumor growth in mice when injected i.p.129 Fusion of the peptide with kringle 5 fragment of human plasminogen inhibited tumor growth and increased survival in tumor-bearing mice after i.p. injection.130 Imaging Applications: The peptide has been used in several different imaging formats.204, 301–304. Gamma imaging of tumor-bearing mice given 99mTc-CTT liposomes encapsulated with 125I-albumin131 and microPET imaging of tumor-bearing mice given 64Cu-DOTA-CTT132 have been performed. Functional Peptide Activity: The peptide itself inhibits cell migration126 and invasion in vitro.133 When injected adjacent to the tumor or i.p in mouse tumor models, the free peptide inhibited tumor growth and improved survival.126 A hydrophilic peptide derivative injected via tail vein inhibited tumor growth and improved survival.133 |
| TAG-72 | FRERCDKHPQKCTKFL29 (pVIII hybrid) DPRHCQKRVLPCPAWL29 (pVIII hybrid) |
Imaging Applications: Peptides were labeled with 99mTc and used for SPECT/CT imaging of tumors in mice, although the tumor to blood ratio was low for both peptides. |
| NPGTCKDKWIECLLNG134 (A3-10) (pVIII hybrid) | Imaging Applications: The A3-10 and A2-6 peptides have been labeled with 99mTc and used for SPECT imaging.135 TAG-72 positive tumors are clearly distinguished from tumors that do not express the biomarker. | |
| GGVSCMQTSPVCENNL136 (A2-6) (pVIII hybrid) | Functional Peptide Activity: Free A2-6 peptide binds TAG-72 positive cells in culture as well as formalin fixed paraffin embedded tumors that are TAG-72 positive. | |
| E-cadherin | SWELYYPLRANL137 (pIII) | Functional Peptide Activity: Peptide also binds to N-cadherin. Cell adhesion is blocked by free peptide. |
| N-cadherin | SWTLYTPSGQSK138 (pIII) | Functional Peptide Activity: Peptide inhibited adhesion and tube formation of HUVECs. |
| Carbonic anhydrase IX | YNTNHVPLSPKY139 (CalX-P1) (pIII) | Note: 131I-CAIX-P1 did not show significant accumulation in carbonic anhydrase IX positive tumors in vivo. |
| Galectin-3 | ANTPCGPYTHDCPVKR140 (G3-C12) (pVIII hybrid) PQNSKIPGPTFLDPH140 (G3-A9) (pVIII hybrid) |
Imaging Applications: G3-C12 peptide has been labeled with 111In and employed as a molecular imaging probe for SPECT/CT & microSPECT/CT imaging of tumors.141,142 |
| PSMA | WQPDTAHHWATL143 (pIII) | Functional Peptide Activity: A dimeric version of the peptide inhibited PSMA peptidase activity. |
| CD133 | LQNAPRS144 (pIII) | |
| VEGFR-3 | CSDSWHYWC145 (pIII) | |
| Phophatidyl serine | CLSYYPSYC90 (pIII) | Imaging Applications: Fluorescein-labeled peptide could detect PS on the cell surface after a single treatment of camptothecin. |
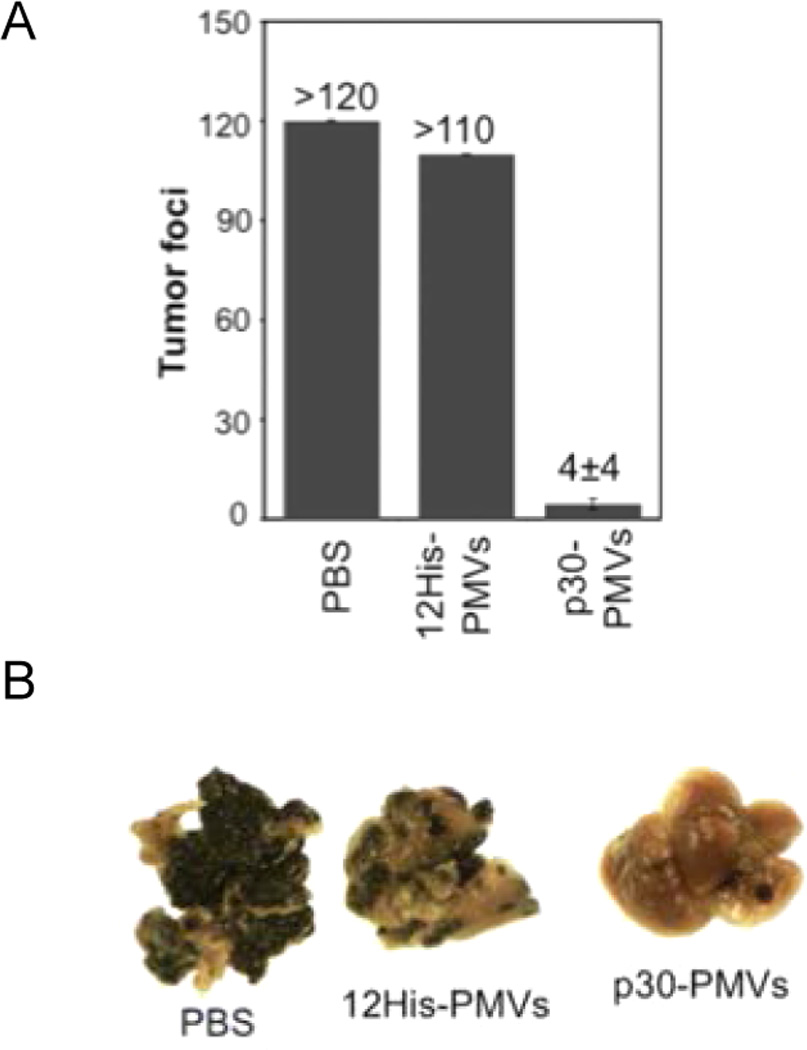
| Galactose β1-3 N-acetyl-galactosamine (Thomsen-Friedenreich carbohydrate antigen) | HGRFILPWWYAFSPS146 (P-30) IVWHRWYAWSPASRI147,148 (P30-1) |
Imaging Applications: P30-1 has been conjugated to NOTA and labeled with 64Cu for PET imaging of TF antigen on MDA-MB-435 tumors.149 Functional Peptide Activity: P-30 inhibited aggregation of MDA-MB-435 cells to each other and blocked adhesion to endothelial cells.150 |
| Mete | YLFSVHWPPLKA70 (pIII) |
Imaging Applications: Radionuclear imaging of tumor-bearing mice injected with 125I-peptide demonstrated low but specific tumor accumulation in a mouse bearing a Met-positive human tumor. Functional Peptide Activity: The peptide competed with HGF/SF binding to Met receptor. |
| Hepsine | IPLVVPL151 (pIII) | Imaging Applications: Peptide was conjugated to fluorescent, cross-linked iron oxide (CLIO) nanoparticle for fluorescence-mediated tomography of tumors in mice. |
Cysteine residues that form disulfrde bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parenthesis next to the peptide sequence.
The phage display library type is indicated in parenthesis. All phage are filamentous unless noted as T7 phage.
Peptides clustered in the same cell in this table were isolated in the same panning experiment
Indicates that the selection was performed on cells that were transfected to overexpress the target protein.
Table 17.
Epithelium-Targeting Peptides
| Isolation methoda | Peptide Sequenceb,c,d | Cellular Receptor and/or Tissue Specificity |
Applications and Notes |
|---|---|---|---|
| In vitro panning against immobilized human polymeric Ig receptor (pIgR) also called membrane secretory component (SC) | CVVWMGFQQVC491 (C9A) | pIgR (SC) on mucosal epithelium | Functional Peptide Activity: The C9A phage clone is translocated through polarized MDCK cells that express the pIgR-receptor. |
| In vitro panning against human bronchial epithelial (16HBE14o−) cell monolayers treated with EGTA | FDFWITP492 | bronchial epithelium | Functional Peptide Activity: A cyclic form of the peptide reduced transepithelial electrical resistance of MDCK cells |
| In vitro panning against human bronchial epithelial (16HBE14o−) cells | CTHALWHTC493 | CD47 (suggested)494 on bronchial epithelium |
Oligonucleotide Delivery: When cyclized and synthesized with (K)16, the peptide delivered a luciferase gene to 16HBE14o- cells.493 Peptide inserted into modified adeno-associated virus (AAV2) encoding luciferase transduced HEK-293, Calu-3, polarized Calu-3, and CFBE41o- cells.494 Note: This peptide sequence was also selected by another group that panned the same library on 16HBE14o− cells.492 |
| In vitro panning against tracheal epithelial cells CFT-2 |
CRFDSLKVC495 CGRGDGDVC495 |
Oligonucleotide Delivery: When fused to a DNA binding peptide, both peptides were able to transfect various cell types with EGFP. | |
| In vitro panning against human airway epithelial (1HAEo−) cells |
CLPHKSMPC496 CSERSMNFC496 CYGLPHKFC496 CPSGAARAC496 CLQHKSMPC496 |
ICAM-1 (suggested) | Oligonucleotide Delivery: All five peptides were individually fused to DNA-binding K16 and mixed with lipofectin and luciferase plasmid in order to deliver a luciferase gene to a variety of cell types.496 Additionally, peptide- K16-luciferase DNA-cationic liposomes transfected rabbit, porcine, and human smooth muscle cells.497 The CSERSMNFC peptide has been employed in numerous other formats for gene delivery,498 including mediating delivery of the cystic fibrosis transmembrane conductance receptor gene (CFTR) via intratracheal administration, resulting in CFTR expression m the lungs.499 Whole body nebulization in mice of peptide-K16luciferase or lacZ DNA-cationic liposomes transfected lungs and trachea.500 |
| In vitro selection against SV40 transformed lung fibroblast VA-13 cells using an invasin displayed bacterial library | CGPSVITSCSIC44 CGKMLFWGGCRADC44 CSNFLTQRVSMC44 CLGPYFMKGMQC44 |
Note: The disulfide bond pattern has not been defined for the peptides containing 3 cysteines. | |
| Ex vivo panning against isolated intestinal mucosal cells from mice with 30% total body surface area steam burn | LTHPQDSPPASA501 | Intestinal lymphatics (suggested) | Functional Peptide Activity: The peptide delivered Qdots to the gut mucosa when injected into the intestinal lumen. |
| Ex vivo panning for phage that penetrate porcine and mouse skin | LVGVFH502 (T2) | Functional Peptide Activity: Pretreating the skin of animals with the peptide enhanced penetration of small molecules, including 5-FU, into the stratum corneum. The peptide interacts with lipids in the skin resulting in a change of lipid organization. | |
| Ex vivo panning against fresh human urothelium derived cells | GGLSGL503 | normal urothelium | |
| HALE503 | normal urothelium and RT4 transitional bladder carcinoma cells | ||
| ISGL503 | normal urothelium and RT4 and T24 transitional bladder carcinoma cells | ||
| In vivo panning in rats by oral administration of phage, isolating phage from the liver, lung, spleen, and kidney | CSKSSDYQC489 | goblet cells (suggested) | Note: Isolated phage from organs known to retain Ml3 bacteriophage in order to isolate phage that cross the intestinal mucosal barrier. |
| In vivo panning in rats applying phage by gavage and isolating phage from the spleen | YPRLLTP490 | Enterocytes and sub mucosal cells in the intestine | Note: Isolated phage from spleen in order to isolate phage that cross the intestinal mucosal barrier. |
| In vivo panning for phage that penetrate mouse skin and enter the bloodstream | ACSSSPSKHCG504 (TD-1) | Functional Peptide Activity: Co-administration of TD-1 with insulin or human growth hormone resulted in transdermal delivery of the protein therapeutics. |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
5.1. Isolation of Cancer-Specific Peptides
Cancer is the leading cause of death for people under 85 in the United States and accounts for one in four total deaths.88 Due to this large clinical problem, the majority of all cell-binding peptides have been isolated against cancer-specific biomarkers, cancer cells, and tumors. Cell-targeting peptides can be used for both therapeutic and diagnostic applications. This field has been driven by the limitations of current chemotherapeutics which have a narrow therapeutic window. As such, drugs are generally given at the maximally tolerated dose, not the maximally effective dose. The ability to deliver a therapeutic to tumor cells while avoiding normal tissues can improve antitumor efficacy while decreasing off-target effects. Additionally, drugs which have been considered too toxic for systemic use may now be viable options. Moreover, as personalized medicine becomes a reality, there is an increasing need for molecular imaging agents that stratify patients by molecular subclasses to guide therapeutic decisions. Cell-binding peptides can be employed to deliver imaging agents to assess expression of particular cancer biomarkers.
Cancer-targeting peptides can be broken down into two main categories: cancer-cell-specific (or tumor-cell-specific) peptides and tumor-vasculature-specific peptides. Both the tumor cells and the vasculature that feeds the tumor are important targets as both contribute to tumor growth and viability. Phage panning on whole cells tends to isolate peptides that are tumor-specific, while in vivo panning generally isolates vasculature-binding ligands. However, there is overlap between the vasculature and tumor cell surface profiles; as a result, some vasculature-targeting peptides bind to the associated tumor cells as well.
5.1.1. Cancer-Specific Peptides Isolated from Phage Libraries
5.1.1.1. Cancer-Specific Peptides Isolated by Panning on Purified Tumor Biomarkers
Panning against purified cancer antigens was one of the first uses of phage-displayed peptide libraries. A suitable cell surface biomarker for targeting must be abundant on the cancer cell but have negligible expression on normal cells. Additionally, the cellular receptor must not be shed as soluble forms will act as a sink for the targeting peptide. Endothelial cell biomarkers of the neovasculature found in tumors can also serve as cancer-specific targets. With this in mind, numerous cancer-specific receptors have been used as bait for panning (Table 2). Peptides have been selected against 19 different target proteins. Isolated peptides range in size from 6 to 20 amino acids, are linear and cyclic, and have little sequence similarity with each other. Additionally, most peptides have no sequence similarity with the native protein ligand of the targeted receptor. A variety of different receptor targets have been used as bait, but receptor tyrosine kinases are over-represented due to their importance in cancer. Significantly, 12 of the peptides have been shown to target tumors in vivo.
It should be noted that not all cell surface markers are proteins. Phage display has been employed to isolate peptides that bind to galactose β1–3 N-acetylgalactosamine, a disaccharide also known as Thomsen–Friedenreich (TF) antigen. TF antigen is found on the majority of human carcinomas but is hidden on normal cells, making it a promising target. Several TF antigen-binding peptides have been isolated, and most contain the sequence WYAW/FSP. More recently, phage display selections have isolated ligands for phospholipids. Phosphatidylserine (PS) is a phospholipid that is normally restricted to the inner leaflet of the cell membrane lipid bilayer. However, under stress conditions such as hypoxia and high levels of reactive oxygen species (ROS), PS flips and is exposed on the cell surface. Similarly, PS is found on the surface of cells undergoing apoptosis.89 Tumor cells and the associated tumor endothelial cells that form the vasculature have been shown to expose PS, and drug or radiation treatment increases PS flipping to the outer leaflet. The cyclic peptide CLSYYPSYC was isolated by panning a heptamer phage-displayed peptide library on PS and was shown to home to a xenograft tumor after a single treatment with the drug camptothecin,90 yet caution must be taken when isolating peptides against hydrophobic targets; numerous other PS-binding peptides have been isolated with varied sequences,91–93 and the specificity of this heptamer has not been fully established. Nonetheless, these reports show the promise of isolating peptides that target nonproteinaceous cell surface biomarkers.
5.1.1.2. Cancer-Specific Peptides Selected by Whole-Cell Panning
Numerous tumor-targeting peptides have been isolated using in vitro panning against cultured cells, as listed in Table 3. All selections were continued for multiple panning rounds until emergence of convergent peptide sequences occurred, resulting in the isolation of a few dominant peptide sequences per panning. Isolated peptides range in size from 7 to 20 amino acids and are both linear and cyclic. The variety of libraries and protocols used for panning and the diversity of cell lines used as targets have resulted in a diverse group of peptides with very little sequence similarity. Additionally, even when the receptor target is the same, there is no sequence overlap between peptides isolated on purified protein and cells. Again, this likely stems from differences in library design and panning protocols. A number of these peptides internalize into their cell types of interest, indicating that they might be useful for drug delivery. Although these peptides were isolated in vitro, many have been shown to target tumors in vivo.
Table 3.
Cancer-Targeting Peptides Selected by Unbiased Panning against Whole Cells
| Cancer Type | Cell Line used for Selectiona |
Peptide Sequenceb,c,d,e | Cellular Receptor |
Applications and Notes |
|---|---|---|---|---|
| Hepatocarcinoma | BEL-7402 | TACHQHVRMVRP152 (pIII) | Therapeutic Applications: The peptide has been employed to deliver gold nanorods to a HepG2 tumor xenograft in vivo. Subsequent treatment with a near-infrared laser resulted in photothermal ablation of the tumor.153 | |
| SMMC-7721 | KSLSRHDHIHHH154 (pIII) |
Therapeutic Applications: A peptide-toxic shock syndrome toxin 1 (TSST-1) fusion protein killed cells in vitro and inhibited tumor growth in mice. Functional Peptide Activity: The peptide inhibited cell migration. |
||
| Mahlavu | SFSIIHTPILPL155 (pIII) | Therapeutic Applications: Virus-like particles modified with the peptide and loaded with doxorubicin, cisplatin, and 5-fluorouracil, or with an siRNA cocktail against cyclins, or with ricin toxin A-chain all selectively killed cells in vitro.156 Peptide-liposomal doxorubicin inhibited tumor growth in mice.155 | ||
| Melanoma | Me6652/4 | CTVALPGGYVRVC157 (phagemid, pVII and pIX) | GRP78157 | Therapeutic Applications: A peptide-taxol conjugate,157 peptide-doxorubicin prodrug, and peptide-taxol prodrug158 exhibited cell cytotoxicity in vitro. |
| B16-F10 (murine) | TRTKLPRLHLQS159 (pIII) | Functional Peptide Activity: Injection of the phage adjacent to the tumor site inhibited tumor growth in mice. | ||
| B16-F10-Nex2 (murine) | CSSRTMHHC160 (pIII) | Cadherins | Functional Peptide Activity: The peptide reduced cell viability and inhibited cell invasion in vitro. I.P. injection of the peptide in a tumor bearing mouse decreased metastatic nodules, delayed tumor growth and improved survival. | |
| B16 cells co-cultured with B-1 lymphocytes (murine) | CLFMRLAWC161 (pIII) | MUC18 | ||
| Prostate | Capan-2 (irradiated) | SHGFSRHSMTLI162 (pIII) | Functional Peptide Activity: The peptide homed to irradiated tumors in vivo. A modest specificity for irradiated Capan-2 tumors over non-irradiated tumors was observed. | |
| LNCaP | DPRATPGS163 (pVIII landscape) | Functional Peptide Activity: In vivo, the peptide increases invasiveness and stimulates MMP-2 production. | ||
| DU-145 | FRPNRAQDYNTN164 (DUP-1) (pIII) | Therapeutic Applications: The peptide has been shown to target tumors in vivo.164,165 A peptide-RNA aptamer- chimera delivered doxorubicin in vitro and super paramagnetic iron nanoparticles in vivo.166 | ||
| PC3 | DTPYDLTG167 DTDSHVNL167 DVVYALSDD167 (pVIII landscape) |
Therapeutic Applications: Landscape phage pVIII coat protein displaying the peptide inserted into liposomal Doxil® specifically killed cells.168 | ||
| PC-1 | GGKRPAR76 (T7 phage) RIGRPLR76 (T7 phage) |
Neuropilin-1 | ||
| Gastric | XGC9811-L4 | GRRTRSRRLRRS169 (pIII) | Functional Peptide Activity: The peptide decreased cell invasion and migration and adherence to Type IV collagen. Cells preincubated with peptide and then implanted orthotopically had decreased incidence of liver metastases. | |
| GC9811-P | SMSIASPYIALE170 (pIII) | Functional Peptide Activity: The peptide decreased invasion and adhesion of cells in vitro. In vivo, i.p delivery of the peptide into mice with peritoneal dissemination models of gastric cancer resulted in reduced metastases to the peritoneum and significantly longer survival. | ||
| HUVEC/SGC701 co-culture | CTKNSYLMC171 (GEBP11) (pIII) | Note: Selected to bind to gastric cancer vasculature, not the actual tumor cells. | ||
| Colon | HT29 | CPIEDRPMC172 (RPMC) (pIII) | α5β1173 |
Therapeutic Applications: The peptide conjugated to D(KLAKLAK)2 selectively killed cells in vitro.172 Imaging Applications: Gamma imaging of 111In-DOTA-peptide in tumor-bearing mice showed tumor homing.173 Fluorescence endoscopy of colon cancer in mice using peptide-FITC.173 |
| WiDr | HEWSYLAPYPWF174 (HEW) (pIII) | Oligonucleotide Delivery: Peptide modified-lacZ adenoviruses selectively infected cells in vitro but did not home to tumors in vivo.175 | ||
| SW480 | VHLGYAT176 (pIII) | |||
| T84 | CQARGDLGKIRC177 (pIII) | |||
| Head and Neck | MDA167Tu | TSPLNIHNGQKL178 (HN-1) (pIII) | Therapeutic Applications: HN-1 peptide homes to its target tumor in vivo.178 HN-1 peptide was conjugated to an inhibitory peptide that blocks protein kinase C-ε. This chimeric peptide inhibited cell motility, invasion, and proliferation in vitro and inhibited tumor growth in mice when injected i.p.179 | |
| HNO223 | SPRGDLAVLGHKY180 (HBP-1) (pIII) | αvβ6 (suggested) | Functional Peptide Activity: Homes to tumor in vivo. | |
| NPC-TW 04 | RLLDTNRPLLPY181 (pIII) | Therapeutic Applications: Peptide-liposomal doxorubicin inhibited tumor growth in mice. | ||
| Hep-2 | CRLTGGKGVGC182 (phagemid pvIII hybrid | Note: Hep-2 cells are now known to be contaminated with HeLa cells. This peptide was also isolated on HPV-16 transformed SiHa cells. The cell specificity of this peptide remains in question. | ||
| Breast | MDA-MB-231 | YQATPARFYTNT174 (pIII) | Note: Not specific for MDA-MB-231 cells | |
| CGWMGLELC174(pIII) | Note: Not specific for MDA-MB-231 cells | |||
| SKBR3 | LTVSPWY183 (pIII) | ErbB2184 |
Therapeutic Applications: Conjugation of the peptide to pro-apoptotic α-tocopheryl succinate selectively killed ErbB2 positive cells in vitro and reduced initial tumor volumes in a transgenic mouse model of ErbB2-positive breast cancer (i.p. injection).184 The peptide has been conjugated to daunomycin. This conjugate was selectively internalized into ErbB2 expressing cells, but IC50 values did not correlate to ErbB2 levels.185 Imaging Applications: A tetrameric far-red fluorescent protein (KatushkaS158A) was used as a scaffold to create an octavalent peptide fluorescent nanoparticle that allowed far-red fluorescent imaging of tumors in mice.186 Peptide coated magnetic nanoparticles were synthesized but active targeting to ErbB2-positive tumors was modest.187 Peptide-coated quantum dots targeted ErbB2-positive SKBR3 cells in vitro.188 Oligonucleotide Delivery: Peptide-antisense oligonucleotide against ErbB2 inhibited ErbB2 gene expression in cells.183 |
|
| WNLPWYYSVSPT183 (pIII) | Note: Isolated in a mixed library panning with the LTVSPWY peptide listed above. Binds to a wide panel of cell lines derived from solid tumors.183 | |||
| MCF-7 | DMPGTVLP189,190 VPTDTDYS190 VEEGGYIAA190 DWRGDSMDS190 (pVIII landscape) |
nucleolin190 |
Therapeutic Applications: Landscape phage pVIII coat protein displaying the DMPGTVLP peptide was incorporated into Doxil® for specific cell killing in vitro.189 Similarly, the peptide-coat protein was incorporated into polymeric micelles loaded with paclitaxel and demonstrated improved efficacy on MCF7 cells in vitro.191 Oligonucleotide Delivery: Landscape phage pVIII coat protein displaying the DMPGTVLP peptide was incorporated into liposomes loaded with PRDM14 siRNA and shown to reduce PRDM14 protein expression.192 |
|
| BT-474 | LSVCVRGLLGCG95 CGDRLCRMLWLC95 DRCWSILATSTF95 CGRVGMDVMGGC95 VWGVFGGCSQRP95 LSVWMQGLSRSL95 CGLGWCGVRWGC95 (phagemid vector that expresses P99 β-lactamase as N-terminal fusion to pIII protein) |
|||
| Nervous System | WAC2 (neuroblastoma) | HLQIQPWYPQIS193 (pIII) VPWMEPAYQRFL193 (p160) (pIII) |
Therapeutic Applications: p 160 peptide-micelles loaded with paclitaxel killed cells in vitro.194 Imaging Applications: p160 homes to WAC2 tumors in vivo as determined by biodistribution of radiolabeled peptide195,196 but has not been used for imaging.463,464 |
|
| RG2 (rat glioma) | DSTKSGNM197 DYDMTKNT197 DLTKSTAP197 ESRGDSYA197 (pVIII landscape) |
|||
| U87-MG (glioma) | MCPKHPLGC198 (pIII) | |||
| VTWTPQAWFQWV199 (VTW) (pIII) | GP130 (suggested) | Note: The peptide can deliver active β-galactosidase into U87MG cells. | ||
| Gli36 (glioma) | LLADTTHHRPWT200 (pIII) | |||
| Mixture of dGli36, SF767, U87MG, U251MG, and U373MG (glioma) | LWATFPPRPPWL201 (pIII) | Oligonucleotide Delivery: Peptide-(K16) complexed with luciferase DNA transfected SF767 glioma cells. | ||
| Glioma stem cells established from human glioblastoma samples | AQYLNPS202 (pIII) | Nestin | Functional Peptide Activity: The peptide homes to nestin positive cells within a glioblastoma tumor in subcutaneous and orthotopic models when injected intravenously. This suggests that the peptide crosses the blood-brain barrier. | |
| GI-LI-N and HTLA-230 (neuroblastoma) | YEGLISR203 HSYWLRS205 WSWPREL203 ALAAHKL203 KSFFLSH203 (pIII) |
Therapeutic Applications: Doxorubicin loaded liposomes conjugated to the WSWPREL or ALAAHKL peptides showed a significant anti-tumor response in two different animal models of neuroblastoma compared to non-targeted lipsomes. | ||
| Cervical | SiHa (HPV-16 infected) |
CRLTGGKGVGC204 CADPNSVRAMC204 CAAHYRVGPWC204 (phagemid pVIII hybrid) |
||
| HeLa |
CSSGKPLVC205 (pIII) CNISRTGTC205 (pIII) CNSTELSGC205 (pIII) |
Oligonucleotide Delivery: The peptides increase transfection of a luciferase DNA polyplex. | ||
| Ovarian Cancer | SK-OV-3 | SVSVGMKPSPRP206 (pIII) | Note: This peptide has been suggested to be a non-specific peptide that is isolated from the NEB 12-mer library most likely due to preferential propagation of this phage clone.207 | |
| Thyroid Cancer | TT | CHTFEPVGC208 (pIII) | ||
| FRO82-2 | EDYELMDLLAYL209 (FROP-1) (pIII) | Note: The peptide binds to many carcinoma cell lines. Radiolabeled peptide homes to FRO82-2 and MCF tumors in mice. | ||
| Rhabdo-myosarcoma | RD |
CQQSNRGDRKRC210 (RMS-I) CMGNKRSAKRPC210 (RMS-II) (T7 phage) |
αvβ3 (RMS-I) | Note: RMS-II shares sequence similarity to the well-studied Lyp-1 peptide. RMS-II targets tumor vasculature in RD tumor xenografts. |
| Osteosarcoma | 143B | ASGALSPSRLDT211 (OSP-1) (pIII) | Heparin sulfate proteoglycans (suggested) | Imaging Applications: MicroPET imaging has been performed with 18F-labeled peptide in tumor-bearing mice. |
| Lymphoma and Leukemia | A20 (murine lymphoma) | SAKTAVSQRVWLPSHRGGEP212 (A20.1) KSREHVNNSACPSKRITAAL212 (A20.2) WLSEAGPVVTVRALRGTGSW212 (PCM.1) (pIII) |
Note: The peptides distinguish A20 cells from a mixture of splenocytes. PCM.l was also isolated from a whole cell biopanning on primary cardiomyoctyes.213 | |
| (lymphoma) | peptide D(KLAKLAK)2 kills cells in vitro. | |||
| CGFYWLRSC215 (p III) | NRP-1 | Therapeutic Applications: Peptide-proapoptotic peptide D(KLAKLAK)2 kills cells in vitro. | ||
| Raji (lymphoma) | CTLPHLKMC216 (pIII) | Variable region of human immunoglobulin heavy chain (suggested) | ||
| Kasumi-1 (leukemia) | CPLDIDFYC217 (pIII) | α4β1 | ||
| Lung Cancer | H1299 (large cell) | VSQTMRQTAVPLLWFWTGSL218 (H1299.1)(pIII) | Therapeutic Applications: Peptide-doxorubicin conjugate selectively killed H1299 cells.219 | |
| YAAWPASGAWTGTAPCSAGT220 (H1299.2) (pIII) | ||||
| EHMALTYPFRPP221 (ZS-1) (pIII) | ||||
| QQMHLMSYAPGP222 (ZT-1) (pIII) | ||||
| H2009 (adenocarcinoma) | RGDLATLRQLAQEDGVVGVR218 (H2009.1)(pIII) | αvβ673 |
Therapeutic Applications: The H2009.1 peptide has been shown to home to αvβ6-positive tumors in NSCLC xenografts.223 A peptide-doxorubicin conjugate,219 a peptide-taxol conjugate,224 a peptide-polyglutamic acid polymer-doxorubicin conjugate,225 peptide-displaying micelles loaded with doxorubicin and SPIO,226 and peptide conjugated to doxorubicin liposomes all selectively kill αvβ6-positive cells.227 Imaging Applications: H2009.1 has been conjugated to SPIO particles for MRI imaging.228 These particles target αvβ6-positive cells in vitro but has not been tested in vivo to date. |
|
| Lung Cancer | A549 (adenocarcinoma) | MTVCNASQRQAHAQATAVSL218 (A549.1) (pIII) | ||
| CL1-5 | TDSILRSYDWTY229 (pIII) | Therapeutic Applications: Peptide-liposomal doxorubicin and liposomal vinorelbine inhibited tumor growth in mice.229 | ||
| Calu-3 (adenocarcinoma) | CDSAFVTVDWGRSMSLC230 (IB1) (pVIII) | Oligonucleotide Delivery: Biotinylated peptide complexed with luciferase/PEI transfected Calu-3 cells with luciferase. | ||
| H460 (large cell) | CSNIDARAC231 (T7) |
Therapeutic Applications: The peptide was coupled to liposomal doxorubicin and found to be more effective than free doxorubicin or non-targeted liposomal doxorubicin in reducing tumor growth in a H460 subcutaneous tumor model. Imaging Applications: Fluorescence imaging of H460 tumors in animals has been accomplished using a FITC conjugate of the peptide. |
||
| Barrett’s Esophagus | OE33 | SNFYMPL232 (pIII) |
All cells lines are of human origin unless stated otherwise.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parenthesis next to the peptide sequence.
The phage display library type is indicated in parenthesis. All phage are filamentous unless noted as T7 phage.
Peptides clustered in the same cell were isolated in the same panning experiment.
In addition to panning on cells maintained in culture, isolated primary cells can be employed. Table 4 lists peptides identified by ex vivo panning on either isolated tumor cells or whole tumors. Significantly, laser capture microdissection (LCM) has recently been used to isolate cancer cells for ex vivo panning. LCM allows separation of cancer cells from other cell types residing in the tumor to ensure that the peptides isolated are binding the cancer cells. Kubo et al. used LCM to isolate colon cancer cells from patients for panning.233 Two of the four isolated peptides have been shown to target tumors in vivo.
Table 4.
Cancer-Targeting Peptides Selected by Panning ex Vivo
| Cancer Type | Cells or Tumor used for Selection |
Peptide Sequence a,b | Receptor Identified |
Applications and Notes |
|---|---|---|---|---|
| Colon | Human colonic adenomas | VRPMPLQ236 (pIII) | Imaging Applications: Topical administration of the peptide labeled with fluorescein was used for fluorescence confocal microendoscope imaging of colonic adenomas in humans. | |
| Resected human colon tumors | SPTKSNS233 (pIII) | Notes: Used laser capture microdissection to isolate cells for biopanning. | ||
| Bladder | Cells from HT-1376 xenograft in mice | CSNRDARRC237 (pIII) | Notes: Peptide homed to HT-1376 xenografts in mice and captured shed bladder cancer cells in the urine of patients with early stage bladder cancer.237,238 | |
| Pancreatic | Pancreatic ductual carcinomas arising from Kras/p52L/L mice | KTLLPTP239 (pIII) | Plectin-1239 | Imaging Applications: The peptide has been conjugated to crosslinked iron oxide-Cy5.5 nanoparticles for intravital confocal microscopy in tumor-bearing mice.239 The 111In labeled peptide has been used for SPECT/CT imaging in tumor-bearing mice.74 |
| Gastric | Freshly harvested human gastric cancer tissue | AADNAKTKSFPV240 (pIII) |
Cysteine residues that form disulfide bonds are indicated in bold.
The phage display library type is indicated in parentheses. All phage are filamentous.
Not included in Tables 3 and 4 are studies that selected for peptide motifs instead of convergent sequences. Instead of panning against a single cell line and selecting for the best binding sequences, these studies focused on generating consensus peptide motifs that bind to a panel of cell lines. In one such study, Arap and Pasqualini and co-workers used the NCI-60, a panel of human cancer cells encompassing different histological types and grades, for peptide selection.234 This panel includes cell lines from kidney cancer, breast cancer, colon cancer, lung cancer, prostate cancer, ovarian cancer, tumors of the nervous system, melanoma, leukemia, and lymphoma. It is a particularly important group of cells to study as protein expression across the cell lines has been extensively examined. A group of 38 tripeptide motifs were identified that bound broadly across the panel of cells and clustered with cell lines known to express the same receptors. Similarly, Shukla and Krag used ex vivo panning on whole breast tumors from patients and examined motifs present among the isolated phage clones.235 Panning for peptide motifs is more likely to help profile cell surfaces and expand knowledge about similarities and differences between cancer cells and less likely to generate high-affinity peptides.
5.1.1.3. Cancer-Specific Peptides Isolated by in Vivo Panning
Table 5 lists peptides identified by in vivo panning in animals. The majority of peptides isolated by in vivo panning in animals bind to the tumor vasculature and not the tumor cells, although some of the peptides bind selectively to tumor cells or to both the vasculature and tumor cells. Tumor-vasculature-specific peptides are identified in the table with a V, tumor-cell-specific peptides with a T, and peptides that bind both vasculature and tumor cells with a T/V. On the basis of the variety of peptides isolated from different tumor models, tumor vasculature is shaped by the specific tumor type. Isolated peptides can even distinguish between drug- or radiation-treated tumor vasculature and their untreated tumor counter-parts. For example, peptides have been isolated that can distinguish between the vasculature of untreated tumors compared to tumors treated with the monoclonal antibody VEGF inhibitor bevacizumab241 or tumors responding to treatment with the small-molecule receptor tyrosine kinase inhibitor sunitinib.242 Hallahan and colleagues also recently identified a peptide that binds specifically to the tumor vasculature of tumors treated with both radiation and the VEGF receptor tyrosine kinase inhibitor SU11248.243 This demonstrates the remarkable discriminating power of the peptides isolated by panning.
Table 5.
Cancer-Targeting Peptides Selected by Panning in Vivo
| Tumor Typea | Peptide Sequenceb,c,d,e,f | Cellular Receptor |
Applications and Notes |
|---|---|---|---|
| Gastric (AZ-P7a) | SWKLPPS259 (g) (pIII, T) | α3β1 (suggested) | Therapeutic Applications: Peptide-liposomal doxorubicin killed AZ-P7a cells better than unconjugated liposomes in vitro. |
| Human gastric adenocarcinoma (propagated in mice) | CGNSNPKSC260 (GX1) (pIII, V) |
Therapeutic Applications: The free peptide fused to recombinant human tumor necrosis factor alpha (rmhTNFα) induced HUVEC apoptosis in vitro.261 This peptide-rmhTNFα fusion also inhibited SGC7901 tumor growth in mice.261 Imaging Applications: 99Tcm-peptide261,262 and 99Tcm-peptide-rmhTNFα261 successfully detected SGC7901 tumors by SPECT imaging. MicroPET imaging using 64Cu-DOTA-peptide detected U87MG tumors in mice.263 Near-infrared fluorescence imaging of Cy5.5-peptide in U87MG tumor-bearing mice demonstrated tumor targeting.264 |
|
| Lung (irradiated and SU11248 treated murine Lewis lung carcinoma) & Glioblastoma (GL261, murine) | HVGGSSV243 (h) (T7 phage, V) | TIP-1265,266 |
Therapeutic Applications: Peptide-nab-paclitaxel inhibited Lewis lung carcinoma and H460 tumor growth in mice in conjunction with irradiation.265 Lewis lung carcinoma and H460 tumor growth inhibition was observed when animals were treated with irradiation and peptide liposomal doxorubicin.267 Imaging applications: Near-infrared fluorescence imaging using dye labeled peptide has been performed for several tumor types and in various types of tumor-bearing mice treated with irradiation or TKI (tyrosine kinase inhibitors) or both.243,265–269 |
| Lung (CL1-5) | SVSVGMKPSPRP249 (pIII, V) |
Therapeutic Applications: Peptide liposomal doxorubicin inhibited CL1-5 tumor growth and increased survival in mice. Note: This peptide has been suggested to be a non-specific peptide that is isolated from the NEB 12-mer library most likely due to preferential propagation of this phage clone.207 |
|
| Lung (H460) | RCPLSHSLICY270 (pIII, T) | ||
| Oral (SAS) | SVSVGMKPSPRP249 (pIII, V) |
Therapeutic Applications: Peptide liposomal doxorubicin inhibited SAS tumor growth and increased survival in mice. Note: This peptide has been suggested to be a non-specific peptide that is isolated from the NEB 12-mer library most likely due to preferential propagation of this phage clone.207 |
|
| SNPFSKPYGLTV244 YPHYSLPGSSTL244 (pIII, V) |
Therapeutic Applications: Peptide-liposomal doxorubicin inhibited tumor growth and increased survival in mice with various tumor types compared to non-conjugated liposomal doxorubicin. Similar growth inhibition was observed for both peptides. | ||
| Nasopharyngeal Carcinoma (CNE-1) | EDIKPKTSLAFR271 (pIII, ND) | ||
| Prostate (PC-3) | IAGLATPGWSHWLAL272 (G1) (pIII, T) |
Imaging applications:
111In-DOTA-Gl peptide has been used for SPECT/CT imaging of PC-3 tumors in mice.273 Functional Peptide Activity: The peptide induced apoptosis of PC-3 cells in culture.273 |
|
| LKGDCTQRYVYCMKSK273 (H5) (pVIII hybrid, T) |
Imaging applications:
111In-DOTA-H5 peptide has been used for SPECT/CT imaging of PC-3 tumors in mice.273 Functional Peptide Activity: The peptide induced apoptosis of PC-3 cells in culture.273 |
||
| CRGDKGPDC274 (iRGD) (T7 phage, T/V) | αvβ3, αvβ5 then cleaved to CRGDK which binds NRP-1 |
Therapeutic Applications: Co-injection of the peptide with Nab-paclitaxel, trastuzumab, liposomal doxorubicin or doxorubicin inhibited BT474 or 22Rv1 orthotopic tumor growth in mice.275 CGKRK-d[KLAKLAK]2-iron oxide nanoworms systemically injected into mice with 005 tumors prolonged survival when co-injected with the peptide.276 Imaging applications: The peptide was conjugated to Cy7 for near-infrared imaging of tumors in mice bearing novo pancreatic ductal adenocarcinoma. MRI of 22Rv1 orthotopic xenografts in mice using peptide-linked SPIO nanoworms has been performed.276 Note: This peptide has been postulated to have a unique mode of action in which the peptide first binds the target integrin and is then cleaved to expose a NRP-1 binding peptide. The binding of NRP-1 increases penetration of cargo throughout the tumor. |
|
| Prostate (TRAMP mice) | CREAGRKAC257 (REA) (i) (T7 phage, L) | Therapeutic Applications: Peptide-D(KLAKLAK)2 conjugate reduced PPC1 orthotopic tumor lymphatic vessels in mice. | |
| Prostate (DU145) | YRCTLNSPFFWEDMTHECHA277 (pIII, T) | CRKL | Therapeutic Applications: Peptide-proapoptotic peptideD(KLAKLAK)2 is cytotoxic to DU145 cells in vitro and inhibits DU145 tumor growth in mice. |
| Prostate (PPC-1)i | RPARPAR278 (T7 phage, T) | Neuropilin-1 (NRP-1)278,279 | |
| Breast (MDA-MB-435) | CNGRCVSGCAGRC112,280 (NGR)j (pIII, V) | Amino-peptidase N (CD13)281 | Therapeutic Applications: A NGR peptide-doxorubicin conjugate inhibited MDA-MB-435 tumor growth & prolonged survival in mice.112 Peptide-liposomal doxorubicin inhibited growth of orthotopic neuroblastoma xenografts in mice282 and increased survival in mouse lung, ovarian, and neuroblastoma xenografts.283 NRG peptide-TNF (tumor necrosis factor α) inhibited tumor growth284 and synergistically increased the effects of various chemotherapy drugs on tumor inhibition in mice (for various tumor types).285–289 Human interferon alpha (hIFN-α2a)-peptide conjugate290 and recombinant hIFN-α2a-peptide291 inhibited tumor growth in mice when injected i.p. in various tumor models. A conjugate of the NRG peptide with a Tum-5-peptide (derived from tumstatin) inhibited S180 tumor growth in mice.292 |
| Breast (MDA-MB-435) | CDCRGDCFC112 (RGD-4C)j (pIII, T/V) | αvβ3,αvβ5,α5β1 |
Therapeutic Applications: A RGD-4C-doxorubicin conjugate inhibited MDA-MB-435 tumor growth and prolonged survival in mice112 and inhibited MH134 orthotopic tumor growth in mice.293 A conjugate of RGD-4C-tumor necrosis factor-α (TNF) inhibited MDA-MB-435 orthotopic tumor growth.136 Conjugation of RGD-4C to adeno-associated virus phage vector (AAVP) allowed for delivery of the HSVtk gene. Treatment of DU145, KS1767, and UC3 xenografts in mice and EF43-FGF4 mouse mammary tumors with RGD-4C-AAVP- HSVtk and gancilovir inhibited tumor growth.294 RGD-4C-Delta-24 adenovirus (an adenovirus with anticancer activity) is cytotoxic to a variety of cancer cell lines.295 Intratumoral injection of RGD-4C-Delta-24 adenovirus into orthotopic U-87 MG tumors in mice increased survival.295 This conjugate has recently begun Phase I clinical trials. Imaging applications: Scintigraphic imaging of DU145 tumors in mice using 99mTc(CO3)-RGD-4C and 99mTc(CO3)-HPMA polymer-RGD-4C has been performed; the polymer conjugate was also imaged in PC-3 tumors.296 MicroPET imaging using 64Cu-DOTA-RGD-4C-TNF-α was able to detect U87MG and MDA-MG-435 tumors in mice.136 Oligonucleotide Delivery: Conjugation of RGD-4C to AAVP resulted in delivery of GFP to KS1767 xenografts in mice & delivery of luciferase to DU145 xenografts as evidenced by bioluminescent imaging.294 RGD-4C-AAVP delivered the HSVtk gene to DU145 xenografts as evidenced by PET imaging with [18F]FEAU.294 |
| CGNKRTRGC255 (LyP-1)i (T7 phage, T/L/M) | p32/gC1qR297 |
Therapeutic Applications: LyP-1 conjugated to liposomes containing doxorubicin are no more effective in reducing tumor growth than non-targeted liposomes.298 However, doxorubicin loaded LyP-1 liposomes injected after gold nanorod-mediated heating of tumors caused tumor regression in mice with MDA-MB-435 tumors.299 Peptide-doxorubicin liposomes killed cells with or without heat treatment in vitro. Peptide coupled to microbubbles containing paclitaxel killed cells when treated in combination with ultrasound but this approach was only tested in vitro.300 Dye-labeled–peptide-abraxane inhibited tumor growth in mice.301 Imaging applications: Near-infrared fluorescence imaging and ex vivo imaging of lymphanogenesis were performed using Cy5.5-peptide injected via the middle phalanges of both upper extremities in 4T1 tumor bearing mice.302 Oligonucleotide Delivery: Baculovirus displaying peptide-VSVG protein fusions and carrying the luciferase gene specifically transduced cells in vitro.303,304 Functional Peptide Activity: The LyP-1 peptide itself induced apoptosis of cancer cell lines in culture and inhibited MDA-MB-435 tumor growth in mice.256 Note: LyP-1 has been shown to home to tumor lymphatics in vivo when used in a variety of formats.255,256,298,299,301,302,304–308 LyP-1 also accumulates in atherosclerotic plaques, primarily in the associated macrophage.309,310 |
|
| Breast (MDA-MB-231 and MCF-7 in mice treated with sunitinib and responding to therapy)h | EGEVGLG242 (T7 phage, V after sunitinib treatment) | Imaging applications: A peptide-AlexaFluor 750 conjugate was used for near-infrared imaging of sunitinib treated MDA-MB-435 and MCF7 tumors in mice. | |
| Medullary Thyroid Carcinoma (TT) | CHTFEPVGC311 (pIII, T) | Oligonucleotide Delivery: Peptide linked to adenovirus vector delivered the GFP gene to cells in vitro resulting in GFP expression but this peptide has not been tested in vivo.311 | |
| Basal cell squamous carcinoma from K14-HPV16 micei | CGKRK312 CDTRL312 (T7 phage, V) |
Therapeutic Applications: The CGKRK peptide was conjugated to a clot inducing nanoworm particle. This conjugate induces clotting in prostate tumors in mice, resulting in a reduction of tumor burden.313 A CGKRK peptide-D[KLAKLAK]2-iron oxide nanoworm decreased the number of blood vessels in bFGF-Matrigel plugs in mice. When systemically injected into mice bearing induced brain tumors, the peptide-conjugate cured most mice.276 CGKRK Peptide-D[KLAKLAK]2-iron oxide nanoworms co-injected with the iRGD peptide into mice with 005 tumors prolonged survival.276 Oligonucleotide Delivery: Baculovirus displaying CGKRK peptide-VSVG protein fusions and carrying the luciferase gene specifically transduced cells in vitro,303 and the CGKRK peptide has been used to transfect cancer cells with a spider silk derived gene carrier.314 Note: Both peptides home to the vasculature of several different tumor types but the CGKRK peptide binds to a broader number of tumor types. |
|
| Melanoma (C8161)i | CLSDGKRKC257 (LSD) (T7 phage, L) | Therapeutic Applications: LSD peptide-D(KLAKLAK)2 conjugate reduced C8161 orthotopic tumor lymphatic vessels in mice. | |
| Melanoma (murine B16-F10)g,i | TRTKLPRLHLQS159 (WDC-2) (pIII, T) | Therapeutic Applications: Phage inhibited B16-F10 tumor growth when injected adjacent to tumor, but the free peptide was not tested. The anti-tumor effect is likely due to infiltration of neutrophils induced by the phage particle. | |
| RIP1-Tag2 mice Pancreatic isletsi | CRGRRST315 (RGR) CRSRKG315 (RSR) CKAAKNK315 (KAA) CKGAKAR315 (KAR) FRVGVADV315 (VGVA) (T7 phage, V/T) |
PDGFRβ is a candidate receptor for RGR peptide |
Therapeutic Applications: Combination treatment with RGR peptide-anti-CD40 antibody and murine IL-2-peptide fusion protein increased survival in RIP1-Tag2 mice. 80% of mice treated with the combination treatment plus adoptive transfers of anti-Tag CD4+ and CD8+ T cells survived long-term.316 Note: KAA and KAR peptides bind to the RIP1-Tag2 tumor vasculature. RSR binds specifically to angiogenic islet vasculature but not to the tumor vasculature. VGVA and RGR bind to both angiogenic and tumor vasculature.315 |
| Dysplastic Colon Mucosa/colon adenomas from CPC;Apc mice | QPIHPNNM317 T7 phage, T) | Imaging applications: FITC-labeled peptide bound adenomas in CPC;Apc mice as viewed by endoscopy317 and microendoscopy.318 | |
| Colorectal (Bevacizumab-treated LS174T) | LLADTTHHRPWT241 (BRP) (h) (pIII, bevacizumab-treated V) | Imaging applications: BRP peptide-IRDye800 conjugate was used for near-infrared imaging of bevacizumab-treated LS174T tumors in mice. PET imaging using a 18F-BRP-peptide detected LS174T tumors in mice treated with bevacizumab.241 | |
| Colorectal (Colon 26 cells orthotopically implanted in BALB/c mice) | CTPSPFSHC319 (TCP-1) (pIII phage, V) |
Therapeutic Applications: The peptide homes to the vasculature of the colon 26 tumor in a mouse but not to normal vasculature. Conjugation of TCP-1 to the proapoptotic peptide D(KLAKLAK)2 conjugate induces apoptosis in the tumor vasculature. Note: The peptide binds to vasculature of resected human colorectal tumors. |
|
| Gliomas (irradiated murine GL261) | GIRLRG320 (h) (T7 phage, irradiated V) | GRP78 |
Therapeutic Applications: Peptide conjugated to a nanoparticle encapsulating paclitaxel inhibits MDA-MB-231 tumor growth in irradiated mice.320 Imaging applications: Peptide-AlexaFluor 750 has been used for near-infrared imaging in mice with irradiated G1261 tumors.320 |
| Hepatocarcinoma (BEL-7402) | AGKGTPSLETTP321 (A54) (pIII, T) | Therapeutic Applications: Peptide-doxorubicin conjugate inhibited tumor growth and increased survival in mice with BEL-7402 tumors.152 | |
| Human Renal Carcinoma Tumor Endothelial Cells | CVGNDNSSC250 (pIII, V) | Therapeutic Applications: Biotinylated peptide conjugated to saporin induced apoptosis in tumor endothelial cells in mice. | |
| Waldenström macro-globulinemia B cell malignancy (from pancreas of a human patient) | CGRRAGGSC77 (pIII, T) | IL-11Rα77,251 |
Therapeutic Applications: A peptide-proapoptotic peptide D(KLAKLAK)2 conjugate induced apoptosis in LNCaP and MDA-PCa-2b prostate cell lines in vitro.152 Imaging applications: 111In-DTPA-IR dye-peptide construct was used for both SPECT/CT and near-infrared imaging in a mouse bearing a MDA-MB-231 tumor.322 |
All tumors are human xenografts unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parenthesis next to the peptide sequence.
The phage display library type is also indicated in parentheses. All phage are filamentous unless noted as T7 phage.
T indicates tumor cell targeting, V indicates tumor vasculature targeting, L indicates tumor lymphatics homing, and M indicates tumor associated macrophage homing. ND means it is unclear whether the peptide binds the tumor cells or tumor vasculature.
Intravenous injections used for selection unless otherwise indicated.
Selection used intraperitoneal injections instead of intravenous injections.
Selection used intracardiac injections instead of intravenous injections.
Combination of ex vivo/in vivo panning employed.
This peptide has been used in numerous other studies not listed.
The selection pressures involved with in vivo panning are very different from those placed on the system during an in vitro panning. As such, in vitro and in vivo panning can lead to the isolation of different peptides, even when the same library and cell line are employed. For example, the same laboratory used a 12-mer library to pan against the CL1-5 NSCLC cell line in vitro and against CL1-5 tumor xenographs in vivo.229,244 Two different peptides were isolated; the peptide TDSILRSYDWTY (SP5-2) was isolated by in vitro panning on the cell line, and the peptide SVSVGMKPSPRP (SP5-52) was identified from the in vivo panning experiment. SP5-52 targets the tumor vasculature, while SP5-2 penetrates the tumor, resulting in diffuse binding throughout the tumor mass. This emphasizes how differing selection methods can identify ligands with different biological characteristics. However, the peptide SVSVGMKPSPRP has been suggested to be a nonspecific peptide that is isolated from the NEB 12-mer library most likely due to preferential propagation of this phage clone.207,245 The peptide sequence has been selected on DNA,246 multiple semiconductor surfaces,247 phosphatidylserine,92 and numerous cell types.248,249 Further studies are required to determine the true specificity of this peptide.
One problem with the use of in vivo panning in animals is that the isolated peptides are binding to mouse or rat and not human vasculature. Additional testing is required to determine whether they also bind human vasculature. One study bypassed the problem of selecting peptides against mouse vasculature by using mice injected with human endothelium for in vivo panning.250 Mice were injected with tumor endothelial cells derived from human renal carcinomas mixed with Matrigel so that they formed human tumor vessels that grew into the murine vessels. In vivo panning in these mice resulted in peptides that targeted human tumor endothelium and not mouse tumor endothelium.
The first in vivo panning in a human was performed by Arap and Pasqualini and involved the identification of tripeptide motifs specific for different organs.77 A patient with the B-cell cancer Waldenström macroglobulinemia was injected intravenously with phage and tissue collected from five areas of the body—bone marrow, fat, skeletal muscle, prostate, and skin—for identification of specific tripeptide motifs. One extended peptide motif was validated as evidence of the specific nature of these motifs. A phage bearing the peptide CGRRAGGSC was isolated from the prostate and shown to recognize the vasculature of human prostate tissue specimens. The CGRRAGGSC peptide binds to IL-11Rα,77,251 which increases in expression during the progression of cancer.252 This peptide is currently in phase I clinical studies for patients with prostate cancer.253 Recently, the Krag laboratory performed a study in which patients with different types of stage IV cancer were intravenously injected with phage libraries for panning followed by biopsy and then reinfusion of the library for further panning and biopsy steps.254 One peptide phage clone isolated from a patient with stage IV melanoma subsequently bound ex vivo to tumor cells from the same patient, but not to tumor cells from other patients. This peptide phage clone did not bind to human melanoctyes and bound only slightly to one of six melanoma cell lines tested. Thus, the peptide appears to be specific for the tumor from which it was isolated.
Selections can also be biased to identify peptides binding to other nonvasculature components of the tumor. Using in vitro or ex vivo phage display in combination with in vivo panning can help this process. Rhouslahti’s group recently used sequential ex vivo and in vivo panning on a mouse xenograft MDA-MB-435 breast cancer model to identify peptides specific for both tumor lymphatics and tumor cells.255–258 The peptides were isolated by using ex vivo panning on cell suspensions of MDA-MB-435 tumors that were depleted of endothelial cells, biasing toward nonvasculature-targeting peptides.255 Subsequent in vivo panning with this phage population led to selection of the cyclic nonamer peptide LyP-1.255 LyP-1 has been shown to translocate to the nucleus upon binding and to home to lymphatic vessels in melanoma, breast, prostate, skin, and osteosarcoma tumors while not binding either tumor vasculature or normal tissue.255–258 Other lymphatic-homing peptides have been identified, as delineated in Table 5. This is significant as tumor lymphatic vessels serve as drainage systems, and some evidence suggests that they are the route through which tumor cells escape to form metastases.
5.1.2. Cancer-Specific Peptides Isolated from Bacterial Libraries
Table 6 lists cancer-specific peptides identified from screening bacterial libraries against either protein or cellular targets. Peptides have been selected against seven cell lines in vitro and against one cell line ex vivo. These peptides range in size from 5 to 18 amino acids, and the majority of the peptides are disulfide-constrained and cyclic. Three of the peptides target tumors in vivo. Compared to phage display, a larger number of clones are isolated and fewer high-frequency clones observed. Consensus sequences are often observed. The library sizes used in these selections are in the 108–109 range, comparable to the diversity found in phage display libraries. As such, diversity size cannot account for the selection of multiple binding clones from bacterial libraries compared to the convergence on a particular sequence during phage display panning. More likely, fewer bacterial clones are lost during the selection process, and there is less amplification bias. The selection of multiple clones does increase the time required for validation and characterization of the peptide sequences. This is especially true when it comes to assessing the binding activity of the free peptides. For example, in a selection on HepG2 cells using the FliTrx peptide library, 700 clones were recovered for characterization.323 Only 28% of the clones contained the whole fusion protein, and of the remaining 200 clones, only 10 had higher binding on HepG2 cells than a control cell line. However, isolation of multiple binding peptides can provide a panel of binding ligands which can be used to profile the tumor cell surface. Case in point, using a suite of 24 cell-binding bacterial clones, a signature for luminal and basal breast cancers has been generated.324 While this binding array has not been tested on a large set of patient samples, it demonstrates the potential of peptide ligands to serve as diagnostic classifiers.
Table 6.
Cancer-Targeting Peptides Isolated from Bacterial Display Libraries
| Cancer Type | Cell Line used for Selectiona |
Peptide Sequenceb,c,d | Library Type | |
|---|---|---|---|---|
| Prostate Cancer | PC-3M-1E8 | NVVRQ325 (TMTP1, targets in vivo) | FliTrx | |
| PC-3 | CPGDRGQRRLFSKIEGPC326 (MM-2, targets in vivo) | FliTrx | ||
| Breast Cancer | ZR-75-1 | TCVLHRQRCLMFTLR84 ICVNIKKSLWACEIR84 |
WARVLLIEGRLIVCE84 WWDMVSDRYIWKPVK84 |
OmpA |
| VPCQKRPGWVCLW84 KWCVIWSKEGCLF84 SSWCMRGQYNKICMW84 VECYLIRDNLCIY84 |
WWCLGERVVRCAH84 FYCVIERLGVCLY84 RVCFLWQDGRCVF84 |
CPX | ||
| MDA-MB-231 | MSCLMNSNSFCSI324 WACLMNMYSFCSS324 LRCLTTLDNFCTI324 LICLHRIDRFCSV324 MECLKSMFTYCDI324 LSCLYSMYSYCDV324 LWCLTDLMGWCTV324 LGCLLDVQSWCIV324 |
LWCLLDLMSWCEI324 LDCFRNIYGFCNI324 LKCLWEMRGFCEI324 VDCLFHTDRFCYI324 WRCLMSLETWCMV324 LACLMSLEQWCAV324 WSCLWDLSQFCNF324 PSCLFNLDSFCEF324 |
CPX | |
| MCF-7 | VECDPVRNNFCWW324 LECHRLRTNMCFL324 EWCGIVRVGYCLG324 DACGIIHVGYCKV324 RVCTWNWSWICKE324 RMCTWNLEWVCDL324 RLCVWDWEWLCRD324 |
RVCTWRMVWVCDY324 NLCRGDLEKLCMK324 YACRGDAYYLCAT324 HSCRGDMALLCWL324 FACRGDRWVLCNS324 GLCVADGRPRCLE324 GWCFRDGRPMCSY324 |
CPX | |
| T47D | FWCMGDGRPRCTG324 VWCYLWKYGYCVY324 |
PICRGDRDWRCRD324 GQIWKGEWVKLWRDV324 |
CPX | |
| Hepatoma (Liver) | HepG2 | IAVAPGWLWEEE323 KELCELDSLLRI323 TRGRPRDVANGH323 |
IRELYSYDDDFG323 ESLSVDFMGERA323 QELAPYSWSEKD323 ARRILKGGGVHT323 |
FliTrx |
| Murine Squamous Carcinoma | SCC VII(e) | CGGRRLGGC327,328 (targets in vivo) | FliTrx | |
All cell lines are of human origin unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Peptides clustered in the same cell in this table were isolated in the same panning experiment.
Peptides that have been confirmed to target the appropriate tumor in vivo are noted.
This was an ex vivo panning using isolated cells.
5.1.3. Cancer-Specific Peptides Isolated from OBOC Libraries
While a few peptides have been selected against purified cell surface cancer biomarkers using OBOC libraries,329 these chemical libraries have been more widely exploited for selections on whole cells (Table 7). This may due in part to the ease with which positive beads can be isolated by visual inspection. Biased selections can be performed in which the target receptor is overexpressed and the selection occurs within the context of whole cells. For example, the cyclic peptide cGRGDdvc was isolated from OBOC screening using αvβ3 integrin-transfected K562 leukemia cells.330 The parental K562 cell line was utilized for negative selections to remove ligands that bound to receptors other than αvβ3. However, most OBOC screens have been performed on cell lines without overexpression of a specific receptor. Nonetheless, all of the peptides isolated from OBOC libraries for which receptors have been identified bind integrin receptors. Much of this is likely due to library synthesis as many of the libraries are designed with a bias toward sequences that are known to bind integrins. It should be noted that receptors were identified by competition experiments using panels of integrin antibodies. As such, it cannot be ruled out that the peptides bind other cellular targets as well. Unlike peptides isolated from phage-displayed libraries, the ligands identified from OBOC libraries frequently bind multiple cancer cell lines. This is not surprising considering that integrins are often upregulated in cancer.331 The vast majority of peptides selected using OBOC libraries contain d-amino acids, other unnatural amino acids, or both. As unnatural amino acids are more resistant to peptidase cleavage, these amino acids may have better stability in vivo. To date, six of the OBOC-selected peptides have been shown to target tumors in vivo.
Table 7.
Cancer-Targeting Peptides Isolated from OBOC Libraries Using Cultured Cells as the Target
| Cancer Type | Cell Line used for Selectiona |
Peptide Sequenceb,c,d,e,f | Cellular Receptor |
Applications and Notes |
|---|---|---|---|---|
| Breast Cancer | MDA-MB-231 |
cdGLGBNc332 (LXY1) cdGTyr(3-NO2)GBNc (LXY3)332 |
α3β1 |
Imaging applications: LXY1 peptide-biotin-streptavidin-Cy5.5 used for near-infrared imaging of U-87MG tumors in mice.333 LXY3 peptide-biotin-streptavidin-Cy5.5 used for near-infrared imaging of orthotopic MDA-MB-231 tumors. Note: LXY3 was identified from a focused peptide library based on the LXY1 sequence. |
| Leukemia | Jurkat | LTGpLDI334 | α4β1 | |
| Ovarian Cancer | CaOV-3, SKOV-3, OVCAR-3, & ES-2 |
cLDWDLIc335 cDGLGDDc335 cDGWGPNc335 |
α3 (DGLG peptide) | |
| ES-2 | cdGHCitGPQc336 (OA02) | α3 |
Therapeutic Applications: OA02 peptide displayed on the surface of paclitaxel loaded micelles improves efficacy in two ovarian cancer models compared to the non-targeted paclitaxel or paclitaxel-micelles.337 Imaging applications: A OA02 peptide-biotin-streptavidin-Cy5.5 and peptide-Cy5.5 have been used for near-infrared imaging of ES-2 tumors in mice.336 [64Cu] DOTA-OA02 peptide was used for microPET imaging of ES-2 tumor and SKOV3 metastatic lesion in mice.18 |
|
| SKOV-3 |
cdG-Cha-G-HCit-Qc18 cdG-Chg-G-Hyp-Nc18 cdG-HCit-GPQc18 cdGIGPQc18 cdGLGQ-Bta-c18 cdG-Phe-GP-Cha-c18 cdG-Tyr-GI-Pra-c18 |
Note: The peptides bind multiple cancer cell lines but most also bind a normal human lung epithelial cell line. | ||
| Lung Cancer | A549 | cNGQGEQc338 (pA) | α3β1 | Note: A consensus sequence of NGXG was observed for most positive hits. |
| HI650 | cNleDNleTHyprc339 (pM2) | α4β1 | ||
| Prostate | DU145 | LNIVSVNGRHX85 (RU-1) DNRIRLQAKXX85 (RX-1) |
||
| kmviywkag340 (RZ-3) kikmviswkg340 (HYD-1) |
α3β1341 (RZ-3) α6β1 and α3β1341 (HYD-1) |
Functional Peptide Activity: Free HYD-1 peptide induced cell death in multiple myeloma cells and inhibited H929 tumor growth in mice when injected i.p.342 RZ-3 inhibited random haptotaxis of PC3N cells on lamin-5.341 HYD-1 blocked random haptotaxis and inhibited invasion of PC3N cells on lamin-5.341 HYD-1 also inhibited adhesion of H929 cells to fibronectin mediated by α4β1.342 | ||
| LNCaP | QMARIPKRLARH50 | Note: The peptide binds to other epithelial derived cancer cell lines and can be used to label LNCaP cells spiked into whole blood. | ||
| Bladder Cancer | 5637 | cQDGRMGFc343 (PLZ4) | Imaging applications: Near-infrared imaging of human bladder tumor-bearing mice was performed with a peptide-biotin-SA-Cy5.5 conjugate. |
All cell lines are of human origin.
Cysteine residues that form disulfide bonds are indicated in bold.
Lowercase indicates d-amino acids.
Abbreviations for unnatural amino acids are as follows: B = hydroxyproline, Cha = cyclohexylalanine, Chg = α-cyclohexylglycine, HCit = homocitrulline, Hyo = hydroxyproline, Bta = benzothioenylalanine, Phe = 4-methylphenylalanine, Pra = propargylglycine, Tyr = 3-nitrotyrosine, and Nle = norleucine.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
5.1.4. Cancer-Specific Peptides Isolated from PS-SPCLs
Cancer-specific peptides have been isolated from PS-SPCLs, yet to date, only one of the peptides isolated from PS-SPCLs has been shown to target tumors in vivo.65,344 PS-SPCLs are ideal for assays screening for cellular effects induced by peptides (Table 8). Kang and co-workers used integrin microarrays to select peptides that inhibit angiogenesis.345,346 They first used an αvβ3 microarray and a PS-SPCL to screen for peptides that interfered with the αvβ3–vitronectin interaction. Fluorescently labeled vitronectin was mixed with the peptide library before addition to the microarray. Library peptides that inhibited the receptor–vitronectin interaction were thus visible by a reduced fluorescent signal. This resulted in the selection of two peptides, HGDVHK and HSDVHK. In a similar fashion, they used an α5β1 microarray and a PS-SPCL to screen for peptides that inhibit α5β1–fibronectin interactions.64
Table 8.
Cancer-Targeting Peptides Isolated from PS-SPCLs
| Protein or Cell Line used for Selectiona |
Peptide Sequenceb,c |
Cellular Receptor | Applications and Notes |
|---|---|---|---|
| NR6 cells transfected to overexpress EGFRvIII (mutation variant III) | H-FALGEA-NH265 H-FALIEA-NH265 |
EGFRvIII | Imaging applications: The labeled 4-[18F]fluorobenzoyl-peptide has been used for microPET imaging of NR6M cells in mice.344 |
| α5β1 (selected for ability to compete with fibronectin binding) | VILVLF64 (A5-1) | α5β1 | Functional Peptide Activity: The synthetic peptide inhibits proliferation, bFGF-induced migration, bFGF-induced tubular network formation of HUVEC cells and bFGF-induced neovascularization in a chorioallantoic membrane angiogenesis assay. |
| αvβ3 (selected for ability to compete with vitronectin binding) | HGDVHK-NH263 HSDVHK-NH263 |
αvβ363,345 | Functional Peptide Activity: Both peptides inhibit bFGF-induced HUVEC cell migration and bFGF-induced neovascularization in a chorioallantoic membrane angiogenesis assay. HSDVHK inhibits HUVEC proliferation.346 |
| U266 B myeloma cells (selected for stimulation of inositol phosphates) | WKYMVM-NH2347 | Formyl peptide receptor-like 1348,349 & N-formyl peptide receptor-like 2349 | Functional Peptide Activity: The peptide stimulated phosphoinositide hydrolysis and release of [Ca2+]i from U266, U937 and HL60 cells.347,350 It also stimulated phosphoinositide hydrolysis and superoxide production in human neutrophils.350 In human peripheral blood monocytes and neutrophils, the peptide induced migration and Ca2+ mobilization.348 |
All cell lines are of human origin.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
5.1.5. Common Peptide Motifs Selected from Different Peptide Libraries
The variety of libraries and protocols used for panning and the assortment of cell lines used as targets have resulted in a diverse group of peptides with very little sequence similarity. However, several peptide motifs have been isolated repeatedly from different panning experiments. Most commonly, peptides containing the integrin-binding motif RGD have been found by panning on purified integrins, on whole cells, and in vivo.351 The specificity of the peptide for a particular integrin varies depending on the flanking sequence around the RGD.73,111,115,180,352,353 High-affinity binding to integrins is achieved with only three amino acids; any library with a diversity of 8.0 × 103 is statistically likely to contain the RGD binding motif. For this reason and the fact that integrins are often overexpressed in cancer cells and tumor vasculature, it is not surprising that RGD sequences are often isolated. Similarly, numerous NGR-containing peptides have been found over the years.280,289 This peptide motif binds to aminopeptidase N (CD13) but can also undergo a spontaneous deamination to form isoDGR, a ligand for αvβ3 and α5β1 integrins.354,355
5.2. Isolation of Cardiac- and Muscle-Binding Peptides
Cardiovascular disease is the leading cause of death in the world.149 The primary forms of cardiovascular disease include coronary heart disease, congenital heart disease, stroke, aortic aneurysm, and hypertensive heart disease. The assessment of cardiovascular disease risk as well as the treatment of cardiac disease will benefit from the generation of new reagents that specifically localize to heart tissue or distinct vascular beds. Targeted delivery would increase the therapeutic window of drugs effecting ion channels or contractility and potentially open avenues for gene therapy to rescue damaged tissue.356 For example, administration of vascular endothelial growth factor (VEGF) may improve revascularization in ischemic heart tissue, but concerns about side effects observed with systemic administration have limited its use.357 In addition, targeted delivery could help overcome problems with present diagnostic and treatment protocols. Contrast media presently used in cardiac catheterization and related techniques have undesirable side effects, require administration directly into the local vasculature, and are quickly flushed from the tissue bed. Agents that localize at the specific tissue could enhance the observation of changes over time and help identify the transition between normal and diseased tissue. Peptides have the potential to serve as cardiac-targeting agents for new drugs and diagnostics. Table 9 lists cardiac-specific peptides identified by in vitro, ex vivo, and in vivo phage panning. As the heart is essentially a large muscle containing many blood vessels, most cardiac-specific peptides fall into one of two categories: cardiomyocyte-binding (heart-muscle-binding) peptides or cardiac-vasculature-binding peptides. Surprisingly, other peptide libraries have not been employed to identify cardiac-specific peptides.
Table 9.
Cardiac- and Muscle-Targeting Peptides
| Cell Type and Isolation Method |
Peptide Sequencea,b,c,d,e,f | Cellular Receptor | Applications and Notes |
|---|---|---|---|
| In vivo panning in normotensive Wistar Kyoto rats, recovering phage from the heart | IPTHIRP369 (cell type not determined) HPWPTSI369 (cell type not determined) (pIII) | ||
| In vivo panning in 3 month old C57B61/L mice, recovering phage from the heart | CQAQGQLVC367 (cardiac endothelium, pSKAN phagemid) | TNF-R1 (suggested) | |
| In vivo panning in aging (18 mo) C57B61/L mice, recovering phage from the heart | CARRGQAVC368 (cardiac endothelium, pSKAN phagemid) | Trk B (suggested) | |
| Dual ex vivo & in vivo panning: first ex vivo panning against cell suspensions from BALB/c murine hearts and then in vivo panning in the mice, recovering phage from the heart | CARPAR359 (cardiac cndorhclium, T7) | Cysteine-rich protein 2 (CRIP2; HLP; ESP-1) |
Therapeutic Applications: The CRPPR peptide linked to the antioxidant peptide gp91 ds increased nitric oxide availability and reduced systolic blood pressure in a SHSRP rat model of genetic hypertension.360 Imaging applications: The CRPPR peptide was displayed on 18F radiolabeled-liposomes for PET imaging and dynamic PET analysis of hearts in FVB mice.370 |
| CARPAR359 (cardiac endothelium, T7) | EST (putative) | ||
| CKRAVR359 (cardiac endothelium, T7) | Single immunoglobulin interlukin-1 receptor-related protein (SIGIRR: TIR8) | ||
| CRSTRANPC359 (cardiac endothelium, T7) | unamed protein product; similar to integral membrane protein CII-3 (MpcII-3) | ||
| CPKTRRVPC359 (cardiac endothelium, T7) | Mus musculus bladder cancer-associated protein (human) homologue (bc10) | ||
| Combined in vitro & in vivo screening: panning in vitro on rat cardiomyoblast H9C2 cells followed by in vivo panning in Balb/c mice, recovering phage from the heart | APWHLSSQYSRT358 (CTP) (cardiomyocytes, pIII) |
Imaging applications: Peptide-biotin labeled neutravidin-fluospheres were used for near-infrared imaging of hearts after intra-cardiac injection in mice. Note: This peptide has been isolated on numerous targets and it has been suggested to be a nonspecific phage that is isolated due to propagation advantages.207 |
|
| Ex vivo panning against murine primary cardiomyocytes | WLSEAGPVVTVRALRGTGSW213 (PCM.1) (cardiomyocytes, pIII) | Oligonucleotide Delivery: Peptide conjugated to polymer-Fas siRNA polyplex knocked down Fas expression and inhibited apoptosis in H9C2 ells.371 | |
| In vitro panning on pluripotent stem cell derived cardiomyocytes | QPFTTSLTPPAR372 NNWSSPPQMISR372 ATFSPPQQSLMM372 ISTSPPQGSTSS372 (pluripotent stem cell derived cardiomyocytes, pIII) |
||
| Combined in vitro & in vivo screening: first screened in vitro against murine C2C12 myotubes & then in vivo in Balb/C mice, isolating phage from skeletal muscle | ASSLNIA373 (MSP) (myoblasts and myotubes, pIII) | Oligonucleotide Delivery: Adeno-associated virus modified with the MSP peptide allowed for luciferase and EGFP transduction into C2C12 myotubes in vitro and transduced cardiac muscle and other striated muscles with luciferase when injected i.v.374 The MSP peptide was conjugated to a phosphrodiamidate morpholino antisense oligonucleotide specific for the dystrophin transcript and produced functional truncated dystrophin transcripts in H2K mdx myoblasts.375 The MSP peptide conjugated to a peptide nucleic acid (PNA) antisense oligonucleotide specific for the dystrophin transcript increased the number of dystrophin-positive muscle fibers when injected into the tibialis anterior muscles of a mdx mouse model of muscular dystrophy.376 | |
| In vitro panning against murine cardiac endothelial cells | CVHSPNKKC365 (VP) (cardiac endothelium, pIII) | VCAM-1 | Imaging applications: A VP peptide-modified magnetofluorescent nanoparticle has been used to image vasculature in the ears of mice with mTNF-α-induced inflammation using intravital confocal microscopy. The nanoparticle also homed to atherosclerotic lesions in cholesterol fed apoE−/− mice.365 |
| In vivo panning against rats with ischemia/reperfusion injury, isolating phage from the ischemic left ventricular tissue | CSTSMLKAC361 (ischemic cardiomyocytes, pIII) | Note: Recombinant peptide conjugated to SUMO-mCherry localized to ischemic myocardium in mice. | |
| In vivo panning against atherosclerosis lesion-bearing ApoE−/− mice, isolating phage from plaque-loaded segments of the aorta | VHPKQHR377 (VINP-28) (atherosclerotic plaques, primarily binds endothelial cells but also has affinity for smooth muscle cells and macrophages, pIII) | VCAM-1 | Imaging applications: VINP-28 peptide conjugated to a crosslinked iron oxide-Cy5.5 nanoparticle was used to image vasculature in the ears of mice with mTNF-α-induced inflammation using intravital laser microscpoy. These nanoparticles were also used for MR imaging of atherosclerotic lesions in cholesterol fed ApoE−/− mice; significantly, the peptide-nanoparticle gave no MRI signal change in stain-treated ApoE−/− mice.378 Dynamic PET/CT imaging of atherosclerotic plaques in ApoE−/− mice has been performed using 18F-labeled tetrameric version of peptidie.379 |
| In vivo panning against atherosclerosis lesion-bearing ApoE−/− mice, isolating phage from atherosclerotic plaques from the aorta | CAPGPSKSC380 (atherosclerotic plaque endothelium, pIII) | Glucose-regulated protein precursor (Grp78) | Oligonucleotide Delivery: Peptide inserted into adeno-associated virus-2 carrying the luciferase gene transduced rat endothelial cells in vitro.381 In vivo, peptide inserted into adeno-associated virus-2 carried eGFP transgene to atherosclerotic plaques in ApoE−/− mice and not to the aorta of non-diseased mice.381 |
| CNQRHQMSC380 (atherosclerotic plaque endothelium, pIII) | |||
| CNHRYMQMC380 (atherosclerotic plaque endothelium pIII) | Membrane type 1 matrix metalloproteinase381 | Oligonucleotide Delivery: Peptdie inserted into adeno-associated virus-2 carrying the luciferase gene transduced human, murine, and rat endothelial cells in vitro and carried eGFP trangene to atherosclerotic plaques in ApoE−/− mice.381 | |
| CYNRSDGMC380 (atherosclerotic plaque endothelium, pIII) | |||
| In vivo panning in LDLr knockout mice on a Western diet, isolating phage from atherosclerotic lesions of vasculature | CLVEAYPGLVRSC382 (ON2604, pVIII display) | ||
| Ex vivo panning against cell suspensions from human atherosclerotic plaque tissue | CRKRLDRNC366 (AP) (atherosclerotic plaques - endothelial cells, macrophages, and smooth muscle cells, T7) | IL-4R |
Therapeutic Applications: The AP peptide has found use in tumor targeting. Peptide conjugated to hydrophobically modified glycol chitosan nanoparticles loaded with paclitaxel inhibited H226 and MDA-MB-231 tumor growth in mice. Cy7.5-labeled versions of the naoparticles were used for near-infrared imaging of the same tumor models in mice.383 AP peptides were placed on pH-sensitive micelles loaded with doxorubicin and shown to inhibit MDA-MB-231 tumor growth in mice.384 Imaging applications: AP peptide was conjugated to hydrophobically modified glycol chitosan-Cy5.5 nanoparticles for near-infrared imaging of atherosclerotic plaques in Ldlr−/− mice.385 The AP peptide was conjugated to pH-sensitive micelles loaded with TRITC and used for near-infrared imaging of MDA-MB-231 tumors in mic.384 |
| In vivo panning in hypertensive SHSRP rats, recovering phage from the heart | DDTRHWG369 (cardiac endothelium) ASAGGPN369 (cell type not determined) VQASNSN369 (cell type not determined) (pIII) |
Oligonucleotide Delivery: The DDTRHWG peptide cloned into the HI loop of the AD5/19p adenovirus vector expressing LacZ is able to transduce cardiac vasculature. | |
| In vitro panning against recombinant human Stabilin-2 | CRTLTVRKC386 (S2P) (atherosclerotic plaques - endothelial cells, macrophages, and smooth muscle cells, T7) | Stabilin-2 | Note: Peptide accumulates in atherosclerotic lesions in Ldlr−/− mice. However, stabilin-2 is not specific to plaques but is expressed widely in sinusoidal endothelial cells. |
| In vitro panning against cultured primary aortic vascular smooth muscle cells followed by in vivo panning in mice with endothelial denudation of the carotid artery, isolating phage from the carotid artery | CNIWGVVLSWIGVFPEF387 CESLWGGLMWTIGLSDC387 (proliferating VSMCs, pComb8 phagemid) |
||
| Ex vivo panning against primary human saphenous vein smooth muscle cells | EYHHYNK388 (saphenous vein smooth muscle, pIII) | Oligonucleotide Delivery: Peptide inserted into adeno-associated virus-2 transduced HSVSMCs and HCASMCs with β-galactosidase. | |
| In vivo panning in Duchenne muscular dystrophy mdx mice, isolating phage from the heart and quadriceps | SKTFNTHPQSTP363 (T9) (cardiomyocytes and quadriceps, pIII) |
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
The phage display library type is indicated in parenthesis. All phage are filamentous unless noted as T7 phage.
The targeted cell type is indicated if determined.
Peptides clustered in the same cell in this table were isolated in the same selection experiment. A dotted line between cells indicates that the peptides were isolated in the same panning but are separated for clarity.
Peptides selected to bind diseased heart arc highlighted in blue.
Peptides specific for cardiomyocytes have primarily been targeted toward normal myocardium, Duchenne muscular dystrophy (DMD) myocardium, or ischemic myocardium. The peptides targeting the normal myocardium include the CTP peptide, isolated by a combination of in vitro and in vivo panning. This peptide binds to normal murine cardiomyoctyes both in vitro and in vivo.358 Six different peptides all targeting normal murine cardiac endothelium were identified by a combination of ex vivo and in vivo panning, and receptors for most of the peptides were identified using a bacterial two-hybrid system.359 While phage displaying all six of these peptides targeted cardiac endothelium in vivo, only one peptide, the CRPPR peptide, has been used for in vivo targeting outside the context of the phage.359,360 Peptides isolated against ischemic myocardium, characterized by reduced blood supply to the heart, include the CSTSMLKAC peptide isolated from myocardium of rats given an ischemic injury.361 A dye-labeled version of this peptide demonstrated in vivo specificity for the ischemic left ventricle.
DMD is a fatal X-linked disease involving progressive muscle degeneration.362 The disease is also associated with dilated cardiomyopathy, and most of those affected die in their late teens or early twenties from pneumonia complicated by cardiac problems.362 One recent study used in vivo phage panning in an mdx mouse model of DMD to isolate peptides specific for the heart and quadriceps.363 The peptide SKTFNTHPQSTP isolated from quadriceps demonstrated specific homing to both the heart and quadriceps when injected in a dye-labeled form into the tail vein of mdx mice.
Peptides specific for cardiac vasculature have been isolated for various disease states: hypertension/stroke, atherosclerotic plaques/inflammation, and aging. Atherosclerosis is the most significant contributor to cardiovascular disease and is a progressive disease driven by inflammation that leads to accumulation of plaque in large arteries and can end with artery rupture and thrombosis.364 As atherosclerosis begins with endothelial cell changes brought about by activation of inflammatory pathways,364 targeting these endothelial cells has the potential to enrich the available therapeutic and diagnostic options. The peptide CVHSPNKKC, specific for vascular cell adhesion molecule-1 (VCAM-1), was isolated from murine cardiac endothelial cells in culture.365 This peptide shows specificity for VCAM-1-expressing endothelial cells and atherosclerotic lesions in vivo and has been used for a variety of ex vivo and in vivo techniques (described in more detail later). The atherosclerotic-plaque-specific peptide CRKRLDRNC was identified after an ex vivo panning on cell suspensions from human atherosclerotic plaques and appears to bind the IL-4R expressed on the endothelial cells, macrophages, and vascular smooth muscle cells of the plaques.366 Significantly, this peptide was able to home to atherosclerotic plaques in mice and to bind to human tissue sections of atherosclerotic tissue. Peptides specific for vasculature of different ages have also been identified. One peptide bound cardiac vasculature of young (3 month old) mice and not that of aging (18 month old) mice,367 while another peptide demonstrated specificity for the aging vasculature.368 Again, phage display panning proves to have the ability to isolate highly specific ligands that can discriminate between healthy and diseased states.
5.3. Isolation of Immune-Cell-Binding Peptides
Peptides that target specific cell types involved in the immune systems have several key uses, including antigen delivery for vaccine development, delivery of immunosuppressant agents for the treatment of autoimmune diseases, and redirection of an immune response to eradicate diseased cells. Identification of immune-cell-specific peptides is challenging. First, there are numerous subclasses of immune cells, and they share overlapping cell surface markers. Furthermore, these subclasses have high plasticity in which the cells can exist in numerous states of activation or differentiation. Second, several cells of the immune system are inherently phagocytic; background uptake of nontargeted ligands can be high. However, the demands on specificity may not be as great for immune cells as for other cell types. In many cases the cargo is not toxic to other cell types. Unlike delivery of a chemotherapeutic or toxin, uptake of a proteinaceous antigen in a nontarget cell is unlikely to cause harm. Instead, targeting is performed to enhance an immune response compared to a nontargeted antigen. Additionally, the inherent amplification of an immune signal lowers the amount of antigen that needs to be delivered. Finally, many cell surface receptors on immune cells have been identified, and antibody reagents for these markers are available; biased selection for a specific cell surface marker can be performed readily. Immune-cell-specific peptides have been identified using in vitro, ex vivo, and in vivo phage panning and from PS-SPCLs (Table 10).
Table 10.
Immune-Cell-Targeting Peptides
| Isolation Methoda | Peptide Sequenceb,c,d | Immune Cell Type |
Applications and Notes |
|---|---|---|---|
| In vitro panning against the heterodimeric protein CD11c/CD18 purified from splenic tissue of a patient with hairy cell leukemia | CGRWSGWPADLC390 | Dendritic Cells | Immunomodulation: C57BL/6 mice vaccinated with peptide-liposomes carrying OVA induced OVA-specific T cell priming and antibody production. The peptide was also engrafted into plasma membrane vesicles derived from B16-OVA cells and used to vaccinate C57BL/6 mice carrying either lung or subcutaneous B16-OVA tumors, inhibiting tumor growth and metastasis in both models391 |
| In vitro panning against immobilized CD40-Ig | ATYSEFPGNLKP399 | Dendritic and B cells | Oligonucleotide Delivery: A bifunctional peptide, created by conjugating this peptide to a peptide that binds the adenoviral knob protein, allowed for specific transduction of adenoviral GFP to human and murine dendritic cells, murine B cell blasts, murine L10A cells, and human B cells. |
| In vitro panning against the murine dendritic cell line JAWSII. Bound phage were eluted with TNFα to identify TNF-R2 ligands. | CYTYQGKLC392 (pTNF) | Dendritic Cells | Oligonucleotide Delivery: Both a K16-G5-peptide fusion and peptide-K16 microspheres transduced JAWSII and BMDC with a GFP expressing plasmid392 Peptide-lipoplexes containing EGFP-siRNA induced silencing of EGFP in EGFP-expressing DC2.4 cells.400 |
| In vitro panning against XS52 dendritic cells | GPEDTSRAPENQQ-KTFHRRW393 (XS52.1) | Dendritic Cells |
Oligonucleotide Delivery: Peptide-liposomes containing luciferase plasmid transduced Langerhans cells when injected into mouse skin. Immunomodulation: The peptide containing phage clone induced a more rapid and robust humoral immune response against phage coat proteins than did control peptide phage clones.393 |
| In vitro panning against LPS-activated U937 hematopoietic precursor cells | PTAPTST401 PSPRSGS401 PAIAHRT401 |
Macrophages (Activated) | |
| In vitro panning against immobilized ganglioside GM1 | AFHKNSQTTTLY398(CL1) TNCLQNCSGVHL398 (CL2) GWKERLSSWNRF398 (CL3) |
M Cells | Immunomodulation: Oral delivery of the CL3 peptide-fused to EGFP induced an EGFP-specific mucosal and systemic immune response in mice (IgG & IgA production, Th2 type response). |
| In vitro panning against human M-like cell model system (co-cultures of Caco-2 and Raji cells) | SFHQLPARSPLP397(Col) | M Cells |
Immunomodulation: Peptide-fused EGFP given orally bound M cells and transported to the mucosal immune induction site, inducing an EGFP-specific mucosal and systemic immune response in mice (IgG and IgA production, Th2 type response). Suggested Receptor: C5aR402 |
| In vitro panning against human M-like cell model (co-cultures of Caco-2 and Raji cells), selecting for transcytosis | CKSTHPLSC403 (CKS9) | M Cells | Functional Peptide Activity: Chitosan nanoparticles that display CKS9 localized in the Peyer's patch when injected into a closed ileal loop. |
| In vitro panning of a T7 phage peptide library against monocultures of Caco-2 cells and co-cultures of Caco-2 and M cells, selecting for phage transcytosis | CTGKSC404 PAVLG404 LRVG404 |
M Cells | Functional Peptide Activity: The peptides transcytose nanoparticles across M-like cells in an in vitro assay. |
| In vivo panning in Winstar rats, delivering the phage library into the closed ileal loop and isolating Peyer's patches | LETTCASLCYPS396 (P8) VPPHPMTYSCQY396 (P25) |
M Cells | Functional Peptide Activity: The d- analog of P8 delivers polystyrene beads to M cells and Peyer's patch when injected locally into the intestine. |
| In vitro panning against immobilized FcγRIIA-R134-GST | WAWVWLTETAV394 | Leukocytes | |
| Ex vivo panning against freshly isolated human neutrophils | FGPNLTGRW25 CKDGLFLGSWLC25 DLVTSKLQV25 |
Polymorphonuclear leukocytes | |
| In vitro screening against neutrophil-like differentiated HL60 cells screening for arachidonic acid release using PS-SPCL | RKYHVM-NH2405 RKYYYM-NH2405 MKYYKM-NH2405 MKYYYM-NH2405 |
Leukocytes |
Functional Peptide Activity: The peptides stimulate superoxide production and induce chemotactic migration. Suggested Receptor: FPRL1 |
| Ex vivo screening against human monocytes using PS-SPCL, screening for superoxide anion generation | HFYLPM406 HFYLPm406 MFYLPM406 |
Monocytes | Functional Peptide Activity: The peptides induce superoxide production and cause an increase in intracellular Ca2+ rise. |
All libraries are pill phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
Dendritic cells are antigen-presenting cells that reside in peripheral tissues such as skin acting like guards on the lookout for molecules of foreign material, or antigens.389 The dendritic cells capture these antigens and process them into small peptides while transforming into mature dendritic cells that migrate toward peripheral lymphoid organs. There mature dendritic cells present these peptides to naïve T cells by displaying peptide–MHC protein complexes on their cell surface, initiating an adaptive immune response against the origin of the antigen. Dendritic cells also activate B-cells to produce antigen-specific antibodies (humoral response) and further activate natural killer (NK) cells and natural killer T (NTK) cells. Due to their ability to activate all of these different immune pathways, dendritic cells are ideal targets for vaccine development. Subsequently, several dendritic-cell-specific peptides have been isolated for the purposes of specifically delivering antigens to dendritic cells or transducing genes into dendritic cells. Most peptides have been selected to bind a known DC marker. One such peptide, the CGRWSGWPADLC peptide, was isolated against the dendritic-cell-specific marker CD11c/CD18390 and was later used for vaccination.391 A TNF-α-competing (TNF-α = tumor necrosis factor-α) peptide, CYTYQGKLC, was isolated by in vitro panning against the murine dendritic cell line JAWSII, using TNF-α to elute bound phage.392 The peptide was shown to specifically deliver genes to dendritic cells (details later). In an unbiased selection, McGuire et al. also identified a novel dendritic-cell-targeting peptide, XS52.1, by in vitro panning on the mouse dendritic cell line XS52, derived from mouse skin.393 A multimeric tetramer version of the peptide specifically bound to XS52 cells in vitro and was further used for gene delivery in vivo.
Dendritic cells fall into the category of leukocytes, or white blood cells, which comprise a category consisting of several other cell types. Several peptides have been isolated against other types of leukocytes, including the leukocyte-binding peptide WAWVWLTETAV isolated against the FcγRIIA receptor expressed by neutrophils and mononuclear phagocytes. 394 Importantly, this peptide bound primary mononuclear (PMN) cells and monocytes isolated from human peripheral blood.
Another immune cell type for which cell-binding peptides have been isolated is the microfold, or M, cell. M cells are antigen-sampling cells located in Peyer’s patches of intestinal epithelium. The intestines are lined with a single layer of epithelium for protection, making it difficult for most things to pass through, or out, of the gut.395 However, M cells sample the antigens in the gut and transport them a short distance to a binding pocket in which immune cells such as lymphocytes or macrophages dock.395 Thus, the M cells can carry antigens out of the gut to immune cells to facilitate an immune response, making M cells desirable targets for orally available vaccines.395 While the oral vaccine will inherently reach the intestines after administration, subsequent delivery of the vaccine to M cells is variable.395 Identification of ligands that could direct the oral vaccine to M cells would allow for their specific delivery to immune cells for initiation of an immune response. In vivo phage display performed by injecting phage directly into a loop of rat intestines identified the P8 and P25 peptides as specific for M cells.396 These peptides were able to specifically bind M cells in vivo when injected into mouse intestine. Importantly, although isolated from rat M cells, these peptides also bound to tissue sections of human intestine as evidenced by immunohistochemistry. Kim et al. used in vitro panning against human M-like cells to identify the Co1 peptide.397 The Co1 peptide was able to bind both human M-like cells and M cells in vivo in mice. As described earlier, not all cellular features are proteins, and an M-cell-targeting peptide was isolated by panning against immobilized ganglioside GM1 (monosialotetrahexosylganglioside). 398 While this peptide can deliver an antigen to M cells in vivo, it is important to note that GM1 is not restricted to M cells.
5.4. Isolation of Pancreatic-Islet-Cell-Binding Peptides
Diabetes is the seventh leading cause of death in the United States and affects 8.3% of the population, or 25.8 million people. There is no cure for diabetes, and complications of the disease can be serious. There are two major types of diabetes: type 1 and type 2. Although the two types of diabetes differ with regard to their origins, both involve the β cells that produce and secrete insulin. The ability to image β cell mass and β cell function as well as delivery of genes and therapeutics to β cells remains a major goal of the biomedical field. β cells comprise only ~2% of the pancreas and are localized in discrete regions known as the islets of Langerhans; thus, the demand for specificity and sensitivity of detection is great. Additionally, few well-characterized β cell lines are available. Only a handful of islet-specific peptides have been identified. Table 11 lists islet-specific peptides identified by in vitro and in vivo phage panning. The use of phage display to isolate islet-specific antibodies and peptides has recently been reviewed elsewhere. 407
Table 11.
Islet-Targeting Peptides
| Isolation methoda | Peptide Sequenceb,c,d | Target Cell | Applications and Notes |
|---|---|---|---|
| Ex vivo panning against freshly isolated rat pancreatic islets | LSGTPERSGQAVKVKLKAIP75 (RIP1) GAWEAVRDRIAEWGSWGIPS75 (RIP2) |
β-cells (RIP1) Non-specific binding (RIP2) |
Note: Radiolabeled RIP1 phage was shown to home to islets in vivo.409 |
| In vivo panning in male C57BL/6 mice followed by laser pressure catapult microdissection (LPCM) of pancreatic islet sections | CVSNPRWKC408 CHVLWSTRC408 |
vasculature of islets of Langerhans |
Oligonucleotide Delivery: Peptide-polymer carrying luciferase DNA transduced MS1 cells in culture.410 Note: EphA4 and EphA2 have been suggested as the receptors. |
| Ex vivo panning on cell suspensions from solid tumors of RIP1-Tag2 mice and then in vivo panning identifying phage homing to tumors using a T7 phage library | CRGRRST315 (RGR) CRSRKG315 (RSR) CKAAKNK315 (KAA) CKGAKAR315 (KAR) FRVGVADV315 (VGVA) |
RGR: hyperplastic & tumor vasculature of pancreatic islets (endothelial cells & pericytes) RSR: hyperplastic vasculature of pancreatic islets (endothelial cells & pericytes) KAA: tumor vasculature of pancreatic islets (endothelial cells & pericytes) KAR: tumor pancreatic islets VGVA: hyperplastic & tumor pancreatic islets |
Therapeutic Applications: Combination treatment with RGR peptide-anti-CD40 antibody and murine IL-2-RGR peptide fusion protein increased survival in RIP1-Tag5 mice, and 80% of mice treated with the combination treatment plus adoptive transfers of anti-Tag CD4+ and CD8+ T cells survived long-term.316 Note: PDGFRβ has been suggested as the receptor for the RGR peptide. |
| Ex vivo panning on cell suspensions from angiogenic islets of RIP1-Tag2 mice and then in vivo panning isolating phage that homed to angiogenic islets | CEYQLDVE315 (EYQ) | hyperplastic pancreatic islets |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
Ex vivo panning against freshly isolated rat pancreatic islets resulted in selection of two islet-binding peptides termed RIP1 and RIP2.75 While both peptide-displaying phage associate with isolated rat islets, the RIP1 phage showed 8-fold higher association with the Ins832/13 β cell line in vitro than the RIP2 phage. Thus, it is assumed that the RIP1 phage binds β cells, while the RIP2 phage may bind some other islet component. Although the peptides have not been tested outside the context of the phage, phage displaying both peptides were able to reach and bind islets in vivo as evidenced by immunohistochemistry on pancreatic sections from rats injected with phage. While the RIP1 phage demonstrated specificity for islets, with some expected clearance in other organs, the RIP2 phage accumulated nonspecifically throughout the rat. Therefore, the RIP1 phage was chosen for further imaging studies, as described later. Importantly, this phage bound only to the islets of normal rats and not to those of a model diabetic rat, suggesting that the RIP1 peptide in the context of the phage can distinguish between normally functioning β cells and dysfunctional β cells.
Peptides specific to islet vasculature have also been identified. In vivo panning in normal mice followed by laser pressure catapult microdissection to specifically isolate pancreatic islets identified the vasculature-binding peptides CVSNPRWKC and CHVLWSTRC.408 Phage were injected intravenously, and immunohistochemistry of pancreas tissue sections was used to assess phage binding. While both phage clones bound normal islet vasculature, they bound more impressively to angiogenic vasculature of islet tumors from RIP-Tag2 mice.
Another study used ex vivo panning on cell suspensions from both angiogenic islets and solid tumors from RIP1-Tag2 mice followed by in vivo panning in the same mice to look for phage homing to the angiogenic islets or tumors.315 As RIP1-Tag2 mice spontaneously develop angiogenic islets that progress into solid tumors, 12 week old mice that have a combination of angiogenic islets and tumors are a good model for isolating stage-specific peptides. While six specific phage were initially isolated, only the RGR, RSR, and KAA phage were used as synthetic peptides for further study. Dye-labeled versions of the peptides were injected intravenously into RIP1-Tag2 mice, and in vivo homing was verified by fluorescent images of pancreatic sections. While all three peptides colocalized with both the endothelial and pericyte cells of islet vasculature, they each bound different stages. RGR bound both angiogenic and tumor vasculature, RSR bound only angiogenic vasculature, and KAA bound only solid tumor vasculature. None of them bound normal islet vasculature. This finding highlights the exquisite differences between the vasculature at different stages of tumor development, making it possible to specifically target the vasculature of tumors at a specific stage. Importantly, the RGR, RSR, and KAA peptides are the only islet-cell-binding peptides that have been tested outside the context of the phage. Further use of these peptides for specific delivery of imaging agents or drugs could allow very precise delivery to different types of islet vasculature.
5.5. Isolation of Adipose-Cell-Binding Peptides
Obesity is a significant problem in the United States, with approximately 33% of the adult population classified as obese.411 For this reason, there is interest in identifying peptides that bind to adipose cells. Table 12 lists adipose-specific peptides identified by in vitro, ex vivo, and in vivo phage panning. To the best of our knowledge, only four adipose-binding peptides have been identified.
Table 12.
Adipose-Targeting Peptides
| Isolation method and cell typea |
Peptide Sequenceb,c | Receptor Identified |
Applications and Notes |
|---|---|---|---|
| In vitro panning against mouse preadipocyte 3T3-L1 cells | CWLGEWLGC413 (mPep) | α5β1 |
Therapeutic Applications: Peptide-D(KLAKLAK)2 killed mouse 3T3-L1 preadipocytes that were induced toward adipocyte differentiation in vitro. Oligonucleotide Delivery: When the peptide was displayed on a chimeric prokaryotic-eukaryotic AAVP vector carrying the Ucp 1 gene, it transduced 3T3-L1 preadipocytes, preventing them from differentiating into adipocytes. |
| Ex vivo panning against adipose stromal cells isolated from white adipose tissue after patient liposuction | CMLAGWIPC413 (hPep) | α5β1 | Functional Peptide Activity: Free peptide increased cell migration of human adipose stem cells in vitro. |
| In vivo panning against white fat in obese leptin-deficient Lepob/ob mice using a T7 phage peptide library | CKGGRAKDC414 (binds white fat vasculature) |
Prohibitin414,416,417 |
Therapeutic Applications: Subcutaneous injection of peptide-GG-D(KLAKLAK)2 into obese mice414,418 or rats418 reversed obesity and reduced white fat mass. Injection of a peptide-BVT.2733 (an 11β-HSD1 inhibitor) conjugate into obese mice reversed obesity, reduced adipose tissue mass and size, and decreased plasma glucose tolerance.419 Subcutaneous injection of peptide-GG-D(KLAKLAK)2 into obese rhesus macaques monkeys decreased white fat mass, total body weight, BMI, abdominal circumference, and insulin resistance.415 Note: The peptide also targets to prohibitin-positive tumors.472 |
| In vivo panning (T7 library) against white adipose tissue in mice followed by FACS to isolate adipose stromal cells | CSWKYWFGEC412 (WAT7) | Truncated decorin lacking glycanation site (ΔDCN) |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Adipose stromal cells are precursors to the cells of white adipose tissue.412 Two adipose stromal cell peptides were isolated from two separate pannings.413 Ex vivo panning against adipose stromal cells isolated from white adipose tissue after patient liposuction identified the CMLAGWIPC peptide named hPep. A second in vitro panning against the mouse preadipocyte cell line 3T3-L1 identified the CWLGEWLGC peptide named mPep. Both peptides bound to cultured adipose stromal cells, and the integrin α5β1 was identified as the receptor for both peptides. Significantly, mPep was used for in vitro therapy, and hPep was shown to have cellular effects (details later). Two other adipose-stromal-cell-binding peptides were isolated using in vivo phage display coupled with FACS.412 After in vivo phage injection and isolation of white adipose tissue, FACS was used to sort for adipose stromal cells. The peptides CSWKYWFGEC (WAT7) and CGQWLGNWLC were isolated from this selection.
In addition, a peptide specific for white fat vasculature was identified from an in vivo screen in an obese mouse model, leptin-deficient (Lepob/ob) mice.414 Although identified from an obese mouse model that is not representative of human obesity, the peptide also homed to the vasculature of white fat in normal wild-type mice that better represent the human condition. Significantly, this CKGGRAKDC peptide was able to specifically decrease amounts of white fat in mice and prevent obesity in these mice as well as in Old World monkeys415 (details later). The receptor for the peptide was identified as prohibitin.
5.6. Isolation of Brain-Specific Peptides
Table 13 lists brain-specific peptides identified by in vitro and in vivo phage panning and from PS-SPCLs. Treatment and diagnosis of all brain-related diseases is greatly hampered by the blood–brain barrier. The blood–brain barrier is composed primarily of brain capillary endothelial cells and is located at the junction of the blood and brain.420 True to its name, it is a barrier that is extremely difficult to pass physically. Very few drugs are able to penetrate this barrier, including peptides, proteins, antibodies, genes, and more than 98% of small molecules.421 Significantly, none of the medium- or large-sized pharmaceutical companies are focused on targeting the blood–brain barrier. There is a clear need for ligands that can specifically home to and deliver cargo to this barrier. With the goal of identifying blood–brain-barrier-specific peptides, in vivo phage display was performed using an in situ brain perfusion of the phage library into normal male C57Bl/6 mice.422 Two of the isolated phage clones (GLA and GYR) demonstrated specificity for the cultured human brain endothelial cell line hCMEC/D3. Importantly, in situ perfusion of both phage into the mouse brain demonstrated specific homing to the brain as compared to that of a control peptide phage. Another study used in vivo phage display to isolate the brain-specific TGN peptide in an effort to find a ligand that can bypass the barrier.206 Interestingly, this study used a 24 h time point for phage isolation during panning as opposed to the usually short 5–15 min circulation time. Significantly, the TGN peptide homed to the mouse brain in vivo. Another method that allows for bypassing the blood–brain barrier is to deliver agents intranasally. Recently, a phage library was panned in vivo in rats by intranasal administration to identify phage clones that reach the cerebrum.423 The clone containing the peptide ACTT-PHAWLCG homes to the olfactory nerve and further into the brain.
Table 13.
Brain-Targeting Peptides
| Isolation Methoda | Peptide Sequenceb,c,d,e | Applications and Notes |
|---|---|---|
| In vitro panning against EphA4 extracellular domain fused to human Fc | APYCVYRGSWSC248 (APY) KYLPYWPVLSSL248 (KYL) VTMEAINLAFPG248 (VTM) |
Functional Peptide Activity: All three peptides block binding of ephrin-A3 to EphA4 but do not activate the receptor. The free peptides disrupted migration of neural crest cells in chick trunk explants.248 The KYL peptide inhibited EphA4-Fc mediated collapse of E17 rat neocortical axonal growth cones and infusion of KYL into spinal cord lesions in rats prevented retrograde degeneration of the corticospinal tract and improved corticospinal tract function.425 When KYL was applied to hippocampal slices from Thyl-mGFPsil mice, the peptide increased the number of terminal aborizations, reduced remodeling at the most plastic terminal aborizations in mossy fibers and disrupted terminal aborization topography.426 |
| In vitro panning against EphA5 extracellular domain fused to human Fc | SLRDTYMRAEVL248 WDCNGPYCHWLG248 WTFPVLWDDKHP248 |
Note: WTFPVLWDDKHP binds both EphA5 and EphA6. |
| In vitro panning against EphA7 extracellular domain fused to human Fc | WASHAPYWPHPP248 SVSVGMKPSPRP248 KHLPFYPHPTSP248 |
Note: These peptides bind multiple members of the EphA receptor family. SVSVGMKPSPRP has been suggested to be a non-specific peptide that is isolated from the NEB 12-mer library most likely due to preferential propagation of this phage clone.207 |
| In vitro panning against a prototypical clone of murine neural stem cells (C17.2) | CGLPYSSVC427 |
Functional Peptide Activity: The peptide induced proliferation of C17.2 neural stem cells and primary neural progenitors isolated from adult mouse brain. Note: The receptor for the peptide is laminin γ1. |
| In vitro panning against cultured mouse cerebellar granule neurons (CGNs) | SLNDWIDWSEPH424 SHSLMTSSSVWT424 SHTTPGKNNDPF424 NYARDPLMSLPQ424 |
|
| Ex vivo panning against cultures of primary neurospheres isolated from hippocampus of adult C57BL/6 mice | QTRFLLH428 VPTQSSG428 |
Oligonucleotide Delivery: The QTRFLLH and VPTQSSG peptides were linked to adenovirus carrying RFP. Both constructs infected hippocampal stem cells after injection into brains of pNestin-GFP transgenic or C57BL/6 mice, but the QTRFLLH peptide was limited to the precursor cells of the hippocampal dentate gyrus. A QTRFLLH-adenovirus construct carrying GFP infected hippocampal stem cells after injection into brains of Wistar rats. This allowed for monitoring of stem cell differentiation.429 |
| Ex vivo selection for the ability to inhibit binding of the cocaine analog [125I]RTI-55 to the dopamine transporter from rat caudates. This selection used a positional scanning D-amino acid library. | Ac-frrwfc-NH262 Ac-frrwrc-NH262 |
Functional Peptide Activity: Microdialysis of the Ac-frrwfc-NH2 peptide into brains of rats increased extracellular dopamine and serotonin levels. |
| In vivo panning in mice using a tail vein injection and isolating phage from the brain | TGNYKALHPHNG206 (TGN) | Imaging Applications The peptide crossed the blood-brain barrier, even when injected via the tail vein, and transported PEG-PLGA nanoparticles containing DiR dye into the brain as determined by near-infrared imaging. |
| In vivo panning via nasal passageways in rats, isolating phage from the cerebrum | ACTTPHAWLCG423 | Note: The peptide enters the brain through the olfactory nerve pathway. Phage do not pass through the blood brain barrier when administered intravenously. |
| In vivo panning in male C57B1/6 mice by in situ brain perfusion of phage, isolating phage from the brain | GLAHSFSDFARDFVA422 (GLA) GYRPVHNIRGHWAPG422 (GYR) |
Functional Peptide Activity: The phage homed to brain endothelium when perfused in situ into the brain of a mouse. The free peptide was not tested. |
| In vivo panning (T7 phage) in Balb/c mice, using a tail vein injection and isolating phage from the brain | CLSSRLDAC67 CNSRLHLRC67 CENWWGDVC67 |
Functional Peptide Activity: The CLSSRLDAC peptide was conjugated to red blood cells resulting in an accumulation of red blood cells in the brain. All phage home to the brain vasculature. |
| WRCVLREGPAGGCAWFNRHRL67 | Functional Peptide Activity: The phage clone binds to the brain vasculature. | |
| In vivo panning in normal mice, using i.v. injection of phage and recovery of phage from the brain | CRTIGPSVC300 |
Therapeutic Applications: A peptide-adeno-associated virus phage vector (AAVP) carrying herpes simplex virus thymidine kinase gene (HSV-TK) transduced intracranial glioblastomas in mice after i.v. injection. This led to tumor inhibition after i.p. ganciclovir administration. Imaging applications: Treatment with an intravenous injection of the above peptide-AAVP allowed for microPET/CT imaging of accumulation in tumors after i.v. injection of [18F]-FEAU. Note: The peptide binds normal brain and glioblastoma vasculature and parenchyma. The cellular receptor is the Transferrin receptor. |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
Lowercase letters indicate d-amino acids and Ac = acetylation.
Several peptides specific for brain vasculature have also been identified. An in vivo panning isolated four peptides, all of which were shown to home to the brain in vivo in the context of the phage.67 Additionally, one peptide, the CLSSRLDAC peptide, was able to mediate brain-specific homing when conjugated to the surface of a red blood cell. Peptides that target other areas of the brain include the APY, KYL, and VTM peptides isolated against the EphA4 receptor that is expressed in the learning and memory portions of the brain—the hippocampus and cortex248—and the SLNDWIDWSEPH, SHSLMTSSSVWT, SHTTPGKNNDPF, and NYARDPLMSLPQ peptides isolated by ex vivo panning against mouse cerebellar granule neurons.424
5.7. Isolation of Liver- and Kidney-Binding Peptides
5.7.1. Liver-Cell-Binding Peptides
Isolation of liver-specific targeting peptides by in vivo phage display has been largely ignored since most intravenously injected phage accumulate nonspecifically in the liver.67 However, treatment and diagnosis of hepatitis and other chronic liver diseases could benefit from the availability of liver-specific agents. A new type of T7 phage library modified to prevent clearance by the immune system, resulting in subsequent nonspecific liver accumulation, was recently used to identify hepatocyte-binding peptides.430 In vivo panning was performed in mice with normal immune systems, resulting in selection of liver-specific peptides. Three peptides in the context of the phage were subsequently able to home to hepatocytes in vivo after intravenous injection in mice (Table 14). Importantly, two of the phage displaying hepatocyte-specific peptides were tested for accumulation in other organs and were shown to maintain liver specificity. These results suggest that the liver may still be a viable target for cell-binding peptides.
Table 14.
Liver- and Kidney-Targeting Peptides
| Isolation Methoda | Peptide Sequenceb,c,d | Target Cell | Applications and Notes |
|---|---|---|---|
| In vivo panning in ICR mice using a T7 phage library injected i.v., isolating phage from the liver | LRRKIVE430 TRRQTIK430 KTHQNSR430 ANQIRSR430 GQKLRYT430 TKQMRKS430 |
hepatocytes hepatocytes hepatocytes sinusoids sinusoids microvilli of hepatocytes |
|
| In vivo panning in Wistar Kyoto rats infusing phage into the femoral vein and selecting for kidney targeting | HTTHREP431 (HTT) HITSLLS431 (HIT) |
tubular epithelium glomeruli | Oligonucleotide Delivery: The HTT peptide inserted into Ad5/19p adenovirus transduced kidneys with β-galactosidase.431 The HIT peptide inserted into Ad5/19p adenovirus transduced fresh human renal tumor cells, and normal human kidney cells in vitro and in vivo with β-galactosidase.431,432 The HIT peptide labeled adenovirus also transduced ACHN subcutaneous or intraperitoneal tumors in mice at levels similar to or above wild type adenovirus and reduced liver uptake of the virus.432 |
| In vivo panning in Balb/c mice, i.v. injecting phage and isolating phage clones from the kidneys | CLPVASC67 | kidney vasculature | |
| Ex vivo panning on microdissected intact cortical collecting ducts from rat kidneys | ELRGDMAAL177 ELRGDRAHW177 |
tubular epithelial cells of the cortical collecting ducts | Note: The peptides are likely to bind an integrin receptor since they contain an RGD motif. The peptides bind preferentially to the basolateral side of the cortical collecting ducts in the kidney. ELRGDMAAL also binds T84 colon carcinoma cells. |
| Ex vivo panning on microdissected intact proximal convoluted tubules from rat kidneys | KMGGTNHPE177 | tubular epithelial cells of the proximal convoluted tubules |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
5.7.2. Kidney-Cell-Binding Peptides
The kidney is involved in a diverse number of diseases. While many peptides nonspecifically accumulate in the kidney, little attention has been focused on isolating specific kidney-targeting ligands. In vivo panning in rats looking for kidney targeting resulted in the isolation of two peptides, termed HTT and HIT (Table 14).431 Rats predosed with a control (no peptide) phage and then injected with the peptide phages demonstrated specific phage targeting to the kidney with some nonspecific liver accumulation similar to that of the control phage. These peptides were then incorporated into a modified adenovirus for specific gene delivery to the kidneys as described in detail later.432 A kidney-vasculature-specific peptide, CLPVASC, was also identified from an in vivo screen in mice and has been shown to home to the kidney on the phage platform.67 Two peptides, ELRGDMAAL and ELRGDRAHW, were isolated by ex vivo panning on microdissected intact cortical collecting ducts (CCDs) from rat kidneys and one peptide, KMGGTNHPE, was isolated by ex vivo panning on microdissected intact proximal convoluted tubules (PCTs) from rat kidneys.177
5.8. Isolation of Stem-Cell-Binding Peptides
Stem cells have the capacity to differentiate into any cell type, and their potential for regenerative medicine has spurred research in this area. Despite this potential, few stem-cell-binding peptides have been identified (Table 15). However, of those peptides identified, many distinguish between pluripotent stem cells and differentiated cells.
Table 15.
Stem-Cell-Binding Peptides
| Isolation methoda | Peptide Sequenceb,c,d | Applications and Notes |
|---|---|---|
| In vitro panning on the extracellular domain of the cancer stem cell marker CD133 | LQNAPRS144 APSPMIW144 |
Functional Peptide Activity: The LQNAPRS peptide inhibits migration of colon and breast cancer cells. Treatment of cells with the peptide reduced c-Met and STAT3 protein levels.437 |
| In vitro whole cell panning on undifferentiated pluripotent P19 cells from a murine teratocarcinoma | ALPSTSSQMPQL438 | Note: The peptide does not bind to differentiated P19 cells. |
| In vitro panning on mouse embryonic stem cells | KHMHWHPPALNT439 (Seq2) | Note: The peptide does not bind to differentiated mouse embryonic stem cells. |
| In vitro panning on an embryonic stem cell line derived from rhesus macaque | APWHLSSQYSRT440 | Note: This peptide has been isolated on numerous targets and is suggested to be a nonspecific phage that is isolated due to propagation advantages.207 |
| In vitro panning on mouse neural stem cells | CGLPYSSVC427 | Note: The peptide shares sequence similarity with netrin-4 and binds laminin γ1. |
| In vitro panning against neural stem cells derived from rheusus monkey embryonic stem cells | HGEVPRFHAVHL441 | |
| In vitro panning on murine neural stem cells | FAQRVPP433 QHLPRDH433 SSLSVND433 |
Functional Peptide Activity: The FAQRVPP peptide promotes cell growth and differentiation. Injection of the peptide fused to a self-assembly peptide domain increases the rate of recovery of spinal injury induced in rats. |
| In vitro panning on undifferentiated murine neural stem cells isolated from the subventricular zone | KLPGWSG442 | Functional Peptide Activity: The KLPGWSG peptide promotes cell differentiation towards a neuronal state. |
| Ex vivo panning against human bone-marrow derived mesenchymal stem cells | EPLQLKM443 (E7) |
Functional Peptide Activity: The E7 peptide was conjugated to polycaprolactone meshes and implanted into cartilage defects created in the knee joints of rats followed by generation of microfractures. The E7-mesh recruited more cells with stem cell markers than that of a control-peptide mesh. Note: E7 was also identified from panning on graphene, calling into question the specificity of the peptide for mesenchymal stem cells.444 |
| Bacterial peptide display selection on hippocampal neural stem cells from a rat brain using a CPX library | WWCDMRGDSRCSG445 DHKFGLVMLNKYAYAG445 KLCCFDKGYYCMR445 |
Functional Peptide Activity: The peptides mediate cell binding when adsorbed onto a solid support or incorporated into a polymer hydrogel. The cells were able to differentiate into different lineages. |
| In vivo panning amplifying the phage from mouse bone marrow and in vitro panning on murine bone marrow stem cells | STFTKSP446 NHWASPR446 |
Functional Peptide Activity: The STFTKSP peptide homes to bone marrow in vivo and reduces homing of stem cells to the bone marrow. |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
Peptides that bind stem cells have the potential to recruit such cells to sites of injury and may stimulate their differentiation. For example, the peptide FAQRVPP was selected for binding to murine-derived neural stem cells from a pIII phage-displayed peptide library.433 This peptide was synthesized as a fusion with a self-assembling peptide and acetylated at the amino terminus. The resultant fusion peptide increased the rate of recovery for rats subjected to spinal cord damage when injected at the site of injury.
Neural stem cells are the multipotent progenitor cells that generate the major cell types of the nervous system. As several diseases such as Parkinson’s, Alzheimer’s, and Huntington’s involve neurodegeneration,434 ligands that could bind to neural stem cells may have the potential to help deliver therapeutics for these diseases. Additionally, diseases such as epilepsy and depression are connected to altered neurogenesis.435,436 Two neural stem-cell-binding peptides, the QTRFLLH and VPTQSSG peptides, were identified from an ex vivo panning against neural stem cells isolated from mouse hippocampus.428 When conjugated to adenoviruses and injected into the brain, these peptides mediated specific binding to neural stem cells.
5.9. Isolation of Vasculature- and Endothelial-Cell-Binding Peptides
Although vasculature flows to all organs, the vasculature varies from organ to organ, with different structure, function, and protein expression profiles.447 The use of in vivo phage display to isolate vasculature-homing ligands has highlighted this diversity.67,253,448,449 Significantly, the vasculature also changes during different disease states.253 Others have reviewed the heterogeneity of the vasculature and the discovery of vasculature-specific peptides.253,449,450 In addition to the vasculature-specific peptides isolated from all of the organs and tumors already described in the subsections above, peptides specific for the vasculature of the lung, skin, pancreas, intestine, uterus, adrenal gland, retina, prostate, breast, and thymus have been isolated.448,451–455 Table 16 lists the vasculature- and endothelial-cell-targeting peptides isolated by in vitro, ex vivo, and in vivo phage panning and from a PS-SPCL.
Table 16.
Vasculature- and Endothelial-Cell-Specific Peptides
| Isolation methoda | Peptide Sequenceb,c,d | Cellular Receptor and/or Vasculature Specificity |
Applications and Notes |
|---|---|---|---|
| In vitro panning on purified Tie2 protein | TMGFTAPRFPHY457 (PH1) | Tie2 is widely expressed in the endothelium as well as in many cancer cell lines |
Therapeutic Applications: This peptide has been conjugated to cisplatin loaded liposomes for specific cell killing in vitro. Note: This peptide has also been isolated from phage displayed peptide selections against the bacterial Murligase enzyme MurF, which is involved in cell wall biosynthesis,458 and against the E. coli protein TonB.459 It was also observed in a panning on purified troponin 1,460 hydroxyapatite,461 and graphene.444 This calls into question the specificity of the peptide. |
| In vitro panning on SMMC772 cells overexpressing Tie2 | NSLSNASEFRAPY462 (GA5) | Tie2 is widely expressed in the endothelium as well as in many cancer cell lines | Oligonucleotide Delivery: The peptide has been conjugated to PET for delivery of the luciferase gene in vitro, and the WT p53 gene was delivered by intratumoral injection in mice. |
| In vitro panning against HUVECs (human umbilical vein endothelial cells) | SIGYPLP463 | Oligonucleotide Delivery: The peptide has been used in numerous formats, including viral constructs and poylcation complexes, to transduce HUVEC cells with eGFP or β-galactosidase.463–466 The peptide also mediates transduction in a panel of 6 cancer cell lines.467 | |
| MSLTTPPAVARP468 (MSL) MTPFPTSNEANL468 (MTP) |
Oligonucleotide Delivery: MSL and MTP peptide-modified adeno-associated virus (AAV-2) transduced HUVECs with β-galactosidase and reduced transduction of HepG2 cells.468 Both peptides were cloned into modified adenovirus and found to transduce HUVECs and HSVECs with EGFP in vitro and abdominal aorta/vena cava with β-galactosidase after tail vein injection into mice.469 | ||
| Ex vivo panning against HUVECs under normoxia | QPWLEQAYYSTF470 (QF) VPWMEPAYQRFL470 (VL) TLPWLEESYWRP470 (TR) |
Functional Peptide Activity: QF, VL and TR peptides induced proliferation and migration of serum starved endothelial cells in vitro. The QF and TR peptides induced endothelial cell tube formation in vitro and increased the number of blood vessels when injected into the ears of BALB/c mice. Note: The TR and VL peptides were isolated from panning of HUVECs under normoxic and hypoxic conditions. |
|
| Ex vivo panning against HUVECs under hypoxia for 3hrs | YPHIDSLGHWRR470 (YR) SAHGTSTGVPWP470 (SP) |
Functional Peptide Activity: YR and SP peptides induced proliferation and migration of serum starved endothelial cells and promoted endothelial cell tube formation under hypoxia. The YR peptide increased the number of blood vessels when injected into the ear of BALB/C mice. Note: The SP peptide was isolated from panning of HUVECs kept under hypoxic conditions for 3 or 24 hours. |
|
| Ex vivo panning against HUVECs under hypoxia for 24hrs | LLADTTHHRPWT470 (LT) SAHGTSTGVPWP470 (SP) VPWMEPAYQRFL470 (VL) TLPWLEESYWRP470 (TR) |
Vimentin is the putative receptor for the SP peptide.471 |
Functional Peptide Activity: The LT, SP, VL, and TR peptides induced proliferation and migration of serum starved endothelial cells. The VL and SP peptides promoted endothelial cell tube formation, although SP only exhibits this effect under hypoxic conditions.471 The VL peptide increased the number of blood vessels when injected into the ear of BALB/C mice. Intramuscular injections of the SP peptide into ischemic hind limbs of the mice increased blood perfusion and the number of capillaries.471 Note: The SP peptide was isolated from panning of HUVECs kept under hypoxic conditions for 3 or 24 hours. The TR and VL peptides were isolated from panning of HUVECs under normoxic and hypoxic conditions. |
| Ex vivo panning against human umbilical cords, isolating endothelial cells | KPSGLTY472 | umbilical vein endothelium | |
| Ex vivo panning against H-2Kb-tsA58 mouse derived lung microvascular endothelial cells | CGSPGWVRC473 | lung vasculature | Therapeutic Applications: Peptide-D(KLAKLAK)2 killed lung endothelial cells in vitro, and i.p. injection of peptide-D(KLAKLAK)2 into C57B16/6J mice induced apoptosis of alveolar cells. |
| Ex vivo panning against primary rat lung alveolar epithelial cell cultures | CTSGTHPRC474 (LTP-1) | lung vasculature | Functional Peptide Activity: Peptide-dendrimer conjugates demonstrated transport across lung airways to pulmonary vasculature in an ex vivo isolated rat perfused lung model. |
| Ex vivo panning against human blood outgrowth endothelial cells | SVPPRYTLTLQW475 TPSLEQRTVYAK475 SPPPSNAGSHHV475MPTLTRAPHTAC475 |
||
| QFPPKLTNNSML476 | Note: The phage clone homes to Lewis lung carcinoma tumors in vivo. | ||
| Combination ex vivo and in vivo panning using a T7 library in K14-HPV16 mice with dysplastic skin. Phage were isolated from the skin of the ears and chest. | CSRPRRSEC312 | neovasculature of dysplastic skin | |
| In vivo panning selecting for pancreas homing followed by in vitro panning against both prolactin-like protein receptor (PLPR) overexpressing COS-1 cells and purified recombinant PRLR | CRVASVLPC452 | PRL receptor on the vasculature of the pancreas and pancreatic islet cells | |
| In vivo panning in Balb/c mice, injecting phage via tail vein injection followed by phage isolation from the lungs |
CGFECVRQCPERC448 (GFE-1) CGFELETC448 (GFE-2) CTLRDRNC448 CIGEVEVC448 |
lung vasculature |
Imaging applications: GFE-1 delivered a quantum dot to lung epithelium in vivo.305 Oligonucleotide Delivery: GFE-1 conjugated to a neutralizing antibody that binds adenovirus (Ad5) transduced MDP expressing cells with β-galactosidase and GFP.477 Functional Peptide Activity: GFE-1 inhibited metastases in vivo when co-injected with C8161 human melanoma cells.456 GFE-1 delivered IFN-α2a to the lung when expressed as a fusion protein but therapeutic efficacy was not tested.478 Note: The receptor for GFE-1 and GFE-2 is membrane dipeptidase (MDP).479 |
| In vivo panning in Balb/c nude mice followed by phage isolation from the skin | CVALCREACGEGC448 | skin vasculature | Functional Peptide Activity: Peptide—GST fusion protein blocked phage binding in vivo. |
| In vivo panning in rats with a skin wound, using tail vein or intracardiac injections and isolating phage from wounded skin | CRKDKC480 (CRK) | Vasculature of wounded skin or tendons, preferentially binds at later stages of wound healing | Imaging applications: Conjugation of CRK to nanoworms (elongated iron oxide nanoparticles) allowed for nanoparticle targeting to tumor vasculature and MRI imaging in an orthotopic prostate tumor model.313 Accumulation of nanoworms also blocked blood flow to the tumor and induced necrosis. |
| In vivo panning of a T7 library in rats with a patellar tendon wound, isolating phage from the patellar tendon | CARSKNKDC480 (CAR) | Heparin sulfate & heparin480 | Functional Peptide Activity: CAR-decorin fusion protein inhibited cell spreading and TGF-β dependent cell proliferation of CHO-K cells.481 Intravenous injection of CAR-decorin fusion protein into mice bearing thick dorsal skin promoted wound healing and reduced scar formation.481 The peptide also homed to lung vasculature in a pulmonary arterial hypertension rat model and spread throughout the lung.482 |
| In vivo panning in CD-I mice selecting for prostate homing | SMSIARL451 | prostate vasculature | Therapeutic Applications: Injection of peptide-D(KLAKLAK)2 into mice caused destruction of prostate glandular epithelial cells and injection into TRAMP mice increased survival.451 Treatment with peptide-PP1/GaDD34 inhibitor peptide (LKARKVRFSEKV) followed by treatment with peptide-D(KLAKLAK)2 increased survival of TRAMP mice.483 |
| In vivo panning of T7 phage library in normal mice selecting for breast homing | CPGPEGAGC453 | Amino-peptidase P in breast vasculature | Functional Peptide Activity: Phage uptake is inhibited by the free peptide. |
| In vivo panning in female lactating rats isolating phage from the mammary tissue | CLHQHNQMC454 (MG1) | Acidic Ribosomal Protein Large P0 (RPLP0) | |
| In vivo panning using a dorsal air sac model of angiogenic vessels | PRPGAPLAGSWPGTS484 DRWRPALPVVFPLH484 ASSSYPLTHWRPWAR484 |
Therapeutic Applications: The peptide fragment APRPG was coupled to a doxorubicin loaded liposome and shown to reduce tumor growth compared to free drug or non-targeted liposome.484 Functional Peptide Activity: All three peptides accumulate in angiogenic vasculature in mouse tumor xenografts. PRPGAPLAGSWPGTS binds vasculature in human tumors.484 ASSSYPLIHWRPWAR suppresses angiogenesis.485 |
|
| In vivo panning in mice selecting for thymus homing | CHAQGSAEC455 | thymus vasculature | Functional Peptide Activity: When injected into mice, the peptide down regulated the expression of T cell receptor circles, suggesting inhibition of thymus bioactivity.455 |
| Screening of a PS-SPCL against murine endothelial MS-1 cells, selecting for an increase in intracellular calcium | SFKLRY-NH2486 (Angio-S) | Functional Peptide Activity: Angio-S induced proliferation, migration, and tube formation of HUVECs as well as sprouting of rat aortic rings. The peptide also protected human dermal fibroblasts and B16 melanoma cells from oxidative stress and increased their survival.487 |
All libraries are pIII phage libraries unless otherwise noted.
Cysteine residues that form disulfide bonds are indicated in bold.
Common peptide names that have been used in the literature are indicated in parentheses next to the peptide sequence.
Peptides clustered in the same cell in this table were isolated in the same selection experiment.
Phage display has been widely used to isolate peptides that bind different vasculature beds with high specificity. For example, the lung-vasculature-specific peptide GFE-1, isolated by in vivo panning, demonstrates specificity for normal lung vasculature over lung tumor vasculature when in the context of the phage.456 Similarly, the prostate-vasculature-specific peptide SMSIARL homes only to prostate vasculature and not prostate tumor vasculature in mice.451 Such distinctions point to the molecular changes evident between normal and tumor vasculature and to the ability of phage panning to isolate ligands that recognize these changes. On the other hand, some peptides, such as the CPGPEGAGC peptide isolated from in vivo homing to breast vasculature, bind to both normal breast vasculature and the vasculature of breast tumors in a mouse model of breast cancer.453
Arap and Pasqualini and co-workers demonstrated the ability to simultaneously pan for peptides specific for different organs.452 During an in vivo panning in mice, six different organs (muscle, bowel, uterus, kidney, pancreas, and brain) were simultaneously isolated and screened for peptide binding. While the peptides were not taken to convergence, tripeptide motifs were identified for each organ using statistics. In an effort to identify the cellular receptors for these peptide motifs, the motifs were BLAST searched for homology to known biological protein ligands. Tripeptide motifs isolated from pancreatic vasculature showed homology to ligands of the prolactin-like protein receptor (PLPR). The narrowed phage library from pancreatic panning rounds 2 and 3 was used for in vitro panning against PRLR-overexpressing COS-1 cells. The peptide CRVASVLPC was subsequently identified as a PRLR-binding peptide and was shown to home to pancreatic vasculature and islets in vivo when in the context of the phage.
5.10. Isolation of Epithelial-Cell-Binding Peptides
In addition to those already discussed, numerous other epithelial-cell-binding peptides have been isolated by in vitro and in vivo phage panning and from bacterial library selection. These peptides have generally been selected for an application specific to a particular cell type. These remaining peptides are highlighted in Table 17. Particularly interesting among this group of peptides are those that mediate transcytosis. During transcytosis, a receptor and its cargo are internalized in a polarized cell at one plasma membrane and transported by vesicles across the cell to the opposite plasma membrane.488 Using an approach to identify peptides that allow for transcytosis across the intestinal mucosal barrier, an in vivo panning against rats by oral administration of phage was recently performed.489 The liver, lung, spleen, and kidney were isolated, and the sequence CSKSSDYQC was identified from all of them. The phage displaying this peptide was also able to bind small intestinal mucosal tissue in vitro. Importantly, this phage accumulated more strongly than a control phage in the liver, lung, spleen, and kidney after oral administration. Immunofluorescence from 30 min after oral administration of the phage showed the phage accumulating in goblet cells. Thus, if the synthetic peptide works as well as the phage, it has the potential to be used for oral delivery of drug conjugates. The YPRLLTP phage clone, isolated by selecting for phage in the spleen after in vivo gavage of a phage library into rats, also demonstrated the ability to transcytose the gastrointestinal mucosal barrier.490
6. PEPTIDES WITH CELLULAR EFFECTS
Often peptides used for specific targeting of diagnostic and therapeutic agents are considered only as delivery agents and not as ligands that can induce cellular effects.72 However, given that these peptides are binding to receptors or other components on the cell surface, they have the potential to induce the effects of these receptors. PS-SPCLs are particularly well suited for isolating peptides with cellular effects due to their ability to be used with almost any readout assay, and the majority of peptides isolated using these libraries induce specific effects. However, there is no guarantee that peptides isolated by other methods will not trigger cellular signaling or other cellular behaviors. Not surprisingly, many of the isolated peptides affect cell adhesion and migration. In some cases, peptide binding produces a beneficial outcome, such as a reduction in migration of cancer cells away from the primary tumor. However, if a peptide stimulates growth, it would be unusable as a tumor-targeting agent. Peptide function has been noted in each table of selected peptides. This section highlights examples of peptides that affect four cellular behaviors: (1) adhesion, migration, and invasion, (2) angiogenesis, (3) proliferation, and (4) viability.
6.1. Peptides That Modulate Adhesion, Migration, and Invasion
Ligand binding to cell surface receptors can induce a wide variety of cellular effects, including adhesion, migration, and invasion.72 This has been most widely studied on tumor or tumor vasculature. Several cancer-specific peptides have demonstrated induction of these pathways.110,126,140,154,160,163,169,170,251,252 Not surprisingly, peptides that bind integrins are often found to inhibit adhesion and migration as this family of receptors are critical for such functions, yet many other ligands are able to block cellular migration. For example, a peptide isolated from phage display against MMP-9 inhibited migration of various tumor cells (fibrosarcoma, melanoma, ovarian carcinoma, and Kaposi’s sarcoma cells) in vitro and inhibited Kaposi’s sarcoma tumor growth when ip injected in vivo.126 Similarly, a peptide selected from GC9811-P gastric cancer cells prevented cell adhesion and invasion in vitro and reduced peritoneal nodules in vivo.170 Another peptide selected against metastatic gastric cancer cells inhibited adhesion and migration in vitro and reduced liver metastasis incidence in vivo.169
Peptides that bind normal vasculature can also prevent metastasis. The lung-vasculature-specific peptide GFE-1, known to bind membrane dipeptidase (MDP), was able to inhibit metastases in vivo when co-injected with tumor cells.456 Mice were injected intravenously either with C8161 human melanoma cells plus the GFE-1 peptide or with the cells plus a control peptide. Mouse lung weight was measured as an indicator of metastasis, and the mice given the GFE-1 peptide had a median weight only 12% above the weight of normal lungs. Significantly, the mice injected with tumor cells plus the control peptide had a median lung weight 88% larger than normal lung weight.
Cardiac-specific peptides also have the potential to induce cellular effects. One such peptide, the VCAM-1-specific peptide CVHSPNKKC, which is known to bind to VCAM-1-expressing cardiac endothelial cells, is able to block VCAM-1-mediated leukocyte–endothelial cell interactions in vitro.365 Murine cardiac endothelial cells activated with TNF-α were preincubated with either the CVHSPNKKC peptide or an anti-VCAM-1 antibody before addition of strain-matched mononuclear cells. The peptide was more efficient at inhibiting leukocyte–endothelial cell interactions than the anti-VCAM-1 antibody, blocking 68% of interactions compared to 52% for the antibody. This finding is particularly interesting as VCAM-1-induced recruitment of monocytes and leukocytes to the cardiac endothelium is an important step during the development of atherosclerosis.
Cell-binding peptides do not always reduce cell adhesion and/or migration. A PS-SPCL screen was used to identify peptides that stimulate arachidonic acid release from neutrophil-like cells. These peptides were later demonstrated to stimulate chemotactic migration of human neutrophils.405 Similarly, the HFYLPM and MFYLPM peptides, isolated from a PS-SPCL screen for the ability to induce superoxide generation from human monocytes, were also able to induce chemotactic migration of the cells.406
6.2. Peptides That Modulate Angiogenesis
Cell-binding peptides can stimulate or inhibit angiogenesis. As angiogenesis is a critical step in tumorigenesis and antiangiogenics are an important class of antitumor drugs, peptides that control this process have been widely sought. One such peptide isolated from a phage display selection against the IL-6 receptor was shown to inhibit angiogenesis in vitro and to inhibit IL-6-mediated tumor growth in a cervical xenograft in vivo.110 Two αvβ3-binding peptides, HGDVHK and HSDVHK, were isolated from a PS-SPCL screen for peptides that interfered with the αvβ3–vitronectin interaction.63 These peptides were subsequently found to inhibit both bFGF-induced human umbilical vein endothelial cell (HUVEC) migration in vitro and bFGF-induced blood vessel formation in a chorioallantoic membrane (CAM) angiogenesis assay. An α5β1 microarray and a PS-SPCL screen were employed to isolate peptides that inhibit α5β1–fibronectin interactions.64 One isolated peptide, VILVLF, subsequently inhibited proliferation of HUVECs and inhibited angiogenesis in a bFGF-induced CAM angiogenesis assay. As α5β1 is expressed at high levels in both angiogenic endothelial cells and several cancer types, this peptide may have future utility for antiangiogenic therapy.
Peptides can also stimulate angiogenesis. The peptide SFKLRY-NH2, isolated by in vitro PS-SPCL screening against the MS-1 cell line, increases angiogenesis as evidenced by its ability to increase proliferation and migration of HUVECs, leading to organization of the HUVECs into tubular formations.486 Additionally, this peptide was able to induce blood vessel sprouting of aortic rings from rats. Mechanistic studies demonstrated that the peptide induces an increase in intracellular Ca2+ via a pertussis-sensitive G protein/phospholipase C-mediated pathway and increases the expression of VEGF, resulting in a pro-angiogenic phenotype. The peptide also exhibits antioxidant activity.505 Importantly, the peptide accelerates wound healing.
6.3. Peptides That Modulate Cellular Proliferation
To date, most peptides isolated against cancer cells reduce cell growth instead of inducing a protumor phenotype. As an example, Matsuo et al. used a unique strategy to specifically screen for a peptide with antitumor activity.160 After in vitro phage display against melanoma B16F10-Nex2 cells, 50 isolated phage were incubated with the cells for 72 h before MTT viability studies. Out of the 50 phage, 7 significantly decreased cell viability, and out of those 7, a peptide with antitumor activity emerged. This peptide was able to inhibit cell invasion and adhesion in vitro and prevent metastasis in vivo (Figure 7).
Figure 7.
A phage-display-selected peptide inhibits tumor metastasis. B16F10-Nex 2 cells were injected intravenously into mice, and the mice were treated via an intraperitoneal injection with peptide 20 (CSSRTMHHC), a scrambled control peptide, or buffer. Images of the lungs show a reduction in metastatic nodules (brown spots) in the animals treated with peptide 20. Reprinted with permission from ref 160. Copyright 2010 Springer-Verlag.
In some cases, peptides selected against cell surface receptors can avoid untoward progrowth cell signaling that occurs when using the native ligand. For example, although only 53 amino acids long, the native EGF ligand cannot be used for targeting because of its role in stimulating EGFR activation. Additionally, EGF binding triggers rapid uptake and degradation of EGFR, resulting in a reduction of the target on the cell surface. By comparison, GE11, an EGFR-binding peptide, does not initiate EGFR signaling. While it is internalized, EGFR is recycled back to the cell surface, making it available to carry in another GE11 peptide (and its cargo). As such, it is the better choice of ligand when targeting EGFR-expressing cells.
On the other hand, the neural-stem-cell-specific peptide CGLPYSSVC (isolated by in vitro panning against murine neural stem cells) increased cellular proliferation when added to the media of either neural stem cells in culture or of freshly isolated neural progenitor cells from the adult mouse brain.427 This peptide’s ability to increase neurogenesis could therefore be explored for the treatment of diseases known to involve loss of neurons, such as Parkinson’s, Alzheimer’s, and Huntington’s diseases.434
6.4. Peptides That Stimulate Apoptosis
Although many peptides reduce cellular proliferation rates, few induce cell death unless conjugated to a cytotoxic agent.256,506 However, the tumor-lymphatic-targeting peptide LyP-1 accumulates in the nucleus and specifically induces apoptosis in cells which have affinity for this peptide.255,256 Importantly, in vivo treatment with LyP-1 results in a reduction of tumor growth, a decrease in tumor lymphatic endothelium, and an increase in apoptotic tumor cells. The mechanism of LyP-1-mediated apoptosis has not been elucidated. The receptor for the peptide has been identified as p32/gC1qR, a mitochondrial protein which is also found on the cell surface of tumor cells. Interestingly, p32/gC1qR mediates ARF-induced apoptosis.507 However, the relationship between peptide binding and internalization and the role of p32/gC1qR in apoptosis has yet to be determined.
7. USE OF PEPTIDES FOR THERAPY
Due to their high cell specificity and ease of manipulation, cell-targeting peptides have been exploited for the delivery of therapeutic agents. While some peptides are biologically active, most peptides require attachment to a drug or other agent to exhibit the desired therapeutic effect. Peptides can deliver small-molecule drugs or biologics. The therapeutic applications for individual peptides have been noted in Tables 2–17 according to their use. In this section we discuss approaches to targeted drug delivery and highlight a few of the successes in developing peptide-targeted therapies. Delivery of small-molecule drugs and therapeutic biologics is covered as well as the use of targeting peptides in immunotherapies. While many proof-of-concept studies have been performed to demonstrate delivery, only studies that address a therapeutic application are discussed in the following subsections.
7.1. Peptides for Small-Molecule Drug Delivery
Peptides as drug-targeting agents can widen the therapeutic window by both increasing drug efficacy and decreasing unwanted side effects. This is of particular importance for drugs with high toxicity profiles, such as many chemotherapeutics. Additionally, peptides that mediate cellular uptake can improve uptake of molecules that do not cross the cell membrane. Peptides are used for drug targeting by either directly conjugating the drug to the peptide or by conjugating the peptide to a drug-carrier platform.508 The ability to be used in multiple platforms again highlights the strengths of peptides as targeting agents. By contrast, using antibodies for drug delivery is hindered by difficulties in chemical conjugation to their cargo. Additionally, antibody size adds ~20 nm to the size of a nanoparticle, which can dramatically affect biodistribution. Before discussion of the successes in peptide-guided drug delivery, it is important to consider the differences between direct drug conjugates and peptide-modified drug carriers.
7.1.1. Approaches for Small-Molecule Delivery: Direct Conjugates vs Drug Carriers
Peptides can be conjugated to a variety of drugs for specific delivery to the desired cell type. These peptide–small-molecule conjugates require chemical groups on both the peptide and the drug that are compatible for conjugation. Additionally, the drug is generally inactive while conjugated to the peptide, meaning that the covalent bond between the peptide and drug must break and release the drug to achieve any therapeutic effects; i.e., they are prodrugs. Ideally, the peptide–drug linkage remains stable under normal bodily conditions, releasing the drug once the conjugate has reached its target. Often acid-labile linkers are employed; these linkers are intact at a pH of 7 but hydrolyze and release under acidic conditions, such as those of the endosome and lysosome. Other types of drug linkers can also be used, such as esters or carbamates;509,510 these linkers are also stable under normal conditions and release after exposure to the high levels of esterases within cells. Similarly, protease cleavage sites can be employed, such as a valine–citrulline linker that is cleaved by the lysosomal cysteine protease cathespin B. The cleavage efficiency by proteases is often diminished by the presence of the drug molecule immediately adjacent to the cleavage site. To overcome this problem, self-immolative linkers have been designed, in which a release site (or trigger site) is placed between the linker and the targeting moiety.511 Upon cleavage, a reactive intermediate forms which then spontaneously decomposes to release free, active drug. Balancing conjugate stability in circulation with rapid release at the target site can be difficult. Premature or inefficient drug release has resulted in poor efficacy for many peptide–drug conjugates. Common drug linkers that have been used to covalently attach peptides to drugs are shown in Figure 8.
Figure 8.
Structures of common cleavable linkers used to attach drugs to targeting peptides. (A) Cleavable linkers are shown along with the conditions which release the drug from the peptide carrier. The acetal, ketal, and hydrazone linkers require incorporation of reactive moieties not found within the 20 naturally occurring amino acids. (B) Two types of commonly used self-immolative linkers are shown along with the mechanism in which drug is released from the carrier. The release is initiated by a trigger, such as a cleavage reaction shown in panel A, where X = O, S, or NH and Y = CH2, NR, or O. The drug serves as a leaving group and is typically attached as an ester or amide. In both cases, the peptide is represented as a squiggly line.
It should be noted that most conjugates are based on the premise that the conjugate will eventually traffic to the lysosome where the low pH and high protease content release the drug. However, it is unlikely that all peptides isolated from the various libraries will direct lysosomal accumulation, and different uptake mechanisms can affect downstream peptide trafficking within the cell. In fact, clatherin-mediated endocytosis, cavelolae-mediated endocytosis, and internalization by macropinocytosis have been observed for different cell-targeting peptides.118,157,214 This stresses the need to characterize the mechanism of cellular uptake and the resultant localization of the peptide before embarking on the synthesis of peptide–drug conjugates.
Both the primary advantages and disadvantages of peptide–drug conjugates lie in their small size. While this small size allows the conjugates better vasculature escape and penetration through tissues,512 it also increases their blood clearance rate. Particles below a molecular weight of 40 000 tend to undergo rapid renal filtration and excretion from the body.513 As most peptide–drug conjugates fall below this molecular weight cutoff, they are only therapeutically beneficial if they accumulate in the desired tissue at effective levels relatively quickly, before the majority of the conjugate is cleared from the body. However, reaching therapeutic levels is possible due to the high specificity and affinity of direct conjugates. Additionally, such conjugates tend to have low background uptake. A significant disadvantage is that peptide–drug conjugates deliver only one drug molecule per targeting event. Thus, to reach an effective dose, the compound must be highly effective at low cellular concentrations or the targeted receptor must be abundant and undergo efficient internalization.
On the other hand, peptides can be conjugated to drug carriers. Most drug carriers fall into one of three categories—polymer scaffolds, micelles, or liposomes—although a variety of other nanoparticles have been created. Liposomes are the most widely used drug carrier for peptide-targeted therapy, and the vast majority of peptides isolated from phage display libraries that have been conjugated to nanoparticles for drug delivery have been conjugated to liposomes.
Liposomes are spherical nanoparticles formed by lipids self-assembling into a bilayer.514 As liposomes are formed from phospholipids and cholesterol that are already normal components of the human body, they are naturally biodegradable. 514 The inner compartment of a liposome is an aqueous phase that can encapsulate hydrophilic agents. However, hydrophobic drugs can also incorporate into the hydrophobic portions of the lipid membrane.515 Early liposome formulations suffered from rapid blood clearance rates due to engulfment by the cells of the reticuloendothelial system, in particular Kupffer cells in the liver. The reticuloendothelial system is the primary means by which foreign macromolecules are eliminated from the body and consists mostly of macrophages in the liver, spleen, and lymph nodes. However, coating the outer lipid membrane with polyethylene glycol (PEG) increases the circulation time of the liposomes by reducing interaction of the liposomes with serum proteins and thus delays clearance by the reticuloendoethial system.516 Several liposome formulations are already clinically approved.517,518
Liposomal formulations are advantageous for a variety of reasons. The large hydrophilic interior of the liposome allows for the encapsulation of thousands of drug molecules, giving a peptide-targeted liposome a much higher peptide:drug ratio than peptide–drug direct conjugates with only a 1:1 peptide:drug ratio. Additionally, hydrophilic drugs can be loaded into liposomes in their natural state and do not require chemical modification for conjugation to the peptide; there is also the potential to encapsulate multiple drugs within the same liposome. The long in vivo circulation time enjoyed by PEGylated liposomes also means that the peptides will have longer to deliver their drug cargo to the tumor than will peptides directly conjugated to a therapeutic. However, liposomes also suffer from several disadvantages. In particular, the larger size (80–200 nm) of liposomes prevents them from penetrating tissue as well as smaller conjugates and can make it more difficult for them to escape from the vasculature.519
Although liposomes are the most extensively used drug carriers for peptide-targeted delivery, polymer scaffolds and micelles are also important drug carriers. Polymer scaffolds are created by attachment of water-soluble polymers to the drug.520 Much like peptide–drug direct conjugates, these drug–polymer scaffolds also require chemically reactive groups and should remain intact until cell internalization, at which point the bond between the polymer and drug breaks, allowing for the drug to release and exert its effects.520 However, unlike peptide–drug direct conjugates, drug–polymer scaffolds have longer in vivo circulation times due to the increased size of the polymer scaffold. Additionally, the size of drug–polymer conjugates can be tailored to reduce renal clearance. A major disadvantage to these conjugates is that it can be difficult to control both the length of the polymers and the amount of drug conjugated to the scaffold. However, polymer scaffolds have progressed extensively through the drug pipeline, with polymers conjugated to paclitaxel, camptothecin, doxorubicin, carboplatin, and 1,2-diaminocyclohexane (DACH)–platinate all in clinical trials.520 Despite these successes, few library-selected peptides have been conjugated to polymer scaffolds.
Micelles are formed by amphiphilic copolymers, which have both a hydrophilic component and a hydrophobic component. 521 Upon exposure to an aqueous solution, the insoluble hydrophobic portions of the polymer aggregate into a core structure, with the hydrophilic polymers forming a shell that surrounds the core. The hydrophobic core of micelles allows them to encapsulate hydrophobic drugs. These self-forming nanoparticles are typically 50–100 nm in size.522 Micelles have many of the same advantages of liposomes, as they are able to incorporate unmodified drugs and can encapsulate multiple agents at once at a fairly high concentration of drug per micelle. As micelles tend to be smaller than liposomes, they cannot incorporate as many drug molecules as the larger nanoparticles and have intermediate circulation times. Their smaller size may allow for better escape from the vasculature and better tumor penetration. To remain intact as nanoparticles, micelles must maintain a critical micelle concentration (cmc).522 The cmc is the concentration of individual amphiphilic copolymers required for the micelle structure to form. Below the cmc, micelles dissociate into the individual copolymers, releasing the entrapped drug. However, micelles can often be designed to remain intact in vivo long enough to reach their target.522 Several micelle formulations are currently in clinical trials.523
7.1.2. Peptide Conjugates Used for Small-Molecule Drug Delivery
Cancer therapies have a narrow therapeutic window as they affect both normal and cancerous cells; most are administered at their maximally tolerated dose and not at their maximally effective dose due to their toxic side effects. Thus, targeting chemotherapeutics specifically to tumors would be of benefit, and small-molecule drug delivery has focused on this disease. Few peptides selected from OBOC libraries, bacterial display libraries, and PS-SPCLs have been used for in vivo therapy in animals.337,342 Peptides selected from phage display libraries, on the other hand, have been used extensively for in vivo therapy.
Several different chemotherapeutic drugs have been directly conjugated to cancer-specific peptides, including doxorubicin, 112,158,166,293,321,524 paclitaxel,118,157,158,224 and a vitamin E analogue.184 While most of these conjugates have only been used for in vitro toxicity studies, several have also been shown to inhibit tumor growth in vivo. The peptide LTVSPWY, isolated from panning a phage display library against cultured SKBR3 breast cancer cells, inhibited breast tumor growth in transgenic mice when conjugated to the proapoptotic vitamin E analogue α-tocopheryl succinate.184 Additionally, three different peptides conjugated to doxorubicin have been used to inhibit tumor growth in mice. The NGR and RGD-4C peptides, both isolated from in vivo phage panning in mice bearing MDA-MB-435 breast xenografts, inhibited MDA-MB-435 tumor growth when conjugated to doxorubicin.112 Of note, both of these peptides target the tumor vasculature and not the tumor cells. However, a peptide specific for tumor cells has also proven effective for drug targeting in vivo. The tumor-cell-targeting A54 peptide, isolated from in vivo panning in mice bearing BEL-7402 hepatocellular carcinoma xenografts, also inhibited tumor growth when conjugated to doxorubicin.321 More recently, a derivative of the EphA2-targeting peptide YSA has been utilized to deliver paclitaxel to prostate and renal carcinomas in vivo.118 The parental peptide was modified by replacement of the amino-terminal l-tyrosine with d-tyrosine and by substitution of l-norleucine and l-homoserine for the two methionine residues (dYNH), resulting in a peptide with dramatically improved serum stability while retaining a low nanomolar affinity for EphA2. This peptide was conjugated to paclitaxel via a carbamate linker to the 2′-hydroxyl of the drug. The conjugate mediated internalization and localized within the lysosome. Treatment with the dYNH–paclitaxel conjugate resulted in a reduction of tumor growth in a subcutaneous prostate tumor model. The conjugate was significantly more effective than free paclitaxel or a scrambled peptide control conjugate. The same conjugate also inhibited growth of an orthotopic renal carcinoma tumor but was no better than free drug.
7.1.3. Peptide-Guided Nanoparticles Used for Small-Molecule Drug Delivery
Most peptides conjugated to liposomes for targeted drug delivery have been conjugated to liposomal forms of doxorubicin. Seventeen different peptides isolated from phage display libraries have been conjugated to liposomal doxorubicin for either in vitro inhibition of cancer cell growth102,168,259,299,427 or in vivo inhibition of tumor growth in rodents.128,155,181,229,244,249,267,282,283,299 All of the targeted peptide–liposomal doxorubicin nanoparticles save one inhibited tumor growth better than liposomes without the targeting peptides. The liposomal studies involving one peptide are particularly worth noting. A peptide derivative of the tumorvasculature-targeting NGR peptide (GNGRG) was conjugated to liposomal doxorubicin.112 NGR–liposomal doxorubicin inhibited the growth of orthotopic neuroblastoma xenografts in mice, leading to tumor eradication. By comparison, treatment with control peptide–liposomal doxorubicin did not alter tumor growth compared to that in control non-drugtreated mice.282 This therapeutic efficacy was the result of greater accumulation of the peptide-targeted liposomes in the tumor.282 NGR–liposomes have been primed for potential future clinical trials by preparation using good manufacturing practices (GMPs),525 and these liposomes have been shown to increase survival in orthotopic mouse lung, ovarian, and neuroblastoma xenografts.283
Despite the many advantages of micelles, fewer libraryselected peptides have been conjugated to this class of nanoparticles. The lung-cancer-targeting peptide H2009.1 has been conjugated to paclitaxel micelles and to doxorubicin/ superparamagnetic iron oxide (SPIO) micelles.226 Both selectively kill cancer cells in vitro, but neither platform has yet met success in vivo. Recently, the ovarian-cancer-targeting peptide OA02 was used to deliver paclitaxel-loaded micelles.337 Importantly, this micellar formulation showed improved antitumor efficacy compared to that of nontargeted micelles. These successes suggest that peptide-targeted micelles should be further explored.
Although less commonly used, a variety of other nanoparticles have been used for peptide-targeted drug delivery. These include nanoworms,103,276 particles created from a phage coat protein,156 nab-paclitaxel,265,274,275,301 microbubbles,300 and polyester-based particles.320 In particular, nab-paclitaxel, a nanoparticle formed by albumin-coated paclitaxel, has been conjugated to several peptides for tumor inhibition in rodents.265,274,275,301 As an untargeted form of nab-paclitaxel, Abraxane, is approved for clinical use, these nanoparticles are an attractive drug-targeting candidate. More recently, the EphB4-binding peptide, referred to as TNYL-RAW, was cyclized and conjugated to hollow gold nanospheres.124 These nanoparticles were loaded with doxorubicin and used to target EphB4-positive tumors in vivo. Irradiation with a near-infrared laser resulted in a dual mode of action: photothermal ablation of the tumor and release of doxorubicin within the tumor environment. Treatment with the TNYL-RAW nanospheres followed by near-infrared irradiation was more effective than the same regimen using nontargeted nanospheres or targeted nanospheres not loaded with doxorubicin. Impressively, complete regression of subcutaneous Hey ovarian tumors was observed in 75% of mice treated with both TNYL-RAW nanospheres and near-infrared irradiation.
An interesting method recently developed involves keeping peptides in the context of the pVIII phage protein for incorporation into nanoparticles.526 Following selection, the isolated phage is disrupted with chlorate buffer so that the peptide–pVIII phage protein conjugate can be isolated and purified. Because the pVIII protein functions as a membrane protein, it is able to incorporate into both liposomes and micelles. Peptide–pVIII fusion proteins insert into preformed liposomes during a short incubation time, making this procedure fairly simple. Peptides selected against both PC3 prostate carcinoma cells and MCF7 breast cancer cells have been used in this method to incorporate peptide–pVIII proteins into doxorubicin liposomes for in vitro cytotoxicity studies.168,189 The MCF-7 peptide–pVIII conjugates have also been incorporated into drug-loaded micelles for in vitro cytotoxicity studies.191
7.2. Peptide Delivery of Biologics
As the field of biologic therapy expands, so does the need to deliver such molecules. Proteins and peptides, either naturally occurring or designed de novo, can have potent biological activities. However, they do not cross the cell membrane. As such they are limited to cell surface targets. Using targeting peptides that mediate both cell-specific binding and internalization can overcome this limitation, opening new therapeutic avenues. Additionally, many peptides and proteins are generated in a local environment; systemic administration results in undesirable outcomes. Redirecting the peptide/protein to its local site of action can mitigate such side effects. This subsection highlights a few examples of successful delivery of therapeutic peptides and proteins.
7.2.1. Delivery of Therapeutic Peptides
Targeting peptides can also be conjugated to other peptides with known cellular activities. By far, the peptide most widely used in this context is the proapoptotic peptide (KLAKLAK)2. Although inactive outside cells, the (KLAKLAK)2 peptide exerts intracellular toxicity by disrupting the mitochondria, leading to subsequent cell death. As (KLAKLAK)2 does not cross the cellular membrane unless attached to an internalizing cell-targeting peptide, it is nontoxic to other cells. This peptide is often synthesized using protease-resistant d-amino acids, increasing its chances of reaching a tumor as an intact active peptide. Numerous cancer-targeting peptides have been fused to the proapoptotic peptide for either in vitro killing of cancer cells104,172,214,215,252,277 or in vivo inhibition of tumor growth in rodents.104,277
The (KLAKLAK)2 peptide is also being developed as an antiobesity therapy. The white-fat-vasculature-specific peptide CKGGRAKDC, isolated from an in vivo screen in the obese leptin-deficient (Lepob/ob) mouse model, was used to ablate white fat in mice.414 This CKGGRAKDC peptide was linked to the d-enantiomer of the proapoptotic (KLAKLAK)2 peptide and injected subcutaneously into wild-type mice forced into obesity by a high-calorie diet. After a month of daily treatment, mice treated with the CKGGRAKDC-GG-d(KLAKLAK)2 peptide conjugate were an average of 15 g lighter than control mice treated with unconjugated CKGGRAKDC and d(KLAKLAK)2. Immunohistochemistry revealed specific apoptosis in the white fat vasculature of the conjugate-treated mice and not in that of control-treated mice. Significantly, fat ablation also led to increased metabolism and reversal of insulin resistance in conjugate-treated mice. Similar results were observed in obese rhesus macaques and baboons.415 The only observed side effect was mild renal degeneration, which was reversible.
Other biologically active peptides can be delivered as well. The cardiac-endothelium-specific CRPPR peptide359 was coupled to the antioxidant peptide gp91ds for antioxidant therapy.360 Gp91ds inhibits the assembly of NAD(P)H oxidase, preventing its production of the reactive oxygen species superoxide.527 As superoxide reacts with nitric oxide, lowering its bioavailable levels, blood vessels no longer relax normally and blood pressure eventually rises.527 Thus, antioxidant therapy with gp91ds should lower blood pressure if the gp91ds is able to reach its biological target in the cardiac endothelium. The CRPPR peptide–gp91ds conjugate was administered to hypertensive and stroke-prone rats through a subcutaneous osmotic minipump. The rats experienced an increase in nitric oxide availability and a decrease in systolic blood pressure consistent with specific delivery of the gp91ds to the cardiac endothelium.360 Although this is unlikely to translate into a therapy available for patients due to the large amount of peptide needed to reduce blood pressure, it is an interesting lead for the development of future therapies.360
7.2.2. Delivery of Therapeutic Proteins
Targeting peptides can deliver therapeutic proteins when created as peptide–therapeutic protein fusion proteins. Unlike the chemical conjugation used to make peptide–small-molecule and peptide–peptide conjugates, most of these conjugates are created genetically. Peptides selected from phage-displayed libraries have been fused to a variety of proteins, including toxic shock syndrome toxin 1,154 vascular endothelial cell growth inhibitor,129 the kringle 5 fragment of human plasminogen,130 a fragment of tumstatin (tum-5),292 interferon α (INFα2a),290,291 and interleukin-2 (IL-2).316 Additionally, three peptides have been conjugated to either TNF-α or a mutant version of the same protein.136,261,284,285,287,288 Significantly, all of these peptide–protein fusions have been used for in vivo therapeutic experiments in rodent cancer models, leading to either inhibition of tumor growth or increased survival. Of particular interest is one of the TNF-α conjugates. A shortened version of the NGR peptide, CNGRCG, expressed as a fusion protein with TNF-α is currently in clinical trials.528 Due to the success of phase I and II trials for this conjugate, it was granted “orphan drug” status in both the European Union and the United States for the treatment of malignant pleural mesothelioma (MPM).528 Phase III trials for NGR–TNF in MPM patients are ongoing.
7.3. Peptides Used for Immunization
As dendritic cells (DCs) are antigen-sampling and -presenting cells that can initiate both adaptive and humoral immune responses, they are attractive targets for targeted delivery of antigens. In the most common approach to DC-based vaccines, DCs are cultured from blood or bone marrow progenitor cells in the presence of cytokines, pulsed with the appropriate tumor antigen, and injected back into the patient. This is a complicated and expensive process.529 However, several recent studies used antibodies, antibody fragments, or other ligands that bind to dendritic cells to specifically deliver antigens in vivo.529–534 Accordingly, peptides can be used to specifically target antigens in vivo. Faham and Altin used the previously isolated dendritic-cell-binding peptide CGRWSGWPADLC390 to specifically target dendritic cells in mice in vivo.391 The peptide was first attached to a liposome carrying OVA as a model antigen. Mice vaccinated with peptide–OVA–liposomes by intravenous injection produced OVA-specific antibodies unlike mice vaccinated with control OVA–liposomes. Additionally, the peptide was grafted onto plasma membrane vehicles derived from B16-OVA cells, a metastatic murine melanoma cell line that secretes OVA. Vaccinating mice with these peptide membrane vehicles before introducing B16-OVA cells into the mice significantly reduced the number of lung metastases (Figure 9). Additionally, mice bearing existing B16-OVA tumors that underwent subsequent vaccination with the peptide membrane vehicles experienced considerable tumor growth inhibition, with four out of five treated mice experiencing complete remission by day 30. The dendritic-cell-specific peptide XS52.1, isolated from in vitro panning against the mouse dendritic cell line XS52, was also isolated with the intent of improving vaccination.393 Although use of the free peptide to elicit an immune response has not been tested, the peptide displayed on the phage was able to induce an immune response. Mice injected intradermally with XS52.1 phage and not those injected with a control peptide-displaying phage developed antiphage serum antibodies. Further studies need to be performed with the synthetic peptide to verify that the peptide itself is able to deliver antigens and elicit an immune response.
Figure 9.
Vaccination with plasma membrane vehicles modified with the dendritic-cell-specific peptide CGRWSGWPADLC (p30) leads to antitumor activity. Naïve mice were injected iv with B16-OVA cells on day 0. At days 2, 8, and 14 different groups of mice (five mice per group) were vaccinated with PBS or B16-OVA-derived plasma membrane vehicles modified with the control peptide 12His or the dendritic-cell-specific peptide p30. At day 21, the lungs were removed from the mice, and tumor foci were counted via microscopy. (A) Bars indicate the mean number of tumor foci for each vaccination group, and this number is indicated above each bar. (B) Representative lung images from each vaccination group. Reprinted with permission from ref 391. Copyright 2010 Union for International Cancer Control (UICC).
The antigen-sampling M cells of the intestinal epithelium sample antigens in the gut and transport them a short distance to a binding pocket in which immune cells such as lymphocytes or macrophages dock.395 Thus, the M cells can carry antigens out of the gut to immune cells to facilitate an immune response.395 Due to these traits, M cells are desirable targets of orally available vaccines.395 Peptides that could direct the oral vaccine to M cells would allow for specific delivery of the vaccine to immune cells for initiation of an immune response. The Co1 peptide identified from in vitro panning against human M-like cells was used to target a model oral vaccine in mice.397 When the Co1 peptide was fused to the fluorescent protein EGFP and given orally to mice, it was able to bind M cells and transport across the intestine as evidenced by fluorescent imaging of Peyer’s patch tissue sections at different times post peptide–EGFP injection. Significantly, after six weeks of oral administration of Co1–EGFP in mice, the mice developed both serum IgG and fecal IgA antibodies against the EGFP, indicating that the vaccine induced an immune response. Importantly, Co1–EGFP induced antibody production that was 2–3-fold higher than that induced by EGFP alone. Since Co1 has also been shown to bind (and was isolated against) human M-like cells in culture, this peptide may translate well into the clinic. Similar immune activation was observed when using the M-cell-targeting peptide CL3 to deliver EGFP, suggesting that targeting M cells is a potential route to develop oral vaccines.398
8. PEPTIDES USED TO DELIVER OLIGONUCLEOTIDES
Delivery of genes, siRNA, and miRNA to cells requires carriers that protect the oligonucleotides during transport and facilitate delivery of the highly charged molecules across the cell membrane. Again, cell-targeting peptides have been used to deliver DNA and RNA to cells. Bacteriophage phage clones that internalize into mammalian cells can deliver DNA; however, the transfection efficiency is low.182,535–540 To overcome low transfection efficiencies, targeting peptides have been spliced onto eukaryotic viral vectors which have high transfection efficiencies or have been incorporated into chemical gene delivery systems. Numerous cell-targeting peptides have been used to transduce specific cells as noted in Tables 2–17. In this section we point out several studies that have either led to in vivo delivery of an oligonucleotide and/or shown a functional biological outcome.
8.1. Engineered Viruses for Delivery of Oligonucleotides
Viral vectors have high transfection efficiencies. However, removal of the native viral tropism must occur to redirect the gene transfer to the targeted cell type.541–543 Most efforts have focused on grafting cell-targeting peptides onto engineered adenovirus as noted below. Recently, peptides have been inserted into adeno-associated virus (AAV) for targeted gene delivery.544 Because it has been difficult to maintain peptide targeting function in the context of the AAV,544 new AAV peptide libraries have been made so that peptides can be isolated directly in the context of the virus in which they will be used.545,546 These AAV libraries were recently reviewed elsewhere and are not covered in this review as the selected peptides have not been tested outside the context of AAV.544
Gene therapy with cardioprotective genes targeted to the cardiac vasculature has the potential to prevent coronary heart disease and to improve the side effects caused by ischemia after heart attacks (myocardial infarction). One recent study isolated the novel peptide DDTRHWG against the cardiac endothelium of hypertensive rats and inserted this peptide into the H1 loop of a modified adenovirus serotype 5 vector (Ad5), termed Ad5/19p, created for its reduced liver tropism.369 A peptide-directed virus expressing LacZ was able to specifically transduce cardiac endothelium in vivo, with no virus detected in the liver. This proof of principle gene transduction suggests that this same peptide–adenovirus vector may be used to deliver a gene directed at improving cardiac vasculature function.
As diseases such as epilepsy and depression are connected to altered neurogenesis,435,436 gene delivery to neural stem cells has the potential for therapeutic effects. The neural-stem-cellbinding peptides QTRFLLH and VPTQSSG were conjugated to AdGFPL adenovirus, which is mutated to ablate natural tropism and contains the GFP gene.428 AdGFPL.QTRFLLH and AdGFPL.VPTQSSG were injected into the mouse brain and shown to specifically transduce neural stem cells in the dentate gyrus, as detected by GFP fluorescence in brain tissue sections. This suggests that these peptides could be used to deliver therapeutic genes to the neural stem cells. However, it will be important to verify that these peptides still bind human neural stem cells.
The kidney-specific peptides HTT and HIT were isolated by in vivo panning in rats.431 Expressing both peptides in the HI loop of the Ad19p adenovirus previously shown to have reduced hepatic specificity547 allowed for specific peptidemediated gene delivery to the kidneys.431 Rats were injected with Ad19p-HTT, Ad19p-HIT, or the control Ad19p, all containing the model gene LacZ. All rats were sacrificed 5 days later, and kidney sections were visualized by immunohistochemistry for β-galactosidase expression. Unlike the control vectors, both the HTT and HIT vectors specifically transduced cells in the kidney, resulting in kidney β-galactosidase expression. Ad19p-HTT-treated rats had β-galactosidase expression in the epithelial cells of the kidney tubules, while Ad19p-HIT-treated rats had β-galactosidase expression within the cells of the glomerulus. Importantly, β-galactosidase was not detected in other nontarget organs, including the liver. These results suggest that the HTT and HIT peptides can be used for specific kidney gene targeting when incorporated into the Ad19p adenovirus.
Arap and Pasqualini took an innovative approach in which the features of the viral vectors were incorporated into an fd-tet bacteriophage.548–550 Specifically, a mammalian transgene cassette from AAV was inserted into a noncoding region of the bacteriophage genome. This vector, referred to as AAVP, has been utilized to deliver several genes to different target cell types. The RGD-4C peptide that binds αvβ3 and α5β1 integrins has been utilized to create chimeric AAVP virus that facilitates functional gene transfer specifically to tumor vasculature and adjacent tumor cells.551 For example, delivery and expression of the tHSVtk gene results in expression of herpes simplex virus type-1 thymidine kinase selectively in the targeted tumor. Remarkably, no significant gene expression is observed in the liver. The TNF-α gene has been delivered to tumors by the same approach, resulting in TNF-α expression and significant reduction in tumor size.552 As a result, RGDdirected AAVP carrying TNF-α is in preclinical development.553
The mPep peptide isolated by panning against the mouse preadipocyte cell line 3T3-L1 was used to specifically deliver the Ucp1 gene to 3T3-L1 cells.413 As Ucp1 has antiobesity properties and can inhibit lipogenesis,554 it was added to an AAVP vector displaying the mPep peptide.413 mPep-AAVP-Ucp1 specifically targeted 3T3-L1 cells in vitro, and 7 days after transduction the cells failed to differentiate into adipocytes. By contrast, nontargeted AAVP-Ucp1 was unable to transduce 3T3-L1 cells, and the cells differentiated into adipocytes.
8.2. Chemical-Based Systems for Targeted Delivery of Oligonucleotides
Oligonucleotides can be condensed on polycation polymers and delivered to cells using a targeting peptide. These chemical-based systems are easier to synthesize than the corresponding viral gene delivery systems and do not suffer from inherent immunogenicity. While their transfection efficiency is lower than that of viral delivery, synthetic gene delivery systems are progressing as viable options for in vivo treatment. For example, a peptide selected against cells overexpressing Tie2 is able to specifically deliver DNA condensed on a poly(ethylenimine) construct to mice bearing SPC-A1 lung cancer.462 This construct was used to deliver WT p53, resulting in a significant decrease in tumor size due to restoration of p53’s tumor suppressor activity.
The EGFR-targeting peptide GE11 was conjugated to linear poly(ethylenimine) to deliver the sodium iodide symporter (NIS) gene for 131I therapy of liver cancer in mice.555 NIS is the protein that allows the thyroid to accumulate iodide, and its expression in thyroid cancer allows for the treatment of thyroid cancer using radioiodine, the most effective radiotherapy currently in clinical use. Liver-tumor-bearing mice injected intravenously with the polyplex and then given 131I 24 h later experienced significant tumor growth delay compared to mice with control treatments.
Gene delivery has not been limited to cancer cells. Nam et al. used the previously identified cardiomyocyte-specific peptide PCM.1213 to specifically deliver cardioprotective siRNA to H9C2 rat cardiac myocytes in vitro.371 The PCM.1 peptide was conjugated to a bioreducible polymer, poly(CBA-DAH), and complexed with Fas siRNA, a known inhibitor of cardiomyocyte apoptosis. The PCM–polymer–siRNA complex knocked down Fas expression in hypoxic H9C2 cells, reducing cell apoptosis. As hypoxia is a side effect of ischemic myocardium, in vivo studies with this complex may provide interesting therapeutic leads for the disease.
The dendritic-cell-specific peptide XS52.1, isolated from in vitro panning against the mouse dendritic cell line XS52, was also used to specifically deliver a gene in vivo.393 The XS52.1 peptide was incorporated into a liposome containing a luciferase gene plasmid and injected into the skin of mice. Immunofluorescence of cell suspensions from lymph nodes isolated 24 h later demonstrated XS52.1-mediated specific delivery of the luciferase gene to the dendritic cells of the skin. Quantification of luciferase expression demonstrated 10-fold more luciferase in dendritic cells targeted with XS52.1–liposomes as opposed to cells given control peptide or no peptide liposomes. This study suggests that the XS52.1 peptide could be used to deliver biologically active genes to the dendritic cells of the skin.
9. USE OF PEPTIDES FOR DIAGNOSTIC APPLICATIONS
Due to the high specificity of targeting peptides, they can be adapted for diagnostic applications in the same manner in which antibodies are currently used. The advantages of using peptides over antibodies for in vitro assays are minimal; as such, most efforts have focused on developing peptides for in vivo diagnostic imaging. In particular, there is a surge of peptides being used as molecular imaging probes. The next sections will highlight recent advances in translating selected cell-binding peptides into useful diagnostics.
9.1. Peptides Used for in Vitro Diagnostics
Peptides can serve as antibody replacements for immunohistochemistry, and since peptides can easily be labeled with a variety of fluorophores, using them in multiplexed assays is possible. However, as the molecular target for most of the selected peptides is unknown, they are unlikely to replace antibodies for pathological classifications. Rather, the ability of these peptides to bind to intact cells in a highly specific fashion makes them ideal for isolation of the target cell from a background of other cells. This is especially important when the cell of interest is a minority population, such as a circulating tumor cell. Several peptides have been used to isolate tumor cells from whole blood. The cDGWGPNc peptide was isolated from an OBOC library screen against multiple ovarian cancer cell lines. When placed on the surface of polystyrene beads, cDGWGPNc is able to bind ovarian cancer cells mixed into blood.335 The pA peptide, selected from an OBOC screen against A549 non-small-cell lung cancer cells, was able to pull down cancer cells from the pleural fluid of a patient with lung adenocarcinoma.338 The QMARIPKRLARH peptide, selected from an OBOC screen against LNCaP cells, also bound LNCaP cells in whole blood.50 The A20 peptide, selected for binding to a B-cell lymphoma cell line from a phage-displayed library, can enrich for the cancerous cells out of a background of normal Bcells. 212
Recently, Lam and co-workers described an interesting method with diagnostic potential for OBOC peptides involving what they term “rainbow beads”.556 After the synthesis of peptides on TentaGel resin beads using standard Fmoc library synthesis, the beads were dyed using commercially available organic dyes. Subsequent washing, drying, and soaking resulted in distinctly colored beads that displayed a given peptide of interest. Using this method, a group of six different peptides and peptidomimetics were synthesized on dye-labeled beads such that each peptide or peptidomimetic had its own distinct bead color. The beads were then mixed and incubated with different cancer cell lines to examine the ability of each peptide to bind to each cell line. Peptide specificity for the cell lines was examined using a standard inverted microscope with white light. The different peptides were easily distinguishable by their different bead colors, and cell specificity for peptides was determined by looking for colored beads that bound cells. This method has the potential to quickly determine peptide specificity for different cell types and can be multiplexed. Similarly, multiplexed assays to determine the binding profile of multiple peptides on a single sample can be performed using peptides conjugated to quantum dots with different emission wavelengths.
Although not diagnostic, another example of using peptides to isolate cancer cells was provided by McDonald and colleagues.119,120 The EphA2-receptor-specific peptide YSA was conjugated to superparamagentic nanoparticles and used to remove ovarian cancer cells in vitro from peritoneal fluids removed by paracentesis. After incubation with the peptide–nanoparticles, a magnet was used to isolate the nanoparticles along with their bound cancer cells, and the remaining peritoneal fluid was drawn off and reintroduced into the mouse. This resulted in tumor growth inhibition and increased survival times compared to those in control mice treated identically without the peptide–nanoparticles.
9.2. Peptides Used for in Vivo Diagnostics
Imaging agents can be incorporated into cell-targeting peptides with relative ease. Peptides have been used for different imaging platforms, including optical, positron emission tomography (PET), single positron emission tomography (SPECT), and magnetic resonance imaging (MRI). These imaging probes have the potential to detect diseases at early stages, locate residual or metastatic disease, and/or provide molecular profiles of cells. Examples of selected peptides used for optical imaging, PET, and MRI are discussed below. Of note, the use of cancerspecific peptides isolated by phage display for molecular imaging has been reviewed elsewhere.557
9.2.1. Peptides Used for Optical Imaging
Progress is being made in the development of near-infrared red reagents,558 yet whole-body fluorescence imaging in humans is currently not feasible due to high background fluorescence, poor light penetration, and inherent light scattering.559 However, it is a valuable research tool in animal models to determine biodistribution of targeting peptides and/or optimization of peptides for improved targeting. Peptides have been attached to a variety of dyes or fluorescent nanoparticles, such as quantum dots, for in vivo optical fluorescence imaging.305,560,561 The numerous peptides that have been used for optical imaging in animals are noted in the tables; peptides selected from phage display, OBOC, and PS-SPCL libraries are represented.
Optical imaging can also be used in conjunction with other diagnostic tools. For example, ex vivo phage display was used to isolate a 6-mer peptide specific for fresh human colon adenomas. This peptide was fluorescently labeled and administered topically to patients undergoing colonoscopy.236 Dysplastic regions of the colon could be distinguished from normal tissue with a sensitivity and specificity of 80% using confocal microendoscopy. Another study used in vivo phage display to isolate a peptide specific for mouse colon dysplasia. A fluorescently labeled version of this peptide bound colonic dysplasia in mice and was easily detected using wide-field endoscopy.317 Although this dysplasia-specific peptide was isolated from a mouse model of the disease, preliminary experiments demonstrate specific binding to human colon dysplastic adenomas over normal colon tissue. Phase I clinical trials have started to determine the utility of this approach in both colon and esophageal cancers. These successes highlight the potential clinical use of peptides for the early diagnosis of cancer. This also points to the possibility of using labeled peptides during surgery to distinguish tumor borders in real time.
Hallahan and co-workers demonstrated use of their TIP-1-receptor-binding peptide HVGGSSV to optically image and improve treatment for irradiated tumors.265,320 A theranosic agent was synthesized in which the peptide was conjugated to an AlexaFluor 750–nab-paclitaxel (nanoparticle albumin-bound paclitaxel) platform for treatment and near-infrared imaging. In a similar study, a peptide isolated from irradiated glioma cells showed specific near-infrared imaging when labeled with AlexaFluor 750 and delayed tumor growth when conjugated to a paclitaxel-containing nanoparticle.320
Near-infrared imaging has also been employed for heart imaging. The CTP peptide, isolated by a combination of in vitro and in vivo panning against normal murine cardiomyoctyes, was conjugated to commercially available 40 nm sized neutravidin–fluorospheres which fluoresce in the near-infrared range.358 Intracardiac injection in mice demonstrated heartspecific imaging for the CTP–fluorospheres as compared to control peptide–fluorospheres (Figure 10). Specificity after intravenous injection of the peptide–nanoparticles remains to be determined.
Figure 10.
The CTP peptide allows for specific cardiac imaging. Mice were imaged by in vivo fluorescent imaging following intracardiac injection of fluorospheres alone, CTP peptide–fluorospheres, or control peptide–fluorospheres. Shown are representative images from the three mice per group at different time points postinjection. Heart accumulation, indicated by the arrow, is only observed for the CTP peptide–fluorospheres. Reprinted with permission from ref 358. Copyright 2010 Public Library of Science (PLoS).
The brain-specific TGN peptide was isolated in an effort to bypass the blood–brain barrier. This peptide was conjugated to ~100 nm sized PEG–poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with the near-infrared dye DiR.206 After tail vein injection into mice, these particles homed to the brain as evidenced by live animal fluorescence imaging. Ex vivo imaging of organs after the mice were sacrificed revealed that control nanoparticles without peptide primarily accumulated in the liver and spleen and did not accumulate in the brain. The targeted TGN nanoparticles had significantly reduced liver and spleen clearance compared to the control nanoparticles.
9.2.2. Peptides Used for Molecular Positron Emission Tomography
PET can detect molecules down to picomolar concentrations and is considered one of the most sensitive molecular imaging techniques.562 As such it is an ideal platform for molecular imaging. The low spatial resolution of PET has been compensated for by the generation of PET/CT scanners that combine the sensitivity of PET with the anatomical resolution of computed tomography (CT). PET radioisotopes can be incorporated directly into targeting peptides (i.e., 18F and 131I), or chelators can be conjugated to the peptides and loaded with radiometals (i.e., 64Cu and 68Ga). SPECT is another imaging modality that can utilize cell-targeting peptides for the delivery of radioisotopes (99Tc, 111In, and 123I). Peptidebased imaging probes are anticipated to increase the sensitivity of detection while providing molecular information without the need for biopsy. However, as targeting peptides exhibit restricted binding profiles, it is reasonable to be cautious about their use as early detection agents. This is especially true for the cancer-binding peptides which have affinity for discrete subsets of tumors. Instead, they are more likely to find utility in providing molecular information about a tumor before or during treatment as well as in detecting micrometastases. Information obtained from PET imaging could aid in determining which ligand(s) should be employed for therapeutic targeting for an individual patient. Of importance, PET imaging can also provide the biodistribution information which is critical in preclinical optimization of cell-targeting peptides.563
Compared to antibodies, peptides are more amenable to the reaction conditions required for chemical modifications and labeling.564,565 Unlike antibodies, the in vivo half-life of the peptides is well matched to the half-life of most commonly used PET radionuclides. Most peptide-based PET imaging has been performed using naturally occurring peptidyl ligands such as bombesin and somatostatin.566–568 Several RGD-containing peptides, including the RGD-4C peptide,296,569 have been used to image αvβ3 in angiogenic vasculature.570,571 RGD peptides are in early clinical trials as molecular PET imaging agents for angiogenesis.572–574
Development of molecular agents for PET requires a fine balance between clearance rates, tumor retention, and nonspecific uptake in other tissues. Radiolabeled peptides must have certain characteristics to work well in vivo: they must have high affinity for their target, exhibit low nonspecific binding, and be stable in serum, maintaining the peptide integrity and retention of the radioisotope. Additionally, the peptide-based imaging agent must clear rapidly from the plasma and excrete in a manner that does not interfere with imaging.575 Kidney accumulation of radiolabeled peptides is a significant problem.576 While different mechanisms have been proposed for this accumulation, the current consensus is that particles below 20–30 kDa in size are filtered by the glomerulus and then reabsorbed by the proximal renal tubules.575,577 Small changes in the peptide sequence, linker, chelator, and isotope can dramatically affect the biodistribution. The empirical process involved in optimizing PET agents is time-consuming and costly. Nonetheless, cell-binding ligands isolated from peptide libraries are emerging as molecular imaging agents.
Many of the cancer-specific peptides have been used for molecular PET and SPECT imaging. For example, a tetrameric, PEGylated version of the plectin-1-binding peptide KTLLPTP was used for SPECT imaging of orthotopic pancreatic tumors in mice.74 Importantly, preinvasive lesions as well as liver metastases were detected. This imaging probe could also distinguish pancreatic cancers from chronic pancreatitis and may be an effective probe to determine whether a patient is a candidate for biopsy. Peptides can also be used to determine receptor expression in a tumor. For example, the EphB4-binding TNYL peptide was labeled with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and loaded with 64Cu.125 This imaging probe was able to distinguish between EphB4-positive and -negative tumors by small-animal PET/CT (Figure 11).
Figure 11.
Small-animal PET/CT imaging of EphB4-positive tumors. The EphB4-binding peptide TNYLFSPNGPIARAW was conjugated to DOTA and loaded with 64Cu. Signal is observed at 4 h in CT-26 and PC-3M but not in the EphB4-negative A549 tumors. Reprinted with permission from ref 125. Copyright 2011 Society of Nuclear Medicine, Inc.
In an extraordinary feat, the Sutcliffe laboratory combined screening of an OBOC peptide library with high-throughput imaging to develop molecular imaging agents for the cancer biomarker αvβ6.578 By screening a limited OBOC peptide library, a total of 55 peptides were identified. This set of peptides were labeled with 4-[19F]fluorobenzoic acid and tested for affinity to αvβ6 as well as specificity for αvβ6 compared to other integrins. On the basis of the in vitro results, 42 peptides were labeled with 4-[18F]fluorobenzoic acid for analysis by PET. Within 11 days, all 42 peptides had been tested, and 4 peptides displayed favorable biodistribution profiles. Surprisingly, little correlation between in vitro affinity and integrin specificity and in vivo tumor accumulation and specificity was observed. Thus, this combination of high-throughput peptide selection and imaging can identify good imaging agents that would otherwise be discarded. However, this approach requires easy access to a source of 18F, large quantities of time on a small-animal PET/CT system, and significant numbers of animals; these resources are expensive and not widely available.
Molecular PET is not limited to cancer imaging. The cardiacendothelium-specific CRPPR peptide359 was coupled to the surface of PEGylated liposomes incorporating 18F-labled lipid for PET imaging.370 Mice injected with the liposomes were imaged by PET for 90 min. The CRPPR-targeted liposomes accumulated in the heart with lower accumulation in the liver, spleen, and bladder. Nontargeted liposomes primarily stayed in circulation, resulting in some signal in the heart as well. In another example, an 18F-labeled tetrameric version of the VCAM-1-binding peptide VINP-28 detected inflammatory atherosclerosis by dynamic PET imaging.379 This probe may find utility in detecting small atherosclerotic plaques.
As properly functioning pancreatic islet β cells are crucial for glucose regulation and prevention and management of diabetes, a great deal of attention has been placed on techniques that could noninvasively monitor β cell mass and function.409 The RIP1 peptide isolated by ex vivo panning against freshly isolated rat pancreatic islets is selective for the β cells of normal rats and does not home to the β cells of a rat diabetic model.75 Thus, it could potentially be used to image properly functioning, normal β cells. While the peptide has not been tested outside the context of the phage, 124I-labeled phage was able to specifically target the pancreas in vivo with efficient clearance from other organs.409 The [124I]RIP1 phage was injected into the tail vain of rats and imaged by PET 4 h later. Although the RIP1 phage appears to accumulate in the pancreas, further studies with radiolabeled RIP1 peptide need to be done to confirm the utility of this peptide for normal β cell imaging.
9.2.3. Peptides Used for Molecular Magnetic Resonance Imaging
MRI yields high-resolution images, but current contrast agents suffer from a lack of sensitivity. Few molecular MR probes have been reported because it is challenging to target enough T1 contrast agent, such as gadoteridol, to the desired cell type to achieve a reasonable signal. For this reason, many have turned to iron oxide particles, which are a highly sensitive T2 agent that darkens the signal in regions in which the nanoparticles accumulate.579 Several nontargeted iron oxide nanoparticles (IONPs) are FDA-approved. 580
A plectin-1-binding peptide that homes to pancreatic ductal adenocarcinomas has been coupled to fluorescent cross-linked iron oxide nanoparticles.561 These peptide–nanoparticles home to pancreatic ductal adenocarcinoma as determined by both intravital fluorescence microscopy and ex vivo MRI. Using the Huisgen cycloaddition, better known as click chemistry, the LyP-1 peptide has been attached to fluorescently labeled, dextran-encapsulated iron oxide particles.306 The total amount of nontargeted and targeted nanoparticle in the tumor was found to be the same, yet the targeted particle penetrated within the tumor while the naked nanoparticle remained localized around the tumor blood vessels. In both of these examples, the peptides home to the appropriate tumors in animals as assessed by ex vivo fluorescent imaging, but in vivo MR imaging was not performed.
To facilitate conjugation of peptides to iron oxide nanoparticles, a one-step procedure for the surface functionalization of SPIO with a targeting peptide has been developed.581 The hydrophobic surfactants on the SPIO nanoparticles can be displaced through ligand exchange with a peptide containing a C-terminal polyethylene glycol-tethered cysteine residue. The resulting SPIO particles are biocompatible and demonstrate high T2 relaxivity. Attachment of the αvβ6-binding peptide H2009.1 resulted in specific targeting of αvβ6-expressing lung cancer cells as demonstrated by in vitro MR imaging and Prussian blue staining. This surface chemistry may expand the use of SPIO for MR imaging, but in vivo compatibility still needs to be determined.
The VCAM-1-specific CVHSPNKKC peptide has been used for a variety of in vivo imaging techniques for both cardiac endothelium in atherosclerotic lesions and for imaging of VCAM-1-expressing endothelial cells in other areas of inflammation.365 The peptide was conjugated to cross-linked iron oxide nanoparticles labeled with Cy5.5 dye. These magnetofluorescent nanoparticles were then used to image both the vasculature of an inflamed ear and the atherosclerotic lesions in the ApoE−/− mouse model of atherosclerosis. Acute inflammation was created in mouse ears by site-specific injection of TNF-α, causing VCAM-1 upregulation on the ear vasculature. Intravenous injection of peptide–nanoparticles led to specific accumulation of the nanoparticles in the inflamed ear vasculature, as evident by fluorescent images from intravital confocal microscopy (Figure 12A). The iron oxide component of the targeted nanoparticles also allowed for their use in MR imaging of atherosclerotic lesions. ApoE−/− mice with atherosclerotic lesions were injected intravenously with the peptide–nanoparticles and the lesions imaged by MRI. Specific darkening of the atherosclerotic lesions was evident in mice given the peptide–nanoparticles, consistent with accumulation of iron oxide in these areas. Areas of the aortic wall with thickening specifically accumulated the targeted nanoparticles, and this accumulation was verified by both ex vivo MRI and ex vivo fluorescent images of excised aortas (Figure 12B). Importantly, the targeted peptide nanoparticles did not accumulate in aorta of wild-type mice without atherosclerotic lesions, and nontargeted nanoparticles did not accumulate in the atherosclerotic areas of the aorta, as evidenced by both ex vivo MRI and ex vivo fluorescent imaging of excised aortas.
Figure 12.
VCAM-1-specific peptide homes to areas of inflammation and atherosclerotic deposits. (A) The VCAM-1-binding peptide CVHSPNKKCGGSKGK was coupled to a magnetofluorescent nanoparticle (VPN). TNF-α-induced inflammation was induced in one ear of a mouse, while the other was left untreated. Intravital microscopy shows clear accumulation of VPN (red) in the inflamed ear at 4 h, while no binding is observed in the normal control ear. (B) Ex vivo imaging by MR and macroscopic fluorescence at 24 h post intravenous injection detects binding of VPN in animals with atherosclerotic plaques (Apo E−/− mice). No signal is detected in wild-type animals with no plaque development or when a nontargeted nanoparticle is used. The high accumulation of VPN in the aortic arch is indicated by the arrows. Adapted with permission from ref 365. Copyright 2005 American Heart Association Inc.
10. TRANSLATION OF TARGETING PEPTIDES FROM LIBRARY SCAFFOLD TO FUNCTIONAL SYNTHETIC PEPTIDES
Despite the ease of identifying cell-binding ligands from peptide libraries, these approaches fell out of favor with some researchers in the community. Specifically, many peptides have had poor affinities when synthesized as free peptides; maintaining the affinity and activity of peptides selected from peptide libraries outside the context of their original scaffold has been an impediment. Many reports utilized monomeric peptides, and this review has highlighted many of them. However, the affinities of numerous peptides are in the micromolar range, which is unsuitable for most clinical applications. This is especially true for peptides selected from phage or OBOC libraries. In both cases, the peptides are displayed in multiple copies on their scaffold; thus, the peptides may bind to their cellular target via a multivalent interaction. The increased affinity due to multivalent binding is lost when the peptides are used in their monomeric forms. Additionally, many endocytotic processes are initiated by receptor multimerization at the cell surface. If internalization of the peptide is desired, the free peptide must facilitate this interaction on the cell surface.
Several different methods have been used to synthesize multimeric peptides to improve affinity (Figure 13). Multimerization of the cell-targeting peptides on a trilysine core is a useful scaffold for retaining the peptide activity outside the context of the phage. The trilysine scaffold mimics the presentation of the peptides on the pIII protein of the phage in both valency and the orientation of the displayed peptides. Additionally, the trilysine core can be modified with a variety of different moieties (drugs, drug carriers, metal chelators, dyes, biotin, and so on) without affecting peptide binding. While these multimeric peptides can be synthesized by linear Fmoc chemistry, we reported a convergent synthesis involving a chemoselective reaction of a cysteine with a maleimide-capped trilysine core.223 This chemistry is facile and is not restricted to laboratories with expertise in synthetic peptide chemistry. We and others have found the tetrameric trilysine framework to be a general platform for cell-targeting peptides selected from phage-displayed peptide libraries.212,218,220,393,582 Tetramerization of a peptide increases its affinity for its target cell 25–100-fold when compared to that of the monomeric peptide, indicating the importance of multivalent binding. For example, we have synthesized a tetrameric peptide that binds αvβ6-expressing cells. The monomeric peptide binds H2009 adenocarcinoma NSCLC cells with a half-maximal binding affinity of 9.2 nM, while the tetrameric peptide increases affinity to 11 pM. This tetrameric peptide has affinities that are competitive with those of antibodies. An additional benefit to tetramerization of the peptides on this core is an increase in serum stability due to a reduced susceptibility to protease degradation.582–585 Although tetrameric peptides are most commonly used, dimeric and trimeric peptides can be synthesized by a similar strategy.223
Figure 13.
Structures of two multimeric peptide scaffolds. Multimeric presentation of the targeting peptides mimics the valency and orientation of the phage particle. The tetrameric peptide based on a trilysine core is shown to the left and the pentavalent dendritic wedge on the right. The targeting peptide is shown as a gray oval. A variety of chemical moieties have been attached to the trilysine core structure, indicated by “R” in the figure.
Another useful scaffold for displaying peptides is a pentavalent dendritic wedge.308,586,587 The AB5 synthon allows for the attachment of five peptides via native chemical ligation or oxime formation. As anticipated, a synergistic boost in affinity is observed due to multivalent binding to the target. Like the trilysine core, the focal point on the dendron can be modified to carry different cargoes, and linkers can be placed between the AB5 core and the targeting peptide as necessary. Several peptides have been placed on the wedge scaffold, including the CREKA and LyP-1 peptides.
It has been assumed that conjugation of multiple copies of a ligand to the surface of a nanoparticle will impart multivalent binding and improve affinity of the ligand for its target,588 yet these platforms are unlikely to display the peptides in an optimal multimeric conformation. Additionally, increasing the copy number of the ligand on the nanoparticle to improve the effects of multivalent binding can result in increased nonspecific binding. We recently reported the effects of peptide valency, density, and affinity on nanoparticle delivery and therapeutic efficacy, using the αvβ6-binding H2009.1 peptide as a model phage-selected peptide and liposomal doxorubicin as a model nanoparticle.227 Liposomes displaying the higher affinity multivalent H2009.1 tetrameric peptide demonstrated 5–10-fold higher drug delivery than liposomes displaying the lower affinity monomeric H2009.1 peptide, even when the same number of peptide subunits were displayed on the liposome. Liposomal targeting also increased with increasing concentrations of H2009.1 tetrameric peptide on the liposome surface. Thus, both the multivalent peptide and the multivalent liposome scaffold worked together to increase targeting to αvβ6-expressing cells.
It should be noted that the boost in affinity for these multimeric peptide platforms is dependent on the receptor density on the surface of cells; the greater the receptor density, the more likely a significant synergistic improvement in affinity will be observed for multimeric peptide platforms.587 Additionally, the optimal valency may not necessarily be 4–5 peptide branches due to the spacing of the receptors on the cell surface. This stresses the importance of optimizing peptide affinity in the context of whole cells where the native receptor landscape is maintained.
11. PERSPECTIVES AND FUTURE CHALLENGES
11.1. Identification of Cellular Receptors
A major challenge in the field is the identification of the cellular targets of peptides selected from unbiased selections on whole cells and from in vivo selections. This is particularly striking when one realizes how few receptors have been identified for the peptides listed in Tables 2–17. Although these ligands can be used without knowledge of their cellular receptors, receptor identification remains a high priority. First, receptor identification can provide knowledge about the cell surface profile and how it differs between different cell types or states. Second, once identified, new ligands can be generated for the receptor. While peptides might be appropriate for some applications, antibodies, aptamers, peptoids, or small molecules may be a better choice for others. Third, FDA approval of any targeting ligand without knowledge of its cellular target will be difficult.
Approaches for receptor identification have focused on biochemical affinity purification and/or protein cross-linking followed by mass spectrometric identification of the isolated protein species.561,589,590 The low success rate is partly due to the inherent nature of membrane proteins. They are present in low abundance, and solubility is an issue. This makes affinity purification and mass spectrometric identification difficult. It is important to remember that the cell surface has a topography in which proteins can multimerize with binding partners or cluster within microdomains. This surface landscape can contribute to the specificity of the peptidic ligands. In other words, peptide cell specificity may not arise from absolute receptor protein expression levels but from an arrangement of the receptors on the cell. This spatial information can be lost upon the preparation of membrane protein for affinity purification and is not borne out in mRNA levels. It is also important to note that while the assumption in the field has been that the peptides bind to protein receptors, they may instead be binding sugar moieties of glycoproteins or glycolipids or to phospholipids.
Protein databases can be searched for sequence similarity to the peptide. This has yielded candidate receptors for a few isolated peptides.591–593 For instance, homology of the lung-cancer-binding peptide H2009.1 to the GH viral coat protein of the foot and mouth disease virus led to the identification of αvβ6 as the cellular receptor for this peptide.592 However, most phage-displayed peptide libraries are chemically synthesized and do not originate from biological sources. Furthermore, the complete sequence coverage of the longer peptides is limited. As such, the probability of the peptide sequence matching a biologically derived sequence is statistically low. Furthermore, many matches do not provide biological insight into the potential receptors. In summary, new techniques are needed to identify the receptor partners for the selected ligands. A combination of cell biology, proteomic, and genomic approaches will be needed to tackle this difficult problem. Finally, a note of caution: Many receptors have been repeatedly identified for different peptide ligands. For example, vimentin and gp78 have been identified repetitively. While these may indeed be the peptides’ binding partners, any initial protein identification needs to be followed by biochemical and molecular biology studies for confirmation.
11.2. Management of the Ever-Growing Peptide Sequence Data
As can be seen from this review, there are numerous peptides selected from different libraries. Furthermore, this list is not comprehensive and does not include peptides selected against antibodies, intracellular targets, inorganic materials, or small organic molecules. Collecting these data is not trivial and becomes more complicated as the field continues to grow, and individual laboratory databases are often incomplete. Recently, several Web-based databases have been created to allow researchers to search for previously isolated peptides. The TumorHoPe database is focused on tumor-homing peptides and is manually curated.594 The MimoDB database is broader and not limited to tumor-targeting peptides. This database allows the user to search by sequence, target, library type, and structure.595 Finally, PepBank contains peptide sequences obtained from text mining of MEDLINE abstracts as well as some manually curated sequences.596 The database has been modified, allowing users to vote in an effort to improve peptide classification.597 PepBank is particularly useful in identifying nonspecific phage clones that are repetitively selected from a particular library.207 It is currently the largest of the peptide databases. Both MimoDB and PepBank have features that allow for BLAST searches of the databases. With these resources available, researchers can determine if a peptide has been previously selected or has sequence similarity to another selected peptide. However, these resources are only as powerful as the completeness of the peptide repository. A random sampling of peptides from Tables 2–17 found many sequences missing from the PepBank database. As such, central data storage of peptide sequences still needs refinement.
11.3. Physiological Challenges in Cell-Targeted Delivery of Biologically Active Cargo
While the selection process focuses on the molecular interactions between a peptide and its cellular receptor, there are numerous physiological parameters that can hinder the efficacy of targeted therapies.598 Many of the pharmacokinetic and pharmacodynamic parameters can be optimized by modification of the peptide, as is touched upon in the next subsection. However, even upon reaching the targeted region, delivery of biologically active cargo faces difficulties, such as efficient escape from the vasculature, available access of the receptors, penetration of the peptide to reach a majority of the cells, and release of the cargo in an active form. Vascular targets which are exposed to the bloodstream are more accessible, and many of the successes in targeted therapies involve endothelial receptors.291,599
Solid tumors present their own set of hurdles that must be dealt with when considering targeted therapy. Primarily, widespread distribution of the drug throughout the tumor such that the majority of tumor cells are eradicated is difficult.600 Tumors possess a poorly organized vasculature with chaotic branching structures. As a result, tumor cells can be up to 100 µm from a functional blood vessel. Once the drug conjugate escapes from the vasculature, there remain long interstitial transport distances to reach cells in certain areas of the tumor, and this transport within the tumor relies primarily on diffusion. Diffusion rates and distances are limited by the dense extracellular matrix and tumor size. Additionally, tumors have dysfunctional lymphatic systems, causing them to have high interstitial pressure; this results in the convection of fluids from the interior of the tumor to its periphery and to surrounding tissues. Finally, a binding site barrier may limit diffusion into the tumor. High-affinity ligands can bind to the first target cells they encounter, and then, due to a rapid internalization and/or a slow off rate from the receptor, the ligand is no longer available to travel further into the tumor.601,602 All together, it is difficult to reach the less vascularized regions of the tumor, and much of the drug remains perivascular. Clearly the magnitude of these challenges will be tumor, receptor, and ligand specific.
Although the challenges are substantial, peptide-targeted tumor therapies are still very promising and may reduce several of these delivery problems. Peptides are smaller than currently used antibodies and are expected to diffuse more rapidly into the tumor. The affinity can be modulated by controlling the valency and chemical composition of the peptide, thus minimizing the binding site barrier. Moreover, peptides that improve tumor penetration have recently been reported, 274,275,278,279,309 and this approach has been recently reviewed.603 Clearly nonsolid tumors such as leukemias and lymphomas do not suffer from these issues, and neither do micrometastases. Additionally, targeting peptides can deliver agents, such as radioisotopes, that have bystander effects; surrounding tumor cells receive ionizing radiation even if they did not directly bind the peptide. Finally, therapeutic approaches to reduce interstitial pressure and/or normalize the tumor vasculature have been show to improve distribution of drugs and nanoparticles throughout a tumor.
A word of caution about targeting larger drug carrier molecules to tumors is required.604 Tumors also possess a leaky vasculature which allows for large particles to extravasate from the vasculature. This coupled with a dysfunctional lymphatic system results in retention of nanoparticles 50–400 nm in size in the tumor.605 This effect, known as the enhanced permeability and retention (EPR) effect, results in an inherent passive targeting and can complicate our understanding of the active targeting of nanoparticle drug carriers. Mathematical modeling of tumor targeting suggests that passive targeting is the driving force for tumor accumulation of nanoparticles.606,607 While increased efficacy of targeted liposomal drugs is often observed over nontargeted liposomal formulation in many cases, the reason for this is debatable. In some cases, especially in vasculature targeting, it appears that more liposomes accumulate in the tumor when targeted.608,609 In others, it appears that the targeting ligand does not increase the liposomal delivery to the tumor, but it facilitates cellular uptake of the drug as well as increases distribution of the liposome throughout the tumor.610,611
11.4. Misperceptions about Peptides as Therapeutics
Perhaps one of the biggest challenges to overcome is the misperception that peptides are not viable therapeutics. Traditionally, pharmaceutical companies have avoided peptides in preference for small molecules, yet there are more than 60 peptide therapeutics approved for clinical use worldwide, 5 of which have sales over $1 billion.612–618 Therapeutic peptides range from 2 to 41 amino acids in length and have varied indications. Furthermore, the number of peptides entering clinical trials has risen to almost 200. To counteract the negative image of peptides as drugs, the Peptide Therapeutic Foundation (www.peptidetherapeutics.org) was founded to promote the development of peptide therapeutics.
The predominate drawbacks attributed to peptides are (1) short biological half-lives due to proteolysis, (2) rapid renal filtration, (3) lack of oral availability, and (4) costs. Proteolysis can be overcome by protection of the free termini, cyclization of the peptide, addition of non-natural amino acids, and elimination of specific cleavage sites. A variety of approaches have been taken to overcome rapid glomerular filtration by the kidneys, including PEGylation, glycosylation, protein conjugation, and hydrophobic depoting. Conjugation of peptides to serum albumin is another approach for extending the peptide half-life in vivo.619 Albumin-binding peptides have been isolated by phage display panning, opening the possibility for a completely peptidic targeting agent with an extended circulation time.620 Methods to improve peptide circulation times in vivo have been reviewed elsewhere.614,621,622 Indeed, peptides are generally not orally bioavailable, and most are administered by injection. However, there have been substantial advances in the development of peptide delivery systems, including controlled release polymeric formulations and osmotic pumps, which can reduce injections from daily to monthly.614,623 Progress is also being made in transdermal, nasal, pulmonary, and oral delivery of peptides as well.624 Finally, the cost of large-scale peptide synthesis has dampened the enthusiasm for the development of peptidic drugs, especially considering the large doses that are likely to be required,625 yet improvements in synthetic methodology and the use of native chemical ligation to synthesize longer peptides have reduced this concern. Peptides are now synthesized in kilogram quantities at costs of ~$1 per gram per amino acid. While more expensive than small-molecule drugs, this cost is substantially lower than that of monoclonal antibody production.
Peptides isolated from phage-displayed peptide libraries are now entering the market. In 2008 the FDA approved Romiplostim (AMG 531, Nplate) for the treatment of thrombocytopenia in patients with immune thrombocytopenia purpura.626–628 The lead peptide was identified by panning a phage display library on the thrombopoietin receptor.629 Romiplostim presents four copies of the peptide on an Fc fragment which extends the peptide’s in vivo half-life. A similar peptibody scaffold is used in AMG-386 (trebananib), Amgen’s antiangiogenic therapeutic. In this case, the peptide prevents binding of angiopoietin-1 and angiopoietin-2 to Tie2.630 AMG-386 is in phase III clinical trials for treatment of a variety of cancer types. The drug is well tolerated,631 and positive signs of efficacy were observed in ovarian cancer patients when used in combination with standard chemotherapeutics.632 Peginesatide (formerly Hematide), is an erythropoietin mimetic peptide selected from a phage-displayed library at Amgen.633,634 The peptide is displayed as a PEGylated dimer to increase its affinity and biological half-life. FDA approval of Peginesatide for the treatment of anemia associated with chronic kidney disease was granted in 2012.
Several of the more recently isolated peptides discussed in this review are in clinical trials. The CD13- and αvβ3-binding peptide NGR has been fused to human tumor necrosis factor (hTNF)635 or truncated tissue factor (tTF).636 NGR–hTNF is in phase III clinical trials for mesothelioma and in phase II trials for a number of solid tumors. Modified adenovirus which displays the RGD-4C peptide on its fiber knob and carries the genes encoding for HSV-TK and the somatostatin receptor has undergone a phase I study.637 Similarly, the fat-targeting peptide CKGGRAKDC linked to the d-enantiomer of the proapoptotic (KLAKLAK)2 peptide entered phase I trial in early 2012 under the name Adipotide.
In summary, these successes indicate that peptides isolated from peptide libraries can translate into useful therapeutics. It is important to note that it took Romiplostim 10 years to go from initial peptide selection to FDA approval. More recently isolated peptides are likely in early stages of preclinical development.
11.5. Conclusions
The number of cell-targeting peptides has expanded greatly in the past 10 years. This expansion has come about primarily from combinatorial peptide libraries and new selection methods. While phage display methods have been the predominant library type used, other peptide library formats have also been used to isolate cell-targeting agents. Cell-targeting peptides can have affinities comparable to those of clinically used antibodies and represent a new tool for directing targeting therapies and imaging agents. The section protocols are robust, allowing for a wide variety of cell types to be targeted. However, it is important to remember that the isolated ligands are rarely the optimal peptide sequence and should be considered lead compounds, a notion that is often overlooked, yet by blending biology and chemistry, peptide leads can be optimized for affinity, specificity, activity, stability, solubility, and biodistribution. These optimized and validated peptides are anticipated to have broad clinical applications. A number of peptides identified from peptide libraries are currently in clinical trials, and more are anticipated to follow.
ACKNOWLEDGMENTS
Work in K.C.B.’s laboratory is supported by the Welch Foundation (I1622) and the National Institutes of Health (Grants 1R01EB014244-01A1 and 1R01CA164447-01A1). B.P.G. was supported by a fellowship from the Cancer Research and Prevention Institute of Texas (Grant RP 101496).
Biographies

Bethany Powell Gray received her B.S. in Chemistry from Abilene Christian University (ACU) in 2005. While at ACU, she began organic synthesis work under the direction of Dr. Perry Reeves. In 2006 she joined Dr. Kathlynn Brown’s laboratory at the University of Texas Southwestern Medical Center and began using phage display selected peptides for drug delivery. She received her Ph.D. in Biological Chemistry in August 2012 and recently began postdoctoral work in Dr. Bruce Sullenger’s laboratory at Duke University Medical Center.

Kathlynn C. Brown is Assistant Professor of Internal Medicine and a member of the Simmons Cancer Center at the University of Texas Southwestern Medical Center. Dr. Brown obtained her Ph.D. in organic chemistry at the University of Texas at Austin, during which time she received fellowships from the Mahler Memorial Foundation and the Organic Division of the American Chemical Society. She continued her training at the University of California at San Francisco, where she was a Damon Runyon Walter Winchell Postdoctoral Fellow. She has utilized her multidisciplinary expertise in organic chemistry, peptide chemistry, biochemistry, and molecular biology to address challenges in biomedical research. Her laboratory was among the early adopters of cell-based biopanning of phage-displayed peptide libraries. Using this approach, she has developed a suite of high-affinity peptides that target a variety of different cell types. Her research team is currently focusing on the development of peptides that target non-small-cell lung cancer for use as delivery vehicles for drugs, nanoparticles, and toxins. Additionally, the peptides are being utilized as molecular imaging agents for diagnosis and classification of lung tumors.
Footnotes
The authors declare no competing financial interests.
REFERENCES
- 1.Strebhardt K, Ullrich A. Nat. Rev. Cancer. 2008;8:473. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM. mAbs. 2013;5:1. doi: 10.4161/mabs.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert JM, Valge-Archer VE. Nat. Rev. Drug Discovery. 2007;6:349. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 4.Tabrizi MA, Tseng C-ML, Roskos LK. Drug DiscoveryToday. 2006;11:81. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 5.Neumeister P, Eibl M, Zinke-Cerwenka W, Scarpatetti M, Sill H, Linkesch W. Ann. Hematol. 2001;80:119. doi: 10.1007/s002770000239. [DOI] [PubMed] [Google Scholar]
- 6.Bray BL. Nat. Rev. Drug Discovery. 2003;2:587. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 7.Nagy A, Schally AV. Curr. Pharm. Des. 2005;11:1167. doi: 10.2174/1381612053507594. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Xu C. Curr. Pharm. Biotechnol. 2011;12:1144. doi: 10.2174/138920111796117427. [DOI] [PubMed] [Google Scholar]
- 9.Schally AV, Engel J, Emons G, Block N, Pinski J. Curr.Drug Delivery. 2011;8:11. doi: 10.2174/156720111793663598. [DOI] [PubMed] [Google Scholar]
- 10.Milletti F. Drug Discovery Today. 2012;17:850. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Smith GP. Science. 1985;228:1315. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe JW, Kay BK. Chem. Rev. 2005;105:4056. doi: 10.1021/cr000261r. [DOI] [PubMed] [Google Scholar]
- 13.Hill HR, Stockley PG. Mol. Microbiol. 1996;20:685. doi: 10.1111/j.1365-2958.1996.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith GP, Petrenko VA. Chem. Rev. 1997;97:391. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 15.Cabilly S. Mol. Biotechnol. 1999;12:143. doi: 10.1385/MB:12:2:143. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga K, Taki M. J. Nucleic Acids. 2012;2012:9. doi: 10.1155/2012/295719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbas CF, Kang AS, Lerner RA, Benkovic SJ. Proc.Natl. Acad. Sci. U.S.A. 1991;88:7978. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aina OH, Liu R, Sutcliffe JL, Marik J, Pan CX, Lam KS. Mol. Pharmaceutics. 2007;4:631. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 19.Mclaughlin M, Sidhu SS. Methods Enzymol. 2013:327. doi: 10.1016/B978-0-12-394292-0.00015-1. [DOI] [PubMed] [Google Scholar]
- 20.Held HA, Sidhu SS. J. Mol. Biol. 2004;340:587. doi: 10.1016/j.jmb.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 21.Scott JK, Smith GP. Science. 1990;249:386. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 22.Kuzmicheva GA, Jayanna PK, Sorokulova IB, Petrenko VA. Protein Eng. Des. Sel. 2009;22:9. doi: 10.1093/protein/gzn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong G, Smith GP, Berry J, Brunham RC. J. Biol. Chem. 1994;269:24183. [PubMed] [Google Scholar]
- 24.Cwirla SE, Peters EA, Barrett RW, Dower WJ. Proc.Natl. Acad. Sci. U.S.A. 1990;87:6378. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzucchelli L, Burritt JB, Jesaitis AJ, Nusrat A, Liang TW, Gewirtz AT, Schnell FJ, Parkos CA. Blood. 1999;93:1738. [PubMed] [Google Scholar]
- 26.Saldanha R, Molloy M, Bdeir K, Cines D, Song X, Uitto P, Weinreb P, Violette S, Baker MS. J. Proteome Res. 2007;6:1016. doi: 10.1021/pr060518n. [DOI] [PubMed] [Google Scholar]
- 27.Steven AC, Trus BL. Electron Microscopy of Proteins. Vol. 5. New York: Academic Press; 1986. p. 2. [Google Scholar]
- 28.Condron BG, Atkins JF, Gesteland RF. J. Bacteriol. 1991;173:6998. doi: 10.1128/jb.173.21.6998-7003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao N, Cheng D, Wang Y, Chen L, Liu X, Dou S, Liu G, Liang M, Hnatowich DJ, Rusckowski M. Cancer Biol. Ther. 2011;11:22. doi: 10.4161/cbt.11.1.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner T, Kolmar H, Haberkorn U, Mier W. Molecules. 2011;16:1625. doi: 10.3390/molecules16021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krumpe LRH, Schumacher KM, Mcmahon JB, Makowski L, Mori T. BMC Biotechnol. 2007;7:65. doi: 10.1186/1472-6750-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derda R, Tang S, Li SC, Ng S, Matochko W, Jafari M. Molecules. 2011;16:1776. doi: 10.3390/molecules16021776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krumpe LRH, Atkinson AJ, Smythers GW, Kandel A, Schumacher KM, Mcmahon JB, Makowski L, Mori T. Proteomics. 2006;6:4210. doi: 10.1002/pmic.200500606. [DOI] [PubMed] [Google Scholar]
- 34.Liu CC, Mack AV, Tsao ML, Mills JH, Lee HS, Choe H, Farzan M, Schultz PG, Smider VV. Proc. Natl. Acad.Sci. U.S.A. 2008;105:17688. doi: 10.1073/pnas.0809543105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daugherty PS. Curr. Opin. Struct. Biol. 2007;17:474. doi: 10.1016/j.sbi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Georgiou G, Stathopoulos C, Daugherty PS, Nayak AR, Iverson BL, Curtiss R. Nat. Biotechnol. 1997;15:29. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Georgiou G. Biotechnol. Bioeng. 2002;79:496. doi: 10.1002/bit.10407. [DOI] [PubMed] [Google Scholar]
- 38.Jostock T, Dubel S. Comb. Chem. High Throughput Screening. 2005;8:127. doi: 10.2174/1386207053258479. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Choi JH, Xu Z. Trends Biotechnol. 2003;21:45. doi: 10.1016/s0167-7799(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 40.Samuelson P, Gunneriusson E, Nygren PA, Stahl S. J.Biotechnol. 2002;96:129. doi: 10.1016/s0168-1656(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z, Murray KS, Van Cleave V, Lavallie ER, Stahl ML, Mccoy JM. Biotechnology. 1995;13:366. doi: 10.1038/nbt0495-366. [DOI] [PubMed] [Google Scholar]
- 42.Bessette PH, Rice JJ, Daugherty PS. Protein Eng. Des. Sel. 2004;17:731. doi: 10.1093/protein/gzh084. [DOI] [PubMed] [Google Scholar]
- 43.Rice JJ, Schohn A, Bessette PH, Boulware KT, Daugherty PS. Protein Sci. 2006;15:825. doi: 10.1110/ps.051897806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima H, Shimbara N, Shimonishi Y, Mimori T, Niwa S, Saya H. Gene. 2000;260:121. doi: 10.1016/s0378-1119(00)00461-3. [DOI] [PubMed] [Google Scholar]
- 45.Lam KS, Lebl M, Krchnak V. Chem. Rev. 1997;97:411. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 46.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. Nature. 1991;354:82. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 47.Lam KS, Liu R, Miyamoto S, Lehman AL, Tuscano JM. Acc. Chem. Res. 2003;36:370. doi: 10.1021/ar0201299. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Marik J, Lam KS. J. Am. Chem. Soc. 2002;124:7678. doi: 10.1021/ja026421t. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Peng L, Liu R, Gill SS, Lam KS. J. Comb. Chem. 2005;7:197. doi: 10.1021/cc049887b. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal S, Janssen S, Wadkins RM, Harden JL, Denmeade SR. Biomaterials. 2005;26:6077. doi: 10.1016/j.biomaterials.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal S, Harden JL, Denmeade SR. Bioconjugate Chem. 2006;17:335. doi: 10.1021/bc0502659. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Tan PH, Zhang Y, Pei D. J. Comb. Chem. 2009;11:604. doi: 10.1021/cc9000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Peng L, Liu R, Xu B, Lam KS. J. Pept. Res. 2005;65:130. doi: 10.1111/j.1399-3011.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 54.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. J. Am. Chem. Soc. 2008;130:5744. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 55.André S, Maljaars CEP, Halkes KM, Gabius HJ, Kamerling JP. Bioorg. Med. Chem. 2007;17:793. doi: 10.1016/j.bmcl.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 56.Hintersteiner M, Knox A, Mudd G, Auer M. J. Chem. Biol. 2012;5:63. doi: 10.1007/s12154-011-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumaresan PR, Wang Y, Saunders M, Maeda Y, Liu R, Wang X, Lam KS. ACS Comb. Sci. 2011;13:259. doi: 10.1021/co100069t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cha J, Lim J, Zheng Y, Tan S, Ang YL, Oon J, Ang MW, Ling J, Bode M, Lee SS. J. Lab. Autom. 2012;17:186. doi: 10.1177/2211068211433503. [DOI] [PubMed] [Google Scholar]
- 59.Dooley CT, Houghten RA. Methods Mol. Biol. 1998;87:13. doi: 10.1385/0-89603-392-9:13. [DOI] [PubMed] [Google Scholar]
- 60.Dooley CT, Houghten RA. Life Sci. 1993;52:1509. doi: 10.1016/0024-3205(93)90113-h. [DOI] [PubMed] [Google Scholar]
- 61.Pinilla C, Appel JR, Blanc P, Houghten RA. Biotechniques. 1992;13:901. [PubMed] [Google Scholar]
- 62.Rothman RB, Baumann MH, Dersch CM, Appel J, Houghten RA. Synapse. 1999;33:239. doi: 10.1002/(SICI)1098-2396(19990901)33:3<239::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Kherman P, Kaye FJ, Lindeman N, Boggon TJ, Naoko K, Sasaki H, Jujji Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. Science. 2004;304:1497. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 64.Kim EY, Bang JY, Chang SI, Kang IC. Biochem. Biophys.Res. Commun. 2008;377:1288. doi: 10.1016/j.bbrc.2008.10.166. [DOI] [PubMed] [Google Scholar]
- 65.Denholt CL, Hansen PR, Pedersen N, Poulsen HS, Gillings N, Kjaer A. Biopolymers. 2009;91:201. doi: 10.1002/bip.21117. [DOI] [PubMed] [Google Scholar]
- 66.Liu R, Enstrom AM, Lam KS. Exp. Hematol. 2003;31:11. doi: 10.1016/s0301-472x(02)01008-1. [DOI] [PubMed] [Google Scholar]
- 67.Pasqualini R, Ruoslahti E. Nature. 1996;380:364. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 68.Barry MA, Dower WJ, Johnston SA. Nat. Med. 1996;2:299. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 69.Goodson RJ, Doyle MV, Kaufman SE, Rosenberg S. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7129. doi: 10.1073/pnas.91.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao P, Grabinski T, Gao C, Skinner RS, Giambernardi T, Su Y, Hudson E, Resau J, Gross M, Vande Woude GF, Hay R, Cao B. Clin. Cancer Res. 2007;13:6049. doi: 10.1158/1078-0432.CCR-07-0035. [DOI] [PubMed] [Google Scholar]
- 71.Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W. Nat. Med. 2001;7:1249. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 72.Brown KC. Curr. Pharm. Des. 2010;16:1040. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, Wistuba Ii, Roth JA, Mcguire MJ, Brown KC. CancerRes. 2007;67:5889. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 74.Bausch D, Thomas S, Mino-Kenudson M, Fernandez-Del CC, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA. Clin. Cancer Res. 2011;17:302. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samli KN, Mcguire MJ, Newgard CB, Johnston SA, Brown KC. Diabetes. 2005;54:2103. doi: 10.2337/diabetes.54.7.2103. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Liu Y, Teesalu T, Sugahara KN, Kotamrajua VR, Adams JD, Ferguson BS, Gong Q, Oh SS, Csordas AT, Cho M, Ruoslahti E, Xiao Y, Soh HT. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6909. doi: 10.1073/pnas.1014753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. Nat. Med. 2002;8:121. [Google Scholar]
- 78.’t Hoen PAC, Jirka SMG, Ten Broeke BR, Schultes EA, Aguilera B, Pang KH, Heemskerk H, Aartsma-Rus A, VanOmmen GJ, Den Dunnen JT. Anal. Biochem. 2012;421:622. doi: 10.1016/j.ab.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Dias-Neto E, Nunes DN, Giordano RJ, Sun J, Botz GH, Yang K, Setubal JC, Pasqualini R, Arap W. PLoS One. 2009;4:e8338. doi: 10.1371/journal.pone.0008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ernst A, Gfeller D, Kan Z, Seshagiri S, Kim PM, Bader GD, Sidhu SS. Mol. Biosyst. 2010;6:1782. doi: 10.1039/c0mb00061b. [DOI] [PubMed] [Google Scholar]
- 81.Ernst A, Sazinsky SL, Hui S, Currell B, Dharsee M, Seshagiri S, Bader GD, Sidhu SS. Sci. Signaling. 2009;2:ra50. doi: 10.1126/scisignal.2000416. [DOI] [PubMed] [Google Scholar]
- 82.Matochko WL, Chu K, Jin B, Lee SW, Whitesides GM, Derda R. Methods. 2012;58:47. doi: 10.1016/j.ymeth.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Ryvkin A, Ashkenazy H, Smelyanski L, Kaplan G, Penn O, Weiss-Ottolenghi Y, Privman E, Ngam PB, Woodward JE, May GD, Bell C, Pupko T, Gershoni JM, Ensoli B. PLoS One. 2012;7:1. doi: 10.1371/journal.pone.0041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dane KY, Chan LA, Rice JJ, Daugherty PS. J. Immunol.Methods. 2006;309:120. doi: 10.1016/j.jim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 85.Pennington ME, Lam KS, Cress AE. Mol. Diversity. 1996;2:19. doi: 10.1007/BF01718696. [DOI] [PubMed] [Google Scholar]
- 86.Aina OH, Sroka TC, Chen ML, Lam KS. Biopolymers. 2002;66:184. doi: 10.1002/bip.10257. [DOI] [PubMed] [Google Scholar]
- 87.Lee Y, Kang DK, Chang SI, Han MH, Kang IC. J.Biomol. Screening. 2004;9:687. doi: 10.1177/1087057104268125. [DOI] [PubMed] [Google Scholar]
- 88.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Ca-Cancer J. Clin. 2012;62:220. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 89.Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US. Molecules. 2009;14:4892. doi: 10.3390/molecules14124892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thapa N, Kim S, So IS, Lee BH, Kwon IC, Choi K, Kim IS. J. Cell. Mol. Med. 2008;12:1649. doi: 10.1111/j.1582-4934.2008.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burtea C, Laurent S, Lancelot E, Ballet S, Murariu O, Rousseaux O, Port M, Vander Elst L, Corot C, Muller RN. Mol.Pharmaceutics. 2009;6:1903. doi: 10.1021/mp900106m. [DOI] [PubMed] [Google Scholar]
- 92.Shao R, Xiong C, Wen X, Gelovani J, Li C. Mol. Imaging. 2007;6:417. [PubMed] [Google Scholar]
- 93.Laumonier C, Segers J, Laurent S, Michel A, Coppeé F, Belayew A, Elst LV, Muller RN. J. Biomol. Screening. 2006;11:537. doi: 10.1177/1087057106288220. [DOI] [PubMed] [Google Scholar]
- 94.Pero SC, Shukla GS, Armstrong AL, Peterson D, Fuller SP, Godin K, Kingsley-Richards SL, Weaver DL, Bond J, Krag DN. Int. J. Cancer. 2004;111:951. doi: 10.1002/ijc.20306. [DOI] [PubMed] [Google Scholar]
- 95.Shukla GS, Krag DN. Protein Eng. Des. Sel. 2010;23:431. doi: 10.1093/protein/gzq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karasseva NG, Glinsky VV, Chen NX, Komatireddy R, Quinn TP. J. Protein Chem. 2002;21:287. doi: 10.1023/a:1019749504418. [DOI] [PubMed] [Google Scholar]
- 97.Kumar SR, Quinn TP, Deutscher SL. Clin. Cancer Res. 2007;13:6070. doi: 10.1158/1078-0432.CCR-07-0160. [DOI] [PubMed] [Google Scholar]
- 98.Deutscher SL, Figueroa SD, Kumar SR. J. Labelled Compd.Radiopharm. 2009;52:583. doi: 10.1002/jlcr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar SR, Gallazzi FA, Ferdani R, Anderson CJ, Quinn TP, Deutscher SL. Cancer Biother. Radiopharm. 2010;25:693. doi: 10.1089/cbr.2010.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Houimel M, Schneider P, Terskikh A, Mach JP. Int. J.Cancer. 2001;92:748. doi: 10.1002/1097-0215(20010601)92:5<748::aid-ijc1258>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 101.Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, Xu Y, Gu J. FASEB J. 2005;19:1978. doi: 10.1096/fj.05-4058com. [DOI] [PubMed] [Google Scholar]
- 102.Song S, Liu D, Peng J, Sun Y, Li Z, Gu JR, Xu Y. Int. J.Pharm. 2008;363:155. doi: 10.1016/j.ijpharm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Y, Meyers JD, Agnes RS, Doane TL, Kenney ME, Broome AM, Burda C, Basilion JP. Small. 2011:2301. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohno M, Horibe T, Haramoto M, Yano Y, Ohara K, Nakajima O, Matsuzaki K, Kawakami K. Eur. J. Cancer. 2011;47:773. doi: 10.1016/j.ejca.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 105.Abourbeh G, Shir A, Mishani E, Ogris M, Rödl W, Wagner E, Levitzki A. IUBMB Life. 2012;64:324. doi: 10.1002/iub.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dejesus O. Curr. Radiopharm. 2012;5:15. doi: 10.2174/1874471011205010015. [DOI] [PubMed] [Google Scholar]
- 107.Schafer A, Pahnke A, Schaffert D, Van Weerden W, DeRidder C, Rodl W, Vetter A, Spitzweg C, R K, Wagner E, Ogris M. Hum. Gene Ther. 2011;22:1463. doi: 10.1089/hum.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Mol. Ther. 2012;21:185. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mickler FM, Möckl L, Ruthardt N, Ogris M, Wagner E, Bräuchle C. Nano Lett. 2012;12:3417. doi: 10.1021/nl300395q. [DOI] [PubMed] [Google Scholar]
- 110.Su JL, Lai KP, Chen CA, Yang CY, Chen PS, Chang CC, Chou CH, Hu CL, Kuo ML, Hsieh CY, Wei LH. Cancer Res. 2005;65:4827. doi: 10.1158/0008-5472.CAN-05-0188. [DOI] [PubMed] [Google Scholar]
- 111.Koivunen E, Wang B, Ruoslahti E. Biotechnology. 1995;13:265. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 112.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 113.Koivunen E, Gay DA, Ruoslahti E. J. Biol. Chem. 1993;268:20205. [PubMed] [Google Scholar]
- 114.Murayama O, Nishida H, Sekiguchi K. J. Biochem. 1996;120:445. doi: 10.1093/oxfordjournals.jbchem.a021431. [DOI] [PubMed] [Google Scholar]
- 115.Kraft S, Diedenbach B, Mehta R, Jonczyk A, Luckenbach GA, Goodman SL. J. Biol. Chem. 1999;274:1979. doi: 10.1074/jbc.274.4.1979. [DOI] [PubMed] [Google Scholar]
- 116.Pameijer CRJ, Navanjo A, Meechoovet B, Wagner JR, Aguilar B, Wright CL, Chang WC, Brown CE, Jensen MC. Cancer Gene Ther. 2007;14:91. doi: 10.1038/sj.cgt.7700993. [DOI] [PubMed] [Google Scholar]
- 117.Koolpe M, Dail M, Pasquale EB. J. Biol. Chem. 2002;277:46974. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 118.Wang S, Noberini R, Stebbins JL, Das S, Zhang Z, Wu B, Mitra S, Billet S, Fernandez A, Bhowmick NA, Kitada S, Pasquale EB, Fisher PB, Pellecchia M. Clin. Cancer Res. 2013;19:128. doi: 10.1158/1078-0432.CCR-12-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scarberry KE, Dickerson EB, Mcdonald JF, Zhang ZJ. J. Am. Chem. Soc. 2008;130:10258. doi: 10.1021/ja801969b. [DOI] [PubMed] [Google Scholar]
- 120.Scarberry KE, Mezencev R, Mcdonald JF. Nanomedicine. 2011;6:69. doi: 10.2217/nnm.10.103. [DOI] [PubMed] [Google Scholar]
- 121.Blackburn WH, Dickerson EB, Smith MH, Mcdonald JF, Lyon LA. Bioconjugate Chem. 2009;20:960. doi: 10.1021/bc800547c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van Geer MA, Bakker CT, Koizumi N, Mizuguchi H, Wesseling JG, Oude Elferink RP, Bosma PJ. World J.Gastroenterol. 2009;15:2754. doi: 10.3748/wjg.15.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koolpe M, Burgess R, Dail M, Pasquale EB. J. Biol. Chem. 2005;280:17301. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 124.You J, Zhang R, Xiong C, Zhong M, Melancon M, Gupta S, Nick AM, Sood AK, Li C. Cancer Res. 2012;72:4777. doi: 10.1158/0008-5472.CAN-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong C, Huang M, Zhang R, Song S, Lu W, Flores L, Gelovani J, Li C. J. Nucl. Med. 2011;52:241. doi: 10.2967/jnumed.110.081943. [DOI] [PubMed] [Google Scholar]
- 126.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Nat. Biotechnol. 1999;17:768. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 127.Medina OP, Soderlund T, Laakkonen LJ, Tuominen EK, Koivunen E, Kinnunen PK. Cancer Res. 2001;61:3978. [PubMed] [Google Scholar]
- 128.Penate Medina O, Haikola M, Tahtinen M, Simpura I, Kaukinen S, Valtanen H, Zhu Y, Kuosmanen S, Cao W, Reunanen J, Nurminen T, Saris PE, Smith-Jones P, Bradbury M, Larson S, Kairemo K. J. Drug Delivery. 2011;2011:160515. doi: 10.1155/2011/160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai J, Wei R, Cheng J. J. Biomed. Biotechnol. 2008;2008:564969. doi: 10.1155/2008/564969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zou Y, Chen Y, Jiang Y, Gao J, Gu J. Cancer Res. 2007;67:7295. doi: 10.1158/0008-5472.CAN-06-3920. [DOI] [PubMed] [Google Scholar]
- 131.Medina OP, Kairemo K, Valtanen H, Kangasniemi A, Kaukinen S, Ahonen I, Permi P, Annila A, Sneck M, Holopainen JM, Karonen SL, Kinnunen PK, Koivunen E. Anticancer Res. 2005;25:33. [PubMed] [Google Scholar]
- 132.Sprague JE, Li WP, Liang K, Achilefu S, Anderson CJ. Nucl. Med. Biol. 2006;33:227. doi: 10.1016/j.nucmedbio.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 133.Heikkila P, Suojanen J, Pirila E, Vaananen A, Koivunen E, Sorsa T, Salo T. Int. J. Cancer. 2006;118:2202. doi: 10.1002/ijc.21540. [DOI] [PubMed] [Google Scholar]
- 134.Rusckowski M, Gupta S, Liu G, Dou S, Hnatowich DJ. Cancer Biother. Radiopharm. 2007;22:564. doi: 10.1089/cbr.2006.307. [DOI] [PubMed] [Google Scholar]
- 135.Chen L, Wang Y, Cheng D, Dou S, Liu X, Liu G, Hnatowich DJ, Rusckowski M. Nucl. Med. Commun. 2011;32:920. doi: 10.1097/MNM.0b013e328348fc64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L, Wang Y, Liu X, Dou S, Liu G, Hnatowich DJ, Rusckowski M. Cancer Lett. 2008;272:122. doi: 10.1016/j.canlet.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Devemy E, Blaschuk OW. Peptides. 2009;30:1539. doi: 10.1016/j.peptides.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 138.Devemy E, Blaschuk OW. Peptides. 2008;29:1853. doi: 10.1016/j.peptides.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 139.Askoxylakis V, Garcia-Boy R, Rana S, Kramer S, Hebling U, Mier W, Altmann A, Markert A, Debus J, Haberkorn U. PLoSOne. 2010;5:e15962. doi: 10.1371/journal.pone.0015962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Carcinogenesis. 2005;26:309. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]
- 141.Kumar SR, Deutscher SL. J. Nucl. Med. 2008;49:796. doi: 10.2967/jnumed.107.048751. [DOI] [PubMed] [Google Scholar]
- 142.Deutscher SL, Figueroa SD, Kumar SR. Nucl. Med. Biol. 2009;36:137. doi: 10.1016/j.nucmedbio.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 143.Aggarwal S, Singh P, Topaloglu O, Isaacs JT, Denmeade SR. Cancer Res. 2006;66:9171. doi: 10.1158/0008-5472.CAN-06-1520. [DOI] [PubMed] [Google Scholar]
- 144.Sun J, Zhang C, Li X. J. Peking Univ. Health Sci. 2008;40:457. [Google Scholar]
- 145.Qin X, Wan Y, Li M, Xue X, Wu S, Zhang C, You Y, Wang W, Jiang C, Liu Y, Zhu W, Ran Y, Zhang Z, Han W, Zhang Y. J. Biochem. 2007;142:79. doi: 10.1093/jb/mvm109. [DOI] [PubMed] [Google Scholar]
- 146.Peletskaya EN, Glinsky VV, Glinsky GV, Deutscher SL, Quinn TP. J. Mol. Biol. 1997;270:374. doi: 10.1006/jmbi.1997.1107. [DOI] [PubMed] [Google Scholar]
- 147.Landon LA, Peletskaya EN, Glinsky VV, Karasseva N, Quinn TP, Deutscher SL. J. Protein Chem. 2003;22:193. doi: 10.1023/a:1023483232397. [DOI] [PubMed] [Google Scholar]
- 148.Landon LA, Zou J, Deutscher SL. Mol. Diversity. 2004;8:35. doi: 10.1023/b:modi.0000006897.40575.41. [DOI] [PubMed] [Google Scholar]
- 149.Kumar SR, Gallazzi FA, Quinn TP, Deutscher SL. J.Nucl. Med. 2011;52:1819. doi: 10.2967/jnumed.111.093716. [DOI] [PubMed] [Google Scholar]
- 150.Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP. Cancer Res. 2000;60:2584. [PubMed] [Google Scholar]
- 151.Kelly KA, Setlur SR, Ross R, Anbazhagan R, Waterman P, Rubin MA, Weissleder R. Cancer Res. 2008;68:2286. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Du B, Qian M, Zhou Z, Wang P, Wang L, Zhang X, Wu M, Zhang P, Mei B. Biochem. Biophys. Res. Commun. 2006;342:956. doi: 10.1016/j.bbrc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 153.Zhan T, Li P, Bi S, Dong B, Song H, Hui R, Wang L. J.Nanosci. Nanotechnol. 2012;12:7198. doi: 10.1166/jnn.2012.6502. [DOI] [PubMed] [Google Scholar]
- 154.Jiang YQ, Wang HR, Li HP, Hao HJ, Zheng YL, Gu J. Mol. Med. 2006;12:81. doi: 10.2119/2006-00011.Jiang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lo A, Lin CT, Wu HC. Mol. Cancer Ther. 2008;7:579. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 156.Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman CL, Brinker CJ, Caldeira Jdo C, Chackerian B, Wharton W, Peabody DS. ACS Nano. 2011;5:5729. doi: 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim Y, Lillo AM, Steiniger SC, Liu Y, Ballatore C, Anichini A, Mortarini R, Kaufmann GF, Zhou B, Felding-Habermann B, Janda KD. Biochemistry. 2006;45:9434. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 158.Yoneda Y, Steiniger SC, Capkova K, Mee JM, Liu Y, Kaufmann GF, Janda KD. Bioorg. Med. Chem. Lett. 2008;18:1632. doi: 10.1016/j.bmcl.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eriksson F, Culp WD, Massey R, Egevad L, Garland D, Persson MA, Pisa P. Cancer Immunol. Immunother. 2007;56:677. doi: 10.1007/s00262-006-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsuo AL, Tanaka AS, Juliano MA, Rodrigues EG, Travassos LR. J. Mol. Med. 2010;88:1255. doi: 10.1007/s00109-010-0671-9. [DOI] [PubMed] [Google Scholar]
- 161.Staquicini FI, Tandle A, Libutti SK, Sun J, Zigler M, Bar-Eli M, Aliperti F, Perez EC, Gershenwald JE, Mariano M, Pasqualini R, Arap W, Lopes JD. Cancer Res. 2008;68:8419. doi: 10.1158/0008-5472.CAN-08-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang C, Liu XY, Rehemtulla A, Lawrence TS. Int. J.Radiat. Oncol. Biol. Phys. 2005;62:1497. doi: 10.1016/j.ijrobp.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 163.Romanov VI, Durand DB, Petrenko VA. Prostate. 2001;47:239. doi: 10.1002/pros.1068. [DOI] [PubMed] [Google Scholar]
- 164.Zitzmann S, Mier W, Schad A, Kinscherf R, Askoxylakis V, Kramer S, Altmann A, Eisenhut M, Haberkorn U. Clin. CancerRes. 2005;11:139. [PubMed] [Google Scholar]
- 165.Askoxylakis V, Zitzmann-Kolbe S, Zoller F, Altmann A, Markert A, Rana S, Marr A, Mier W, Debus J, Haberkorn U. Molecules. 2011;16:1559. doi: 10.3390/molecules16021559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Min K, Jo H, Song K, Cho M, Chun YS, Jon S, Kim WJ, Ban C. Biomaterials. 2011;32:2124. doi: 10.1016/j.biomaterials.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 167.Jayanna PK, Bedi D, Deinnocentes P, Bird RC, Petrenko VA. Protein Eng. Des. Sel. 2010;23:423. doi: 10.1093/protein/gzq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jayanna PK, Bedi D, Gillespie JW, Deinnocentes P, Wang T, Torchilin VP, Bird RC, Petrenko VA. Nanomedicine. 2010;6:538. doi: 10.1016/j.nano.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu S, Guo X, Xie H, Du Y, Pan Y, Shi Y, Wang J, Hong L, Han S, Zhang D, Huang D, Zhang K, Bai F, Jiang H, Zhai H, Nie Y, Wu K, Fan D. Biochem. Biophys. Res. Commun. 2006;341:964. doi: 10.1016/j.bbrc.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 170.Bai F, Liang J, Wang J, Shi Y, Zhang K, Liang S, Hong L, Zhai H, Lu Y, Han Y, Yin F, Wu K, Fan D. J. Mol. Med. 2007;85:169. doi: 10.1007/s00109-006-0115-8. [DOI] [PubMed] [Google Scholar]
- 171.Liang S, Lin T, Ding J, Pan Y, Dang D, Guo C, Zhi M, Zhao P, Sun L, Hong L, Shi Y, Yao L, Liu J, Wu K, Fan D. J.Mol. Med. 2006;84:764. doi: 10.1007/s00109-006-0064-2. [DOI] [PubMed] [Google Scholar]
- 172.Kelly KA, Jones DA. Neoplasia. 2003;5:437. doi: 10.1016/s1476-5586(03)80046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Cancer Res. 2004;64:6247. doi: 10.1158/0008-5472.CAN-04-0817. [DOI] [PubMed] [Google Scholar]
- 174.Rasmussen UB, Schreiber V, Schultz H, Mischler F, Schughart K. Cancer Gene Ther. 2002;9:606. doi: 10.1038/sj.cgt.7700476. [DOI] [PubMed] [Google Scholar]
- 175.Rittner K, Schreiber V, Erbs P, Lusky M. Cancer Gene Ther. 2007;14:509. doi: 10.1038/sj.cgt.7701036. [DOI] [PubMed] [Google Scholar]
- 176.Zhang Y, Chen J, Hu Z, Hu D, Pan Y, Ou S, Liu G, Yin X, Zhao J, Ren L, Wang J. J. Biomol. Screening. 2007;12:429. doi: 10.1177/1087057106299164. [DOI] [PubMed] [Google Scholar]
- 177.Odermatt A, Audige A, Frick C, Vogt B, Frey BM, Frey FJ, Mazzucchelli L. J. Am. Soc. Nephrol. 2001;12:308. doi: 10.1681/ASN.V122308. [DOI] [PubMed] [Google Scholar]
- 178.Hong FD, Clayman GL. Cancer Res. 2000;60:6551. [PubMed] [Google Scholar]
- 179.Bao L, Gorin MA, Zhang M, Ventura AC, Pomerantz WC, Merajver SD, Teknos TN, Mapp AK, Pan Q. CancerRes. 2009;69:5829. doi: 10.1158/0008-5472.CAN-08-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nothelfer EM, Zitzmann-Kolbe S, Garcia-Boy R, Kramer S, Herold-Mende C, Altmann A, Eisenhut M, Mier W, Haberkorn U. J. Nucl. Med. 2009;50:426. doi: 10.2967/jnumed.108.058123. [DOI] [PubMed] [Google Scholar]
- 181.Lee TY, Wu HC, Tseng YL, Lin CT. Cancer Res. 2004;64:8002. doi: 10.1158/0008-5472.CAN-04-1948. [DOI] [PubMed] [Google Scholar]
- 182.Ivanenkov VV, Felici F, Menon AG. Biochim. Biophys.Acta,Gene Struct. Expression. 1999;1448:463. doi: 10.1016/s0167-4889(98)00163-3. [DOI] [PubMed] [Google Scholar]
- 183.Shadidi M, Sioud M. FASEB J. 2003;17:256. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- 184.Wang XF, Birringer M, Dong LF, Veprek P, Low P, Swettenham E, Stantic M, Yuan LH, Zobalova R, Wu K, Ledvina M, Ralph SJ, Neuzil J. Cancer Res. 2007;67:3337. doi: 10.1158/0008-5472.CAN-06-2480. [DOI] [PubMed] [Google Scholar]
- 185.Orbán E, Manea M, Marquadt A, Bánóczi Z, CsíK G, Fellinger E, Bősze S, Hudecz F. Bioconjugate Chem. 2011;22:2154. doi: 10.1021/bc2004236. [DOI] [PubMed] [Google Scholar]
- 186.Luo H, Yang J, Jin H, Huang C, Fu J, Yang F, Gong H, Zeng S, Luo Q, Zhang Z. FASEB J. 2011;25:1865. doi: 10.1096/fj.10-174318. [DOI] [PubMed] [Google Scholar]
- 187.Jie L, Cai L, Wang L, Ying X, Yu R, Zhang M, Du Y. Int. J. Nanomed. 2012;7:3981. doi: 10.2147/IJN.S33593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haglund E, Seale-Goldsmith M, Dhawan D, Stewart J, Ramos-Vara J, Cooper C, Reece L, Husk T, Bergstrom D, Knapp D, Leary J. In: Colloidal Quantum Dots for BiomedicalApplications III, Proceedings of SPIE. Osinski M, Jovin T, Yamamoto K, editors. Vol. 6866. Bellingham, WA: SPIE; 2008. [Google Scholar]
- 189.Wang T, D’souza GG, Bedi D, Fagbohun OA, Potturi LP, Papahadjopoulos-Sternberg B, Petrenko VA, Torchilin VP. Nanomedicine. 2010;5:563. doi: 10.2217/nnm.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fagbohun OA, Bedi D, Grabchenko NI, Deinnocentes PA, Bird RC, Petrenko VA. Protein Eng. Des. Sel. 2012;25:271. doi: 10.1093/protein/gzs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang T, Petrenko VA, Torchilin VP. Mol. Pharmaceutics. 2010;7:1007. doi: 10.1021/mp1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bedi D, Musacchio T, Fagbohun OA, Gillespie JW, Deinnocentes P, Bird RC, Bookbinder L, Torchilin VP, Petrenko VA. Nanomedicine. 2011;7:315. doi: 10.1016/j.nano.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang J, Spring H, Schwab M. Cancer Lett. 2001;171:153. doi: 10.1016/s0304-3835(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 194.Shahin M, Ahmed S, Kaur K, Lavasanifar A. Biomaterials. 2011;32:5123. doi: 10.1016/j.biomaterials.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 195.Askoxylakis V, Zitzmann S, Mier W, Graham K, Kramer S, Von Wegner F, Fink RH, Schwab M, Eisenhut M, Haberkorn U. Clin. Cancer Res. 2005;11:6705. doi: 10.1158/1078-0432.CCR-05-0432. [DOI] [PubMed] [Google Scholar]
- 196.Askoxylakis V, Mier W, Zitzmann S, Ehemann V, Zhang J, Kramer S, Beck C, Schwab M, Eisenhut M, Haberkorn U. J.Nucl. Med. 2006;47:981. [PubMed] [Google Scholar]
- 197.Samoylova TI, Petrenko VA, Morrison NE, Globa LP, Baker HJ, Cox NR. Mol. Cancer Ther. 2003;2:1129. [PubMed] [Google Scholar]
- 198.Spear MA, Breakefield XO, Beltzer J, Schuback D, Weissleder R, Pardo FS, Ladner R. Cancer Gene Ther. 2001;8:506. doi: 10.1038/sj.cgt.7700334. [DOI] [PubMed] [Google Scholar]
- 199.Wu C, Lo SL, Boulaire J, Hong ML, Beh HM, Leung DS, Wang S. J. Controlled Release. 2008;130:140. doi: 10.1016/j.jconrel.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 200.Ho IA, Hui KM, Lam PY. Peptides. 2010;31:644. doi: 10.1016/j.peptides.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 201.Ho IA, Lam PY, Hui KM. Hum. Gene Ther. 2004;15:719. doi: 10.1089/1043034041648372. [DOI] [PubMed] [Google Scholar]
- 202.Beck S, Jin X, Yin J, Kim SH, Lee NK, Oh SY, Jin X, Kim MK, Kim EB, Son JS, Kim SC, Nam DH, Kim S-H, Kang SK, Kim H, Choi YJ. Biomaterials. 2011;32:8518. doi: 10.1016/j.biomaterials.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 203.Loi M, Di Paolo D, Soster M, Brignole C, Bartolini A, Emionite L, Sun J, Becherini P, Curnis F, Petretto A, Sani M, Gori A, Milanese M, Gambini C, Longhi R, Cilli M, Allen TM, Bussolino F, Arap W, Pasqualini R, Corti A, Ponzoni M, Marchiò S, Pastorino F. J. Controlled Release. 2013;170:233. doi: 10.1016/j.jconrel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Robinson P, Stuber D, Deryckere F, Tedbury P, Lagrange M, Orfanoudakis G. J. Mol. Recognit. 2005;18:175. doi: 10.1002/jmr.723. [DOI] [PubMed] [Google Scholar]
- 205.Morpurgo M, Kirschner M, Radu A. J. Biochem. Biophys.Methods. 2002;52:31. doi: 10.1016/s0165-022x(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 206.Zhang L, Yin G, Yan D, Wei Y, Ma C, Huang Z, Liao X, Yao Y, Chen X, Hao B. Biotechnol. Lett. 2011;33:1729. doi: 10.1007/s10529-011-0634-4. [DOI] [PubMed] [Google Scholar]
- 207.Vodnik M, Zager U, Strukelj B, Lunder M. Molecules. 2011;16:790. doi: 10.3390/molecules16010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bockmann M, Drosten M, Putzer BM. J. Gene Med. 2005;7:179. doi: 10.1002/jgm.648. [DOI] [PubMed] [Google Scholar]
- 209.Zitzmann S, Kramer S, Mier W, Hebling U, Altmann A, Rother A, Berndorff D, Eisenhut M, Haberkorn U. J. Nucl. Med. 2007;48:965. doi: 10.2967/jnumed.106.036699. [DOI] [PubMed] [Google Scholar]
- 210.Witt H, Hajdin K, Iljin K, Greiner O, Niggli FK, Schafer BW, Bernasconi M. Int. J. Cancer. 2009;124:2026. doi: 10.1002/ijc.24170. [DOI] [PubMed] [Google Scholar]
- 211.Sun X, Niu G, Yan Y, Yang M, Chen K, Ma Y, Chan N, Shen B, Chen X. Clin. Cancer Res. 2010;16:4268. doi: 10.1158/1078-0432.CCR-10-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mcguire MJ, Samli KN, Chang YC, Brown KC. Exp.Hematol. 2006;34:443. doi: 10.1016/j.exphem.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 213.Mcguire MJ, Samli KN, Johnston SA, Brown KC. J.Mol. Biol. 2004;342:171. doi: 10.1016/j.jmb.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 214.Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, O’brien S, Koivunen E, Arap W, Pasqualini R, Nakayama H, Kuniyasu A. J. Biol. Chem. 2008;283:11752. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Karjalainen K, Jaalouk DE, Bueso-Ramos CE, Zurita AJ, Kuniyasu A, Eckhardt BL, Marini FC, Lichtiger B, O’brien S, Kantarjian HM, Cortes JE, Koivunen E, Arap W, Pasqualini R. Blood. 2011;117:920. doi: 10.1182/blood-2010-05-282921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Choi JH, Lee WK, Han SH, Ha S, Ahn SM, Kang JS, Choi YJ, Yun CH. Int. Immunopharmacol. 2008;8:852. doi: 10.1016/j.intimp.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 217.Jager S, Jahnke A, Wilmes T, Adebahr S, Vogtle FN, Delima-Hahn E, Pfeifer D, Berg T, Lubbert M, Trepel M. Leukemia. 2007;21:411. doi: 10.1038/sj.leu.2404548. [DOI] [PubMed] [Google Scholar]
- 218.Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Cancer Lett. 2003;202:219. doi: 10.1016/j.canlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 219.Zhou X, Chang YC, Oyama T, Mcguire MJ, Brown KC. J. Am. Chem. Soc. 2004;126:15656. doi: 10.1021/ja0446496. [DOI] [PubMed] [Google Scholar]
- 220.Oyama T, Rombel IT, Samli KN, Zhou X, Brown KC. Biosens. Bioelectron. 2006;21:1867. doi: 10.1016/j.bios.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 221.Zang L, Shi L, Guo J, Pan Q, Wu W, Pan X, Wang J. Cancer Lett. 2009;281:64. doi: 10.1016/j.canlet.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 222.Tu X, Zang L, Lan D, Liang W. Mol. Med. Rep. 2009;2:1005. doi: 10.3892/mmr_00000206. [DOI] [PubMed] [Google Scholar]
- 223.Li S, Mcguire MJ, Lin M, Liu YH, Oyama T, Sun X, Brown KC. Mol. Cancer Ther. 2009;8:1239. doi: 10.1158/1535-7163.MCT-08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li S, Gray BP, Mcguire MJ, Brown KC. Bioorg. Med.Chem. 2011;19:5480. doi: 10.1016/j.bmc.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Guan H, Mcguire MJ, Li S, Brown KC. BioconjugateChem. 2008;19:1813. doi: 10.1021/bc800154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guthi JS, Yang SG, Huang G, Li S, Khemtong C, Kessinger CW, Peyton M, Minna JD, Brown KC, Gao J. Mol.Pharmaceutics. 2010;7:32. doi: 10.1021/mp9001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gray BP, Li S, Brown KC. Bioconjugate Chem. 2013;24:85. doi: 10.1021/bc300498d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huang G, Zhang C, Li S, Khemtong C, Yang SG, Tian R, Minna JD, Brown KC, Gao J. J. Mater. Chem. 2009;19:6367. doi: 10.1039/b902358e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chang DK, Lin CT, Wu CH, Wu HC. PLoS One. 2009;4:e4171. doi: 10.1371/journal.pone.0004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Florea BI, Molenaar TJ, Bot I, Michon IN, Kuiper J, Van Berkel TJ, Junginger HE, Biessen EA, Borchard G. J. DrugTargeting. 2003;11:383. doi: 10.1080/10611860310001642389. [DOI] [PubMed] [Google Scholar]
- 231.He X, Na MH, Kim JS, Lee GY, Park JY, Hoffman AS, Nam JO, Han SE, Sim GY, Oh YK, Kim IS, Lee B-H. Mol. Pharmaceutics. 2011;8:430. doi: 10.1021/mp100266g. [DOI] [PubMed] [Google Scholar]
- 232.Li M, Anastassiades CP, Joshi B, Komarck CM, Piraka C, Elmunzer BJ, Turgeon DK, Johnson TD, Appelman H, Beer DG, Wang TD. Gastroenterology. 2010;139:1472. doi: 10.1053/j.gastro.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kubo N, Akita N, Shimizu A, Kitahara H, Parker AL, Miyagawa S. J. Drug Targeting. 2008;16:396. doi: 10.1080/10611860802088796. [DOI] [PubMed] [Google Scholar]
- 234.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, Cardo-Vila M, Giordano RJ, Jaalouk DE, Ozawa MG, Moya CA, Souza GR, Staquicini FI, Kunyiasu A, Scudiero DA, Holbeck SL, Sausville EA, Arap W, Pasqualini R. Cancer Res. 2006;66:34. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 235.Shukla GS, Krag DN. Oncol. Rep. 2005;13:757. [PubMed] [Google Scholar]
- 236.Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Nat. Med. 2008;14:454. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee SM, Lee EJ, Hong HY, Kwon MK, Kwon TH, Choi JY, Park RW, Kwon TG, Yoo ES, Yoon GS, Kim IS, Ruoslahti E, Lee BH. Mol. Cancer Res. 2007;5:11. doi: 10.1158/1541-7786.MCR-06-0069. [DOI] [PubMed] [Google Scholar]
- 238.Jia X, Yu Q, Zhang Z, Yang X. OncoTargets Ther. 2012;5:85. doi: 10.2147/OTT.S31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, Depinho RA, Mahmood U, Weissleder R. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang W, Sui Y, Budha A, Zheng J, Sun X, Hou Y, Wang T, Lu S. World J. Gastroenterol. 2012;18:2053. doi: 10.3748/wjg.v18.i17.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cao Q, Liu S, Niu G, Chen K, Yan Y, Liu Z, Chen X. Amino Acids. 2010;41:1103. doi: 10.1007/s00726-010-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Passarella RJ, Zhou L, Phillips JG, Wu H, Hallahan DE, Diaz R. Clin. Cancer Res. 2009;15:6421. doi: 10.1158/1078-0432.CCR-09-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Han Z, Fu A, Wang H, Diaz R, Geng L, Onishko H, Hallahan DE. Nat. Med. 2008;14:343. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 244.Chang DK, Chiu CY, Kuo SY, Lin WC, Lo A, Wang YP, Li PC, Wu HC. J. Biol. Chem. 2009;284:12905. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Estephan E, Dao J, Saab M, Panayotov I, Martin M, Larroque C, Gergely C, Cuisinier FJG, Levallois B. Biomed.Tech. 2012;57:481. doi: 10.1515/bmt-2011-0109. [DOI] [PubMed] [Google Scholar]
- 246.Wölcke J, Weinhold E. Nucleosides, Nucleotides Nucleic Acids. 2001;20:1239. doi: 10.1081/NCN-100002526. [DOI] [PubMed] [Google Scholar]
- 247.Estephan E, Larroque C, Bec N, Martineau P, Cuisinier FJG, Cloitre T, Gergely C. Biotechnol. Bioeng. 2009;104:1121. doi: 10.1002/bit.22478. [DOI] [PubMed] [Google Scholar]
- 248.Murai KK, Nguyen LN, Koolpe M, Mclennan R, Krull CE, Pasquale EB. Mol. Cell. Neurosci. 2003;24:1000. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 249.Lee TY, Lin CT, Kuo SY, Chang DK, Wu HC. Cancer Res. 2007;67:10958. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
- 250.Bussolati B, Grange C, Tei L, Deregibus MC, Ercolani M, Aime S, Camussi G. J. Mol. Med. 2007;85:897. doi: 10.1007/s00109-007-0184-3. [DOI] [PubMed] [Google Scholar]
- 251.Cardo-Vila M, Zurita AJ, Giordano RJ, Sun J, Rangel R, Guzman-Rojas L, Anobom CD, Valente AP, Almeida FC, Lahdenranta J, Kolonin MG, Arap W, Pasqualini R. PLoS One. 2008;3:e3452. doi: 10.1371/journal.pone.0003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zurita AJ, Troncoso P, Cardo-Vila M, Logothetis CJ, Pasqualini R, Arap W. Cancer Res. 2004;64:435. doi: 10.1158/0008-5472.can-03-2675. [DOI] [PubMed] [Google Scholar]
- 253.Pasqualini R, Moeller BJ, Arap W. Semin. Thromb.Hemostasis. 2010;36:343. doi: 10.1055/s-0030-1253456. [DOI] [PubMed] [Google Scholar]
- 254.Krag DN, Shukla GS, Shen GP, Pero S, Ashikaga T, Fuller S, Weaver DL, Burdette-Radoux S, Thomas C. Cancer Res. 2006;66:7724. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- 255.Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. Nat.Med. 2002;8:751. doi: 10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- 256.Laakkonen P, Akerman ME, Biliran H, Yang M, Ferrer F, Karpanen T, Hoffman RM, Ruoslahti E. Proc. Natl. Acad. Sci.U.S.A. 2004;101:9381. doi: 10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang L, Giraudo E, Hoffman JA, Hanahan D, Ruoslahti E. Cancer Res. 2006;66:5696. doi: 10.1158/0008-5472.CAN-05-3876. [DOI] [PubMed] [Google Scholar]
- 258.Laakkonen P, Zhang L, Ruoslahti E. Ann. N. Y. Acad. Sci. 2008;1131:37. doi: 10.1196/annals.1413.003. [DOI] [PubMed] [Google Scholar]
- 259.Akita N, Maruta F, Seymour LW, Kerr DJ, Parker AL, Asai T, Oku N, Nakayama J, Miyagawa S. Cancer Sci. 2006;97:1075. doi: 10.1111/j.1349-7006.2006.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhi M, Wu KC, Dong L, Hao ZM, Deng TZ, Hong L, Liang SH, Zhao PT, Qiao TD, Wang Y, Xu X, Fan DM. Cancer Biol. Ther. 2004;3:1232. doi: 10.4161/cbt.3.12.1223. [DOI] [PubMed] [Google Scholar]
- 261.Chen B, Cao S, Zhang Y, Wang X, Liu J, Hui X, Wan Y, Du W, Wang L, Wu K, Fan D. BMC Cell Biol. 2009;10:63. doi: 10.1186/1471-2121-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hui X, Han Y, Liang S, Liu Z, Liu J, Hong L, Zhao L, He L, Cao S, Chen B, Yan K, Jin B, Chai N, Wang J, Wu K, Fan D. J. Controlled Release. 2008;131:86. doi: 10.1016/j.jconrel.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 263.Chen K, Sun X, Niu G, Ma Y, Yap LP, Hui X, Wu K, Fan D, Conti PS, Chen X. Mol. Imaging Biol. 2011;14:96. doi: 10.1007/s11307-011-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen K, Yap LP, Park R, Hui X, Wu K, Fan D, Chen X, Conti PS. Amino Acids. 2011;42:1329. doi: 10.1007/s00726-010-0827-5. [DOI] [PubMed] [Google Scholar]
- 265.Hariri G, Yan H, Wang H, Han Z, Hallahan DE. Clin.Cancer Res. 2010;16:4968. doi: 10.1158/1078-0432.CCR-10-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang H, Yan H, Fu A, Han M, Hallahan D, Han Z. PLoS One. 2010;5:e12051. doi: 10.1371/journal.pone.0012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lowery A, Onishko H, Hallahan DE, Han Z. J. ControlledRelease. 2011;150:117. doi: 10.1016/j.jconrel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hariri G, Wellons MS, Morris WH, 3rd, Lukehart CM, Hallahan DE. Ann. Biomed. Eng. 2011;39:946. doi: 10.1007/s10439-010-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Reference deleted on revision. [Google Scholar]
- 270.Liu J, Chu L, Wang Y, Duan Y, Feng L, Yang C, Wang L, Kong D. Int. J. Nanomed. 2011;6:59. doi: 10.2147/IJN.S14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sun L, Chu T, Wang Y, Wang X. Acta Biochim. Biophys.Sin. 2007;39:624. doi: 10.1111/j.1745-7270.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 272.Newton JR, Kelly KA, Mahmood U, Weissleder R, Deutscher SL. Neoplasia. 2006;8:772. doi: 10.1593/neo.06331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Newton-Northup JR, Figueroa SD, Deutscher SL. Comb.Chem. High Throughput Screening. 2011;14:9. doi: 10.2174/1386207311107010009. [DOI] [PubMed] [Google Scholar]
- 274.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Cancer Cell. 2009;16:510. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Science. 2010;328:1031. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Agemy L, Friedmann-Morvinski D, Kotamraju VR, Roth L, Sugahara KN, Girard OM, Mattrey RF, Verma IM, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17450. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mintz PJ, Cardo-Vila M, Ozawa MG, Hajitou A, Rangel R, Guzman-Rojas L, Christianson DR, Arap MA, Giordano RJ, Souza GR, Easley J, Salameh A, Oliviero S, Brentani RR, Koivunen E, Arap W, Pasqualini R. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2182. doi: 10.1073/pnas.0807543105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16157. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Haspel N, Zanuy D, Nussinov R, Teesalu T, Ruoslahti E, Aleman C. Biochemistry. 2011;50:1755. doi: 10.1021/bi101662j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Corti A, Curnis F, Arap W, Pasqualini R. Blood. 2008;112:2628. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Cancer Res. 2000;60:722. [PMC free article] [PubMed] [Google Scholar]
- 282.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. CancerRes. 2003;63:7400. [PubMed] [Google Scholar]
- 283.Pastorino F, Di Paolo D, Piccardi F, Nico B, Ribatti D, Daga A, Baio G, Neumaier CE, Brignole C, Loi M, Marimpietri D, Pagnan G, Cilli M, Lepekhin EA, Garde SV, Longhi R, Corti A, Allen TM, Wu JJ, Ponzoni M. Clin. Cancer Res. 2008;14:7320. doi: 10.1158/1078-0432.CCR-08-0804. [DOI] [PubMed] [Google Scholar]
- 284.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Nat. Biotechnol. 2000;18:1185. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 285.Curnis F, Sacchi A, Corti A. J. Clin. Invest. 2002;110:475. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zarovni N, Monaco L, Corti A. Hum. Gene Ther. 2004;15:373. doi: 10.1089/104303404322959524. [DOI] [PubMed] [Google Scholar]
- 287.Sacchi A, Gasparri A, Curnis F, Bellone M, Corti A. Cancer Res. 2004;64:7150. doi: 10.1158/0008-5472.CAN-04-1445. [DOI] [PubMed] [Google Scholar]
- 288.Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Clin. Cancer Res. 2006;12:175. doi: 10.1158/1078-0432.CCR-05-1147. [DOI] [PubMed] [Google Scholar]
- 289.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Med. Res. Rev. 2012;32:1078. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 290.Meng J, Yan Z, Wu J, Li L, Xue X, Li M, Li W, Hao Q, Wan Y, Qin X, Zhang C, You Y, Han W, Zhang Y. Cytotherapy. 2007;9:60. doi: 10.1080/14653240601094322. [DOI] [PubMed] [Google Scholar]
- 291.Zhang B, Gao B, Dong S, Zhang Y, Wu Y. Regul. Toxicol.Pharmacol. 2011;60:73. doi: 10.1016/j.yrtph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 292.Meng J, Ma N, Yan Z, Han W, Zhang Y. J. Biochem. 2006;140:299. doi: 10.1093/jb/mvj152. [DOI] [PubMed] [Google Scholar]
- 293.Kim JW, Lee HS. Int. J. Mol. Med. 2004;14:529. [PubMed] [Google Scholar]
- 294.Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC, 3rd, Restel BH, Ozawa MG, Moya CA, Rangel R, Sun Y, Zaoui K, Schmidt M, Von Kalle C, Weitzman MD, Gelovani JG, Pasqualini R, Arap W. Cell. 2006;125:385. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 295.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, Sawaya R, Curiel DT, Yung WK, Lang FF. J. Natl. CancerInst. 2003;95:652. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 296.Line B, Mitra A, Nan A, Ghandehari H. J. Nucl. Med. 2005;46:1552. [PubMed] [Google Scholar]
- 297.Fogal V, Zhang L, Krajewski S, Ruoslahti E. Cancer Res. 2008;68:7210. doi: 10.1158/0008-5472.CAN-07-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Herringson TP, Altin JG. Int. J. Pharm. 2011;411:206. doi: 10.1016/j.ijpharm.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 299.Park JH, Von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Proc. Natl. Acad. Sci.U.S.A. 2010;107:981. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Staquicini FI, Ozawa MG, Moya CA, Driessen WH, Barbu EM, Nishimori H, Soghomonyan S, Flores LG, 2nd, Liang X, Paolillo V, Alauddin MM, Basilion JP, Furnari FB, Bogler O, Lang FF, Aldape KD, Fuller GN, Hook M, Gelovani JG, Sidman RL, Cavenee WK, Pasqualini R, Arap W. J. Clin. Invest. 2011;121:161. doi: 10.1172/JCI44798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Karmali PP, Kotamraju VR, Kastantin M, Black M, Missirlis D, Tirrell M, Ruoslahti E. Nanomedicine. 2009;5:73. doi: 10.1016/j.nano.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhang F, Niu G, Lin X, Jacobson O, Ma Y, Eden HS, He Y, Lu G, Chen X. Amino Acids. 2011;42:2343. doi: 10.1007/s00726-011-0976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Makela AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. J. Virol. 2006;80:6603. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Makela AR, Enback J, Laakkonen JP, Vihinen-Ranta M, Laakkonen P, Oker-Blom C. J. Gene Med. 2008;10:1019. doi: 10.1002/jgm.1222. [DOI] [PubMed] [Google Scholar]
- 305.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12617. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J, Fogal V, Sailor MJ, Ruoslahti E, Bhatia SN. Bioconjugate Chem. 2008;19:1570. doi: 10.1021/bc800077y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sharma G, Karmali P, Ramirez M, Xie H, Kotamraju VR, Ruoslahti E, Smith JW. NSTI-Nanotech. Vol. 3. Anaheim, CA; Austin, TX: Nano Science and Technology Insitute (NSTI); 2010. p. 382. [Google Scholar]
- 308.Lempens EH, Merkx M, Tirrell M, Meijer EW. Bioconjugate Chem. 2011;22:397. doi: 10.1021/bc100403e. [DOI] [PubMed] [Google Scholar]
- 309.Hamzah J, Kotamraju VR, Seo JW, Agemy L, Fogal V, Mahakian LM, Peters D, Roth L, Gagnon MKJ, Ferrara KW, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7154. doi: 10.1073/pnas.1104540108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Uchida M, Kosuge H, Terashima M, Willits DA, Liepold LO, Young MJ, Mcconnell MV, Douglas T. ACS Nano. 2011;5:2493. doi: 10.1021/nn102863y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Böckmann M, Drosten M, Pützer BM. J. Gene Med. 2005;7:179. doi: 10.1002/jgm.648. [DOI] [PubMed] [Google Scholar]
- 312.Hoffman JA, Giraudo E, Singh M, Zhang L, Inoue M, Porkka K, Hanahan D, Ruoslahti E. Cancer Cell. 2003;4:383. doi: 10.1016/s1535-6108(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 313.Agemy L, Sugahara KN, Kotamraju VR, Gujraty K, Girard OM, Kono Y, Mattrey RF, Park JH, Sailor MJ, Jimenez AI, Cativiela C, Zanuy D, Sayago FJ, Aleman C, Nussinov R, Ruoslahti E. Blood. 2010;116:2847. doi: 10.1182/blood-2010-03-274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Numata K, Reagan MR, Goldstein RH, Rosenblatt M, Kaplan DL. Bioconjugate Chem. 2011;22:1605. doi: 10.1021/bc200170u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Joyce JA, Laakkonen P, Bernasconi M, Bergers G, Ruoslahti E, Hanahan D. Cancer Cell. 2003;4:393. doi: 10.1016/s1535-6108(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 316.Hamzah J, Nelson D, Moldenhauer G, Arnold B, Hammerling GJ, Ganss R. J. Clin. Invest. 2008;118:1691. doi: 10.1172/JCI33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Miller SJ, Joshi BP, Feng Y, Gaustad A, Fearon ER, Wang TD. PLoS One. 2011;6:e17384. doi: 10.1371/journal.pone.0017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Elahi SF, Miller SJ, Joshi B, Wang TD. Biomed. Opt.Express. 2011;2:981. doi: 10.1364/BOE.2.000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Li ZJ, Wu WKK, Ng SSM, Yu L, Li HT, Wong CCM, Wu YC, Zhang L, Ren SX, Sun XG, Chan KM, Cho CH. J. Controlled Release. 2010;148:292. doi: 10.1016/j.jconrel.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 320.Passarella RJ, Spratt DE, Van Der Ende AE, Phillips JG, Wu H, Sathiyakumar V, Zhou L, Hallahan DE, Harth E, Diaz R. Cancer Res. 2010;70:4550. doi: 10.1158/0008-5472.CAN-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Du B, Han H, Wang Z, Kuang L, Wang L, Yu L, Wu M, Zhou Z, Qian M. Mol. Cancer Res. 2010;8:135. doi: 10.1158/1541-7786.MCR-09-0339. [DOI] [PubMed] [Google Scholar]
- 322.Wang W, Ke S, Kwon S, Yallampalli S, Cameron AG, Adams KE, Mawad ME, Sevick-Muraca EM. Bioconjugate Chem. 2007;18:397. doi: 10.1021/bc0602679. [DOI] [PubMed] [Google Scholar]
- 323.Li W, Lei P, Yu B, Wu S, Peng J, Zhao X, Zhu H, Kirschfink M, Shen G. Acta Biochim. Biophys. Sin. 2008;40:443. doi: 10.1111/j.1745-7270.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 324.Dane KY, Gottstein C, Daugherty PS. Mol. Cancer Res. 2009;8:1312. doi: 10.1158/1535-7163.MCT-08-1105. [DOI] [PubMed] [Google Scholar]
- 325.Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, Zhu T, Ma X, Liu R, Xu G, Meng L, Lu Y, Zhou J, Ma D. Clin.Cancer Res. 2008;14:5494. doi: 10.1158/1078-0432.CCR-08-0233. [DOI] [PubMed] [Google Scholar]
- 326.Zitzmann S, Kramer S, Mier W, Mahmut M, Fleig J, Altmann A, Eisenhut M, Haberkorn U. J. Nucl. Med. 2005;46:782. [PubMed] [Google Scholar]
- 327.Brown CK, Modzelewski RA, Johnson CS, Wong MK. Ann. Surg. Oncol. 2000;7:743. doi: 10.1007/s10434-000-0743-0. [DOI] [PubMed] [Google Scholar]
- 328.Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, Villanueva FS. Cancer Res. 2005;65:533. [PubMed] [Google Scholar]
- 329.Lam KS, Lou Q, Zhao ZG, Smith J, Chen ML, Pleshko E, Salmon SE. Biomed. Pept. Proteins Nucleic Acids. 1995;1:205. [PubMed] [Google Scholar]
- 330.Xiao W, Wang Y, Lau EY, Luo J, Yao N, Shi C, Meza L, Tseng H, Maeda Y, Kumaresan P, Liu R, Lightstone FC, Takada Y, Lam KS. Mol. Cancer Ther. 2010;9:2714. doi: 10.1158/1535-7163.MCT-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rathinam R, Alahari S. Cancer Metastasis Rev. 2010;29:223. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 332.Yao N, Xiao W, Wang X, Marik J, Park SH, Takada Y, Lam KS. J. Med. Chem. 2009;52:126. doi: 10.1021/jm801062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Xiao W, Yao N, Peng L, Liu R, Lam KS. Eur. J. Nucl.Med. Mol. Imaging. 2009;36:94. doi: 10.1007/s00259-008-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Park SI, Renil M, Vikstrom B, Amro N, Song LW, Xu BL, Lam KS. Pept. Res. Ther. 2002;8:171. [Google Scholar]
- 335.Aina OH, Marik J, Liu R, Lau DH, Lam KS. Mol.Cancer Ther. 2005;4:806. doi: 10.1158/1535-7163.MCT-05-0029. [DOI] [PubMed] [Google Scholar]
- 336.Aina OH, Marik J, Gandour-Edwards R, Lam KS. Mol.Imaging. 2005;4:439. doi: 10.2310/7290.2005.05169. [DOI] [PubMed] [Google Scholar]
- 337.Xiao K, Li Y, Lee JS, Gonik AM, Dong T, Fung G, Sanchez E, Xing L, Cheng HR, Luo J, Lam KS. Cancer Res. 2012;72:2100. doi: 10.1158/0008-5472.CAN-11-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lau D, Guo L, Liu R, Marik J, Lam K. Lung Cancer. 2006;52:291. doi: 10.1016/j.lungcan.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 339.Mikawa M, Wang H, Guo L, Liu R, Marik J, Takada Y, Lam K, Lau D. Mol. Cancer Ther. 2004;3:1329. [PubMed] [Google Scholar]
- 340.Deroock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, Cress AE. Cancer Res. 2001;61:3308. [PubMed] [Google Scholar]
- 341.Sroka TC, Pennington ME, Cress AE. Carcinogenesis. 2006;27:1748. doi: 10.1093/carcin/bgl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nair RR, Emmons MF, Cress AE, Argilagos RF, Lam K, Kerr WT, Wang HG, Dalton WS, Hazlehurst LA. Mol.Cancer Ther. 2009;8:2441. doi: 10.1158/1535-7163.MCT-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhang H, Aina OH, Lam KS, De Vere White R, Evans C, Henderson P, Lara PN, Wang X, Bassuk JA, Pan CX. Urol.Oncol. 2012;30:635. doi: 10.1016/j.urolonc.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Denholt CL, Binderup T, Stockhausen MT, Poulsen HS, Spang-Thomsen M, Hansen PR, Gillings N, Kjaer A. Nucl.Med. Biol. 2011;38:509. doi: 10.1016/j.nucmedbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 345.Choi Y, Kim E, Lee Y, Han MH, Kang IC. Proteomics. 2010;10:72. doi: 10.1002/pmic.200900146. [DOI] [PubMed] [Google Scholar]
- 346.Bang JY, Kim EY, Kang DK, Chang SI, Han MH, Baek KH, Kang IC. Mol. Cell. Proteomics. 2011;10:M110 005264. doi: 10.1074/mcp.M110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Baek SH, Seo JK, Chae CB, Suh PG, Ryu SH. J.Biol. Chem. 1996;271:8170. doi: 10.1074/jbc.271.14.8170. [DOI] [PubMed] [Google Scholar]
- 348.Le Y, Gong W, Li B, Dunlop NM, Shen W, Su SB, Ye RD, Wang JM. J. Immunol. 1999;163:6777. [PubMed] [Google Scholar]
- 349.Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. J. Biol. Chem. 2001;276:21585. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 350.Seo JK, Choi SY, Kim Y, Baek SH, Kim KT, Chae CB, Lambeth JD, Suh PG, Ryu SH. J. Immunol. 1997;158:1895. [PubMed] [Google Scholar]
- 351.Ruoslahti E. Annu. Rev. Cell Dev. Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 352.Koivunen E, Gay DA, Ruoslahti E. J. Biol. Chem. 1993;268:20205. [PubMed] [Google Scholar]
- 353.Koivunen E, Wang B, Ruoslahti E. J. Cell Biol. 1994;124:373. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Curnis F, Cattaneo A, Longhi R, Sacchi A, Gasparri AM, Pastorino F, Di Matteo P, Traversari C, Bachi A, Ponzoni M, Rizzardi GP, Corti A. J. Biol. Chem. 2010;285:9114. doi: 10.1074/jbc.M109.044297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Curnis F, Longhi R, Crippa L, Cattaneo A, Dondossola E, Bachi A, Corti A. J. Biol. Chem. 2006;281:36466. doi: 10.1074/jbc.M604812200. [DOI] [PubMed] [Google Scholar]
- 356.Galagudza M. In: Novel Strategies in Ischemic Heart Disease. Lakshmanadoss U, editor. Rijeka, Croatia: InTech; 2012. p. 253. [Google Scholar]
- 357.Scott RC, Rosano JM, Ivanov Z, Wang B, Chong PL-G, Issekutz AC, Crabbe DL, Kiani MF. FASEB J. 2009;23:3361. doi: 10.1096/fj.08-127373. [DOI] [PubMed] [Google Scholar]
- 358.Zahid M, Phillips BE, Albers SM, Giannoukakis N, Watkins SC, Robbins PD. PLoS One. 2010;5:e12252. doi: 10.1371/journal.pone.0012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang L, Hoffman JA, Ruoslahti E. Circulation. 2005;112:1601. doi: 10.1161/CIRCULATIONAHA.104.529537. [DOI] [PubMed] [Google Scholar]
- 360.Greig JA, Shirley R, Graham D, Denby L, Dominiczak AF, Work LM, Baker AH. J. Cardiovasc. Pharmacol. 2010;56:642. doi: 10.1097/FJC.0b013e3181f8f19f. [DOI] [PubMed] [Google Scholar]
- 361.Kanki S, Jaalouk DE, Lee S, Yu AY, Gannon J, Lee RT. J. Mol. Cell. Cardiol. 2011;50:841. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Emery AE. Lancet. 2002;359:687. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 363.Seow Y, Yin H, Wood MJ. Peptides. 2010;31:1873. doi: 10.1016/j.peptides.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 364.Libby P. Nature. 2002;420:868. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 365.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Circ. Res. 2005;96:327. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 366.Hong HY, Lee HY, Kwak W, Yoo J, Na MH, So IS, Kwon TH, Park HS, Huh S, Oh GT, Kwon IC, Kim IS, Lee BH. J. Cell. Mol. Med. 2008;12:2003. doi: 10.1111/j.1582-4934.2008.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Cai D, Xaymardan M, Holm JM, Zheng J, Kizer JR, Edelberg JM. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H463. doi: 10.1152/ajpheart.00144.2003. [DOI] [PubMed] [Google Scholar]
- 368.Cai D, Holm JM, Duignan IJ, Zheng J, Xaymardan M, Chin A, Ballard VL, Bella JN, Edelberg JM. Physiol. Genomics. 2006;24:191. doi: 10.1152/physiolgenomics.00165.2005. [DOI] [PubMed] [Google Scholar]
- 369.Nicol CG, Denby L, Lopez-Franco O, Masson R, Halliday CA, Nicklin SA, Kritz A, Work LM, Baker AH. FEBS Lett. 2009;583:2100. doi: 10.1016/j.febslet.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 370.Zhang H, Kusunose J, Kheirolomoom A, Seo JW, Qi J, Watson KD, Lindfors HA, Ruoslahti E, Sutcliffe JL, Ferrara KW. Biomaterials. 2008;29:1976. doi: 10.1016/j.biomaterials.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Nam HY, Mcginn A, Kim PH, Kim SW, Bull DA. Biomaterials. 2010;31:8081. doi: 10.1016/j.biomaterials.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Li Z, Fan J, Zhao W, Jin L, Ma L. J. Pept. Sci. 2011;17:771. doi: 10.1002/psc.1401. [DOI] [PubMed] [Google Scholar]
- 373.Samoylova TI, Smith BF. Muscle Nerve. 1999;22:460. doi: 10.1002/(sici)1097-4598(199904)22:4<460::aid-mus6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 374.Yu CY, Yuan Z, Cao Z, Wang B, Qiao C, Li J, Xiao X. Gene Ther. 2009;16:953. doi: 10.1038/gt.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wang Q, Yin H, Camelliti P, Betts C, Moulton H, Lee H, Saleh AF, Gait MJ, Wood MJ. J. Gene Med. 2010;12:354. doi: 10.1002/jgm.1446. [DOI] [PubMed] [Google Scholar]
- 376.Yin H, Lu Q, Wood M. Mol. Ther. 2008;16:38. doi: 10.1038/sj.mt.6300329. [DOI] [PubMed] [Google Scholar]
- 377.Kelly KA, Nahrendorf M, Yu AM, Reynolds F, Weissleder R. Mol. Imaging Biol. 2006;8:201. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 378.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Circulation. 2006;114:1504. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 379.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. JACC: Cardiovasc. Imaging. 2009;2:1213. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Liu C, Bhattacharjee G, Boisvert W, Dilley R, Edgington T. Am. J. Pathol. 2003;163:1859. doi: 10.1016/S0002-9440(10)63545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.White K, Buning H, Kritz A, Janicki H, Mcvey J, Perabo L, Murphy G, Odenthal M, Work LM, Hallek M, Nicklin SA, Baker AH. Gene Ther. 2008;15:443. doi: 10.1038/sj.gt.3303077. [DOI] [PubMed] [Google Scholar]
- 382.Houston P, Goodman J, Lewis A, Campbell CJ, Braddock M. FEBS Lett. 2001;492:73. doi: 10.1016/s0014-5793(01)02191-3. [DOI] [PubMed] [Google Scholar]
- 383.Kim JH, Bae SM, Na MH, Shin H, Yang YJ, Min KH, Choi KY, Kim K, Park RW, Kwon IC, Lee BH, Hoffman AS, Kim IS. J. Controlled Release. 2012;157:493. doi: 10.1016/j.jconrel.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 384.Wu XL, Kim JH, Koo H, Bae SM, Shin H, Kim MS, Lee BH, Park RW, Kim IS, Choi K, Kwon IC, Kim K, Lee DS. Bioconjugate Chem. 2010;21:208. doi: 10.1021/bc9005283. [DOI] [PubMed] [Google Scholar]
- 385.Park K, Hong HY, Moon HJ, Lee BH, Kim IS, Kwon IC, Rhee K. J. Controlled Release. 2008;128:217. doi: 10.1016/j.jconrel.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 386.Lee GY, Kim JH, Oh GT, Lee BH, Kwon IC, Kim IS. J. Controlled Release. 2011;155:211. doi: 10.1016/j.jconrel.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 387.Michon IN, Hauer AD, Von Der Thusen JH, Molenaar TJ, Van Berkel TJ, Biessen EA, Kuiper J. Biochim. Biophys. Acta. 2002;1591:87. doi: 10.1016/s0167-4889(02)00254-9. [DOI] [PubMed] [Google Scholar]
- 388.Work LM, Nicklin SA, Brain NJ, Dishart KL, VonSeggern DJ, Hallek M, Buning H, Baker AH. Mol. Ther. 2004;9:198. doi: 10.1016/j.ymthe.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 389.Banchereau J, Palucka AK. Nat. Rev. Immunol. 2005;5:296. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 390.Frick C, Odermatt A, Zen K, Mandell KJ, Edens H, Portmann R, Mazzucchelli L, Jaye DL, Parkos CA. Eur. J.Immunol. 2005;35:3610. doi: 10.1002/eji.200425914. [DOI] [PubMed] [Google Scholar]
- 391.Faham A, Altin JG. Int. J. Cancer. 2010;129:1391. doi: 10.1002/ijc.25810. [DOI] [PubMed] [Google Scholar]
- 392.Chamarthy SP, Jia L, Kovacs JR, Anderson KR, Shen H, Firestine SM, Meng WS. Mol. Immunol. 2004;41:741. doi: 10.1016/j.molimm.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 393.Mcguire MJ, Sykes KF, Samli KN, Timares L, Barry MA, Stemke-Hale K, Tagliaferri F, Logan M, Jansa K, Takashima A, Brown KC, Johnston SA. DNA Cell Biol. 2004;23:742. doi: 10.1089/dna.2004.23.742. [DOI] [PubMed] [Google Scholar]
- 394.Berntzen G, Andersen JT, Ustgard K, Michaelsen TE, Mousavi SA, Qian JD, Kristiansen PE, Lauvrak V, Sandlie I. J.Biol. Chem. 2009;284:1126. doi: 10.1074/jbc.M803584200. [DOI] [PubMed] [Google Scholar]
- 395.Clark MA, Jepson MA, Hirst BH. Adv. Drug Delivery Rev. 2001;50:81. doi: 10.1016/s0169-409x(01)00149-1. [DOI] [PubMed] [Google Scholar]
- 396.Higgins LM, Lambkin I, Donnelly G, Byrne D, Wilson C, Dee J, Smith M, O’mahony DJ. Pharm. Res. 2004;21:695. doi: 10.1023/b:pham.0000022418.80506.9a. [DOI] [PubMed] [Google Scholar]
- 397.Kim SH, Seo KW, Kim J, Lee KY, Jang YS. J.Immunol. 2010;185:5787. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 398.Kim SH, Lee KY, Kim J, Park SM, Park BK, Jang YS. Mol. Cell. 2006;21:244. [PubMed] [Google Scholar]
- 399.Richards JL, Abend JR, Miller ML, Chakraborty-Sett S, Dewhurst S, Whetter LE. Eur. J. Biochem. 2003;270:2287. doi: 10.1046/j.1432-1033.2003.03596.x. [DOI] [PubMed] [Google Scholar]
- 400.Herringson TP, Altin JG. J. Controlled Release. 2009;139:229. doi: 10.1016/j.jconrel.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 401.Kelly KA, Clemons PA, Yu AM, Weissleder R. Mol.Imaging. 2006;5:24. [PubMed] [Google Scholar]
- 402.Kim SH, Jung DI, Yang IY, Kim J, Lee KY, Nochi T, Kiyono H, Jang YS. Eur. J. Immunol. 2011;41:3219. doi: 10.1002/eji.201141592. [DOI] [PubMed] [Google Scholar]
- 403.Yoo MK, Kang SK, Choi JH, Park IK, Na HS, Lee HC, Kim EB, Lee NK, Nah JW, Choi YJ, Cho CS. Biomaterials. 2010;31:7738. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 404.Fievez V, Plapied L, Plaideau C, Legendre D, Des Rieux A, Pourcelle V, Freichels H, Jerome C, Marchand J, Preat V, Schneider YJ. Int. J. Pharm. 2010;394:35. doi: 10.1016/j.ijpharm.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 405.Bae YS, Park EY, Kim Y, He R, Ye RD, Kwak JY, Suh PG, Ryu SH. Biochem. Pharmacol. 2003;66:1841. doi: 10.1016/s0006-2952(03)00552-5. [DOI] [PubMed] [Google Scholar]
- 406.Bae YS, Bae H, Kim Y, Lee TG, Suh PG, Ryu SH. Blood. 2001;97:2854. doi: 10.1182/blood.v97.9.2854. [DOI] [PubMed] [Google Scholar]
- 407.Ueberberg S, Schneider S. Regul. Pept. 2010;160:1. doi: 10.1016/j.regpep.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 408.Yao VJ, Ozawa MG, Trepel M, Arap W, Mcdonald DM, Pasqualini R. Am. J. Pathol. 2005;166:625. doi: 10.1016/S0002-9440(10)62283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Lin M, Lubag A, Mcguire MJ, Seliounine SY, Tsyganov EN, Antich PP, Sherry AD, Brown KC, Sun X. Front. Biosci. 2008;13:4558. doi: 10.2741/3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Blevins KS, Jeong JH, Ou M, Brumbach JH, Kim SW. J. Controlled Release. 2012;158:115. doi: 10.1016/j.jconrel.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Flegal KM, Carroll MD, Ogden CL, Curtin LR. JAMA,J. Am. Med. Assoc. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 412.Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. Cell Stem Cell. 2011;9:74. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 413.Nie J, Chang B, Traktuev DO, Sun J, March K, Chan L, Sage EH, Pasqualini R, Arap W, Kolonin MG. Stem Cells. 2008;26:2735. doi: 10.1634/stemcells.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Nat. Med. 2004;10:625. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 415.Barnhart KF, Christianson DR, Hanley PW, Driessen WHP, Bernacky BJ, Baze WB, Wen S, Tian M, Ma J, Kolonin MG, Saha PK, Do KA, Hulvat JF, Gelovani JG, Chan L, Arap W, Pasqualini R. Sci. Transl. Med. 2011;3:108. doi: 10.1126/scitranslmed.3002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Hossen MN, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. J. Controlled Release. 2010;147:261. doi: 10.1016/j.jconrel.2010.07.100. [DOI] [PubMed] [Google Scholar]
- 417.Staquicini FI, Cardo-Vila M, Kolonin MG, Trepel M, Edwards JK, Nunes DN, Sergeeva A, Efstathiou E, Sun J, Almeida NF, Tu SM, Botz GH, Wallace MJ, O’connell DJ, Krajewski S, Gershenwald JE, Molldrem JJ, Flamm AL, Koivunen E, Pentz RD, Dias-Neto E, Setubal JC, Cahill DJ, Troncoso P, Do KA, Logothetis CJ, Sidman RL, Pasqualini R, Arap W. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18637. doi: 10.1073/pnas.1114503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Kim DH, Woods SC, Seeley RJ. Diabetes. 2010;59:907. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Liu J, Wang L, Zhang A, Di W, Zhang X, Wu L, Yu J, Zha J, Lv S, Cheng P, Hu M, Li Y, Qi H, Ding G, Zhong Y. Endocr. J. 2011;58:199. doi: 10.1507/endocrj.k10e-318. [DOI] [PubMed] [Google Scholar]
- 420.De Boer AG, Gaillard PJ. Annu. Rev. Pharmacol. Toxicol. 2007;47:323. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 421.Pardridge WM. Drug Discovery Today. 2007;12:54. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 422.Van Rooy I, Cakir-Tascioglu S, Couraud PO, Romero IA, Weksler B, Storm G, Hennink WE, Schiffelers RM, Mastrobattista E. Pharm. Res. 2010;27:673. doi: 10.1007/s11095-010-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wan XM, Chen YP, Xu WR, Yang WJ, Wen LP. Peptides. 2009;30:343. doi: 10.1016/j.peptides.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 424.Hou ST, Dove M, Anderson E, Zhang J, Mackenzie CR. J. Neurosci. Methods. 2004;138:39. doi: 10.1016/j.jneumeth.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 425.Fabes J, Anderson P, Brennan C, Bolsover S. Eur. J.Neurosci. 2007;26:2496. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 426.Galimberti I, Bednarek E, Donato F, Caroni P. Neuron. 2010;65:627. doi: 10.1016/j.neuron.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 427.Staquicini FI, Dias-Neto E, Li J, Snyder EY, Sidman RL, Pasqualini R, Arap W. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2903. doi: 10.1073/pnas.0813286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Schmidt A, Haas SJ, Hildebrandt S, Scheibe J, Eckhoff B, Racek T, Kempermann G, Wree A, Putzer BM. Stem Cells. 2007;25:2910. doi: 10.1634/stemcells.2007-0238. [DOI] [PubMed] [Google Scholar]
- 429.Hildebrandt S, Schmidt A, Stoll A, Schmitt O, Kohling R, Wree A, Haas SJ, Putzer BM. Brain Struct. Funct. 2010;215:105. doi: 10.1007/s00429-010-0275-8. [DOI] [PubMed] [Google Scholar]
- 430.Ludtke JJ, Sololoff AV, Wong SC, Zhang G, Wolff JA. Drug Delivery. 2007;14:357. doi: 10.1080/10717540601098765. [DOI] [PubMed] [Google Scholar]
- 431.Denby L, Work LM, Seggern DJ, Wu E, Mcvey JH, Nicklin SA, Baker AH. Mol. Ther. 2007;15:1647. doi: 10.1038/sj.mt.6300214. [DOI] [PubMed] [Google Scholar]
- 432.Diaconu I, Denby L, Pesonen S, Cerullo V, Bauerschmitz GJ, Guse K, Rajecki M, Dias JD, Taari K, Kanerva A, Baker AH, Hemminki A. Hum. Gene Ther. 2009;20:611. doi: 10.1089/hum.2008.108. [DOI] [PubMed] [Google Scholar]
- 433.Gelain F, Cigognini D, Caprini A, Silva D, Colleoni B, Donega M, Antonini S, Cohen BE, Vescovi A. Nanoscale. 2012;4:2946. doi: 10.1039/c2nr30220a. [DOI] [PubMed] [Google Scholar]
- 434.Winner B, Kohl Z, Gage FH. Eur. J. Neurosci. 2011;33:1139. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 435.Parent JM. Epilepsy Res. 2002;50:179. doi: 10.1016/s0920-1211(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 436.Kempermann G, Kronenberg G. Biol. Psychiatry. 2003;54:499. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 437.Sun J, Zhang C, Liu G, Liu H, Zhou C, Lu Y, Zhou C, Yuan L, Li X. Clin. Exp. Metastasis. 2012;29:185. doi: 10.1007/s10585-011-9440-6. [DOI] [PubMed] [Google Scholar]
- 438.Morita Y, Mamiya K, Yamamura S, Tamiya E. Biotechnol.Prog. 2006;22:974. doi: 10.1021/bp060112f. [DOI] [PubMed] [Google Scholar]
- 439.Zhao S, Zhao W, Ma L. Peptides. 2010;31:2027. doi: 10.1016/j.peptides.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 440.Lu S, Xu X, Zhao W, Wu W, Yuan H, Shen H, Zhou C, Li LS, Ma L. PLoS One. 2010;5:e12075. doi: 10.1371/journal.pone.0012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zhao W, Yuan H, Xu X, Ma L. J. Biomol. Screening. 2010;15:687. doi: 10.1177/1087057110370997. [DOI] [PubMed] [Google Scholar]
- 442.Caprini A, Silva D, Zanoni I, Cunha C, Volontè C, Vescovi A, Gelain F. New Biotechnol. 2013;30:552. doi: 10.1016/j.nbt.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 443.Shao Z, Zhang X, Pi Y, Wang X, Jia Z, Zhu J, Dai L, Chen W, Yin L, Chen H, Zhou C, Ao Y. Biomaterials. 2012;33:3375. doi: 10.1016/j.biomaterials.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 444.Cui Y, Kim SN, Jones SE, Wissler LL, Naik RR, Mcalpine MC. Nano Lett. 2010;10:4559. doi: 10.1021/nl102564d. [DOI] [PubMed] [Google Scholar]
- 445.Little LE, Dane KY, Daugherty PS, Healy KE, Schaffer DV. Biomaterials. 2011;32:1484. doi: 10.1016/j.biomaterials.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Nowakowski GS, Dooner MS, Valinski HM, Mihaliak AM, Quesenberry PJ, Becker PS. Stem Cells. 2004;22:1030. doi: 10.1634/stemcells.22-6-1030. [DOI] [PubMed] [Google Scholar]
- 447.Pasqualini R, Arap W, Mcdonald DM. Trends Mol. Med. 2002;8:563. doi: 10.1016/s1471-4914(02)02429-2. [DOI] [PubMed] [Google Scholar]
- 448.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. J. Clin. Invest. 1998;102:430. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Trepel M, Arap W, Pasqualini R. Curr. Opin. Chem. Biol. 2002;6:399. doi: 10.1016/s1367-5931(02)00336-8. [DOI] [PubMed] [Google Scholar]
- 450.Giordano RJ, Edwards JK, Tuder RM, Arap W, Pasqualini R. Proc. Am. Thorac. Soc. 2009;6:411. doi: 10.1513/pats.200903-014AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1527. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Kolonin MG, Sun J, Do KA, Vidal CI, Ji Y, Baggerly KA, Pasqualini R, Arap W. FASEB J. 2006;20:979. doi: 10.1096/fj.05-5186fje. [DOI] [PubMed] [Google Scholar]
- 453.Essler M, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2252. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Lee NK, Kim M, Choi JH, Kim EB, Lee HG, Kang SK, Choi YJ. Peptides. 2010;31:2247. doi: 10.1016/j.peptides.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 455.Yang Y, Zizheng W, Tongxin D. J. Biomol. Screening. 2008;13:968. doi: 10.1177/1087057108326537. [DOI] [PubMed] [Google Scholar]
- 456.Oh Y, Mohiuddin I, Sun Y, Putnam JB, Jr, Hong WK, Arap W, Pasqualini R. Chest. 2005;128:596S. doi: 10.1378/chest.128.6_suppl.596S. [DOI] [PubMed] [Google Scholar]
- 457.Mai J, Song S, Rui M, Liu D, Ding Q, Peng J, Xu Y. J.Controlled Release. 2009;139:174. doi: 10.1016/j.jconrel.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 458.Paradis-Bleau C, Lloyd A, Sanschagrin F, Clarke T, Blewett A, Bugg T, Levesque R. BMC Biochem. 2008;9:33. doi: 10.1186/1471-2091-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Carter DM, Gagnon JN, Damlaj M, Mandava S, Makowski L, Rodi DJ, Pawelek PD, Coulton JW. J. Mol. Biol. 2006;357:236. doi: 10.1016/j.jmb.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 460.Park JP, Cropek DM, Banta S. Biotechnol. Bioeng. 2010;105:678. doi: 10.1002/bit.22597. [DOI] [PubMed] [Google Scholar]
- 461.Chung WJ, Kwon KY, Song J, Lee SW. Langmuir. 2011;27:7620. doi: 10.1021/la104757g. [DOI] [PubMed] [Google Scholar]
- 462.Wu X, Li Z, Yao M, Wang H, Qu S, Chen X, Li J, Sun Y, Xu Y, Gu J. Acta Biochim. Biophys. Sin. 2008;40:217. doi: 10.1111/j.1745-7270.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 463.Nicklin SA, White SJ, Watkins SJ, Hawkins RE, Baker AH. Circulation. 2000;102:231. doi: 10.1161/01.cir.102.2.231. [DOI] [PubMed] [Google Scholar]
- 464.Nicklin SA, Buening H, Dishart KL, De Alwis M, Girod A, Hacker U, Thrasher AJ, Ali RR, Hallek M, Baker AH. Mol.Ther. 2001;4:174. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- 465.Nicklin SA, Von Seggern DJ, Work LM, Pek DC, Dominiczak AF, Nemerow GR, Baker AH. Mol. Ther. 2001;4:534. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- 466.Parker AL, Fisher KD, Oupicky D, Read ML, Nicklin SA, Baker AH, Seymour LW. J. Drug Targeting. 2005;13:39. doi: 10.1080/10611860400020449. [DOI] [PubMed] [Google Scholar]
- 467.Nicklin SA, Dishart KL, Buening H, Reynolds PN, Hallek M, Nemerow GR, Von Seggern DJ, Baker AH. CancerLett. 2003;201:165. doi: 10.1016/j.canlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 468.White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH. Circulation. 2004;109:513. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- 469.Nicklin SA, White SJ, Nicol CG, Von Seggern DJ, Baker AH. J. Gene Med. 2004;6:300. doi: 10.1002/jgm.526. [DOI] [PubMed] [Google Scholar]
- 470.Hardy B, Raiter A, Weiss C, Kaplan B, Tenenbaum A, Battler A. Peptides. 2007;28:691. doi: 10.1016/j.peptides.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 471.Glaser-Gabay L, Raiter A, Battler A, Hardy B. Microvasc.Res. 2011;82:221. doi: 10.1016/j.mvr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 472.Maruta F, Parker AL, Fisher KD, Murray PG, Kerr DJ, Seymour LW. J. Drug Targeting. 2003;11:53. doi: 10.1080/1061186031000086063. [DOI] [PubMed] [Google Scholar]
- 473.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. J. Biol. Chem. 2008;283:29447. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Morris CJ, Smith MW, Griffiths PC, Mckeown NB, Gumbleton M. J. Controlled Release. 2011;151:83. doi: 10.1016/j.jconrel.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 475.Veleva AN, Cooper SL, Patterson C. Biotechnol. Bioeng. 2007;98:306. doi: 10.1002/bit.21420. [DOI] [PubMed] [Google Scholar]
- 476.Veleva AN, Nepal DB, Frederick CB, Schwab J, Lockyer P, Yuan H, Lalush DS, Patterson C. Molecules. 2011;16:900. doi: 10.3390/molecules16010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Trepel M, Grifman M, Weitzman MD, Pasqualini R. Hum. Gene Ther. 2000;11:1971. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 478.Yan Z, Lu L, Shi J, Bao C, Han W, Wu Y, Zhang Y. Appl. Biochem. Biotechnol. 2006;133:149. doi: 10.1385/abab:133:2:149. [DOI] [PubMed] [Google Scholar]
- 479.Rajotte D, Ruoslahti E. J. Biol. Chem. 1999;274:11593. doi: 10.1074/jbc.274.17.11593. [DOI] [PubMed] [Google Scholar]
- 480.Jarvinen TA, Ruoslahti E. Am. J. Pathol. 2007;171:702. doi: 10.2353/ajpath.2007.061251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Jarvinen TA, Ruoslahti E. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21671. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Urakami T, Järvinen TaH, Toba M, Sawada J, Ambalavanan N, Mann D, Mcmurtry I, Oka M, Ruoslahti E, Komatsu M. Am. J. Pathol. 2011;178:2489. doi: 10.1016/j.ajpath.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Obeid M. Mol. Cancer Ther. 2009;8:2693. doi: 10.1158/1535-7163.MCT-09-0228. [DOI] [PubMed] [Google Scholar]
- 484.Oku N, Asai T, Watanabe K, Kuromi K, Nagatsuka M, Kurohane K, Kikkawa H, Ogino K, Tanaka M, Ishikawa D, Tsukada H, Momose M, Nakayama J, Taki T. Oncogene. 2002;21:2662. doi: 10.1038/sj.onc.1205347. [DOI] [PubMed] [Google Scholar]
- 485.Asai T, Nagatsuka M, Kuromi K, Yamakawa S, Kurohane K, Ogino K, Tanaka M, Taki T, Oku N. FEBS Lett. 2002;510:206. doi: 10.1016/s0014-5793(01)03265-3. [DOI] [PubMed] [Google Scholar]
- 486.Lee CH, Lee MS, Kim SJ, Je YT, Ryu SH, Lee T. Peptides. 2009;30:409. doi: 10.1016/j.peptides.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 487.Lee SJ, Park SG, Chung HM, Choi JS, Kim DD, Sung JH. Int. J. Pept. Res. Ther. 2009;15:281. [Google Scholar]
- 488.Okamoto CT. Adv. Drug Delivery Rev. 1998;29:215. doi: 10.1016/s0169-409x(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 489.Kang SK, Woo JH, Kim MK, Woo SS, Choi JH, Lee HG, Lee NK, Choi YJ. J. Biotechnol. 2008;135:210. doi: 10.1016/j.jbiotec.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 490.Duerr DM, White SJ, Schluesener HJ. J. Virol. Methods. 2004;116:177. doi: 10.1016/j.jviromet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 491.Braathen R, Sandvik A, Berntzen G, Hammerschmidt S, Fleckenstein B, Sandlie I, Brandtzaeg P, Johansen FE, Lauvrak V. J. Biol. Chem. 2006;281:7075. doi: 10.1074/jbc.M508509200. [DOI] [PubMed] [Google Scholar]
- 492.Herman RE, Makienko EG, Prieve MG, Fuller M, Houston ME, Jr, Johnson PH. J. Biomol. Screening. 2007;12:1092. doi: 10.1177/1087057107310216. [DOI] [PubMed] [Google Scholar]
- 493.Jost PJ, Harbottle RP, Knight A, Miller AD, Coutelle C, Schneider H. FEBS Lett. 2001;489:263. doi: 10.1016/s0014-5793(00)02236-5. [DOI] [PubMed] [Google Scholar]
- 494.White AF, Mazur M, Sorscher EJ, Zinn KR, Ponnazhagan S. Hum. Gene Ther. 2008;19:1407. doi: 10.1089/hum.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Vaysse L, Burgelin I, Merlio JP, Arveiler B. Biochim.Biophys. Acta. 2000;1475:369. doi: 10.1016/s0304-4165(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 496.Writer MJ, Marshall B, Pilkington-Miksa MA, Barker SE, Jacobsen M, Kritz A, Bell PC, Lester DH, Tabor AB, Hailes HC, Klein N, Hart SL. J. Drug Targeting. 2004;12:185. doi: 10.1080/10611860410001724459. [DOI] [PubMed] [Google Scholar]
- 497.Irvine SA, Meng QH, Afzal F, Ho J, Wong JB, Hailes HC, Tabor AB, Mcewan JR, Hart SL. Mol. Ther. 2008;16:508. doi: 10.1038/sj.mt.6300381. [DOI] [PubMed] [Google Scholar]
- 498.Wong JB, Grosse S, Tabor AB, Hart SL, Hailes HC. Mol. Biosyst. 2008;4:532. doi: 10.1039/b719782a. [DOI] [PubMed] [Google Scholar]
- 499.Tagalakis AD, Mcanulty RJ, Devaney J, Bottoms SE, Wong JB, Elbs M, Writer MJ, Hailes HC, Tabor AB, O’callaghan C, Jaffe A, Hart SL. Mol. Ther. 2008;16:907. doi: 10.1038/mt.2008.38. [DOI] [PubMed] [Google Scholar]
- 500.Manunta MDI, Mcanulty RJ, Tagalakis AD, Bottoms SE, Campbell F, Hailes HC, Tabor AB, Laurent GJ, O’callaghan C, Hart SL. PLoS One. 2011;6:e26768. doi: 10.1371/journal.pone.0026768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Costantini TW, Putnam JG, Sawada R, Baird A, Loomis WH, Eliceiri BP, Bansal V, Coimbra R. Surgery. 2009;146:206. doi: 10.1016/j.surg.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Kumar S, Sahdev P, Perumal O, Tummala H. Mol.Pharmaceutics. 2012;9:1320. doi: 10.1021/mp200594z. [DOI] [PubMed] [Google Scholar]
- 503.Ardelt PU, Wood CG, Chen L, Mintz PJ, Moya C, Arap MA, Wright KC, Pasqualini R, Arap W. J. Urol. 2003;169:1535. doi: 10.1097/01.ju.0000055477.37115.66. [DOI] [PubMed] [Google Scholar]
- 504.Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, Liu S, Zhang M, Wen LP. Nat. Biotechnol. 2006;24:455. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 505.Lee SJ, Park S, Chung HM, Choi JS, Kim DD, Sung JH. Int. J. Pept. Res. Ther. 2009;15:281. [Google Scholar]
- 506.Li B, Russell SJ, Compaan DM, Totpal K, Marsters SA, Ashkenazi A, Cochran AG, Hymowitz SG, Sidhu SS. J. Mol.Biol. 2006;361:522. doi: 10.1016/j.jmb.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 507.Itahana K, Zhang Y. Cancer Cell. 2008;13:542. doi: 10.1016/j.ccr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Prokop A, Davidson JM. J. Pharm. Sci. 2008;97:3518. doi: 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Van Hensbergen Y, Broxterman HJ, Elderkamp YW, Lankelma J, Beers JC, Heijn M, Boven E, Hoekman K, Pinedo HM. Biochem. Pharmacol. 2002;63:897. doi: 10.1016/s0006-2952(01)00928-5. [DOI] [PubMed] [Google Scholar]
- 510.Meyer-Losic F, Quinonero J, Dubois V, Alluis B, Dechambre M, Michel M, Cailler F, Fernandez AM, Trouet A, Kearsey J. J. Med. Chem. 2006;49:6908. doi: 10.1021/jm0606591. [DOI] [PubMed] [Google Scholar]
- 511.Kratz F, Müller IA, Ryppa C, Warnecke A. ChemMedChem. 2008;3:20. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 512.Jain RK. J. Controlled Release. 1998;53:49. doi: 10.1016/s0168-3659(97)00237-x. [DOI] [PubMed] [Google Scholar]
- 513.Torchilin VP, Lukyanov AN. Drug Discovery Today. 2003;8:259. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 514.Allen TM, Cheng WW, Hare JI, Laginha KM. Anticancer Agents Med. Chem. 2006;6:513. doi: 10.2174/187152006778699121. [DOI] [PubMed] [Google Scholar]
- 515.Torchilin VP. Nat. Rev. Drug Discovery. 2005;4:145. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 516.Owens DE, 3rd, Peppas NA. Int. J. Pharm. 2006;307:93. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 517.Chang HI, Yeh MK. Int. J. Nanomed. 2012;2:49. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Duggan S, Keating G. Drugs. 2011;71:2531. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 519.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Cancer Res. 1994;54:3352. [PubMed] [Google Scholar]
- 520.Vicent MJ, Duncan R. Trends Biotechnol. 2006;24:39. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 521.Sutton D, Nasongkla N, Blanco E, Gao J. Pharm. Res. 2007;24:1029. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 522.Torchilin VP. J. Controlled Release. 2001;73:137. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 523.Matsumura Y, Kataoka K. Cancer Sci. 2009;100:572. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Zhou X, Chang YC, Oyama T, Mcguire MJ, Brown KC. J. Am. Chem. Soc. 2004;126:15656. doi: 10.1021/ja0446496. [DOI] [PubMed] [Google Scholar]
- 525.Garde SV, Forte AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, Wu JJ. Anti-Cancer Drugs. 2007;18:1189. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- 526.Jayanna PK, Torchilin VP, Petrenko VA. Nanomedicine. 2009;5:83. doi: 10.1016/j.nano.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Circ. Res. 2001;89:408. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 528.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Med. Res. Rev. 2012;32:1078. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 529.Van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Cancer Res. 2004;64:4357. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 530.Reddy ST, Swartz MA, Hubbell JA. Trends Immunol. 2006;27:573. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 531.Proudfoot O, Apostolopoulos V, Pietersz GA. Mol.Pharmaceutics. 2007;4:58. doi: 10.1021/mp0601087. [DOI] [PubMed] [Google Scholar]
- 532.Van Broekhoven CL, Altin JG. Int. J. Cancer. 2002;98:63. doi: 10.1002/ijc.10157. [DOI] [PubMed] [Google Scholar]
- 533.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. J. Exp. Med. 2004;199:815. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. Eur. J. Immunol. 2008;38:2263. doi: 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- 535.Larocca D, Burg MA, Jensen-Pergakes K, Ravey EP, Gonzalez AM, Baird A. Curr. Pharm. Biotechnol. 2002;3:45. doi: 10.2174/1389201023378490. [DOI] [PubMed] [Google Scholar]
- 536.Larocca D, Jensen-Pergakes K, Burg MA, Baird A. Mol.Ther. 2001;3:476. doi: 10.1006/mthe.2001.0284. [DOI] [PubMed] [Google Scholar]
- 537.Larocca D, Jensen-Pergakes K, Burg MA, Baird A. Methods Mol. Biol. 2002;185:393. doi: 10.1385/1-59259-241-4:393. [DOI] [PubMed] [Google Scholar]
- 538.Larocca D, Kassner PD, Witte A, Ladner RC, Pierce GF, Baird A. FASEB J. 1999;13:727. doi: 10.1096/fasebj.13.6.727. [DOI] [PubMed] [Google Scholar]
- 539.Larocca D, Witte A, Johnson W, Pierce GF, Baird A. Hum. Gene Ther. 1998;9:2393. doi: 10.1089/hum.1998.9.16-2393. [DOI] [PubMed] [Google Scholar]
- 540.Poul MA, Marks JD. J. Mol. Biol. 1999;288:203. doi: 10.1006/jmbi.1999.2678. [DOI] [PubMed] [Google Scholar]
- 541.Trepel M, Grifman M, Weitzman M, Pasqualini R. Hum.Gene Ther. 2000;11:1971. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 542.Makela A, Matilainen H, White D, Ruoslahti E, Oker-Blom C. J. Virol. 2006;80:6603. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Mizuguchi H, Hayakawa Y. Hum. Gene Ther. 2004;15:1034. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 544.Michelfelder S, Trepel M. Adv. Genet. 2009;67:29. doi: 10.1016/S0065-2660(09)67002-4. [DOI] [PubMed] [Google Scholar]
- 545.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. Nat. Biotechnol. 2003;21:1040. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 546.Perabo L, Buning H, Kofler DM, Ried MU, Girod A, Wendtner CM, Enssle J, Hallek M. Mol. Ther. 2003;8:151. doi: 10.1016/s1525-0016(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 547.Denby L, Work LM, Graham D, Hsu C, Von Seggern DJ, Nicklin SA, Baker AH. Hum. Gene Ther. 2004;15:1054. doi: 10.1089/hum.2004.15.1054. [DOI] [PubMed] [Google Scholar]
- 548.Hajitou A, Lev D, Hannay J, Korchin B, Staquicini F, Soghomonyan S, Alauddin M, Benjamin R, Pollock R, Gelovani J, Pasqualini R, Arap W. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4471. doi: 10.1073/pnas.0712184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Hajitou A, Rangel R, Trepel M, Soghomonyan S, Gelovani J, Alauddin M, Pasqualini R, Arap W. Nat. Protoc. 2007;2:523. doi: 10.1038/nprot.2007.51. [DOI] [PubMed] [Google Scholar]
- 550.Hajitou A, Trepel M, Lilley C, Soghomonyan S, Alauddin M, Marini F, Restel B, Ozawa M, Moya C, Rangle R, Sun Y, Zaoui K, Schmidt M, Von Kalle C, Weitzman M, Gelovani J, Pasqualini R, Arap W. Cell. 2006;125:385. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 551.Soghomonyan S, Hajitou A, Rangel R, Trepel M, Pasqualini R, Arap W, Gelovani J, Alauddin M. Nat. Protoc. 2007;2:416. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 552.Tandle A, Hanna E, Lorang D, Hajitou A, Moya CA, Pasqualini R, Arap W, Adem A, Starker E, Hewitt S, Libutti SK. Cancer. 2009;115:128. doi: 10.1002/cncr.24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Paoloni MC, Tandle A, Mazcko C, Hanna E, Kachala S, Leblanc A, Newman S, Vail D, Henry C, Thamm D, Sorenmo K, Hajitou A, Pasqualini R, Arap W, Khanna C, Libutti SK. PLoS One. 2009;4:1. doi: 10.1371/journal.pone.0004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Hansen JB, Kristiansen K. Biochem. J. 2006;398:153. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Klutz K, Schaffert D, Willhauck MJ, Grunwald GK, Haase R, Wunderlich N, Zach C, Gildehaus FJ, Senekowitsch-Schmidtke R, Goke B, Wagner E, Ogris M, Spitzweg C. Mol.Ther. 2011;19:676. doi: 10.1038/mt.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Luo J, Zhang H, Xiao W, Kumaresan PR, Shi C, Pan CX, Aina OH, Lam KS. J. Comb. Chem. 2008;10:599. doi: 10.1021/cc8000663. [DOI] [PubMed] [Google Scholar]
- 557.Deutscher SL. Chem. Rev. 2010;110:3196. doi: 10.1021/cr900317f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Weiedenmann B, Grotzinger C. Nat. Biotechnol. 2001;19:327. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 559.Pierce M, Javier D, Richards-Kortum R. Int. J. Cancer. 2008;123:1979. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Park JH, Von Maltzahn G, Ruoslahti E, Bhatia S, Sailor M. Angew. Chem. Int. Ed. 2008;47:7284. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Kelly K, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, Depinho R, Mahmood U, Weissleder R. PLoS Med. 2008;5:657. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 563.Van Dongen G, Visser G, Lub-De Hooge M, De Vreis E, Perk L. Oncologist. 2007;12:1379. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 564.Okarvi SM. Cancer Treat. Rev. 2008;34:13. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 565.Okarvi SM. Med. Res. Rev. 2004;24:357. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 566.Reubi J, Maecke H. J. Nucl. Med. 2008;49:1735. doi: 10.2967/jnumed.108.053041. [DOI] [PubMed] [Google Scholar]
- 567.Wangler C, Buchmann I, Eisenhut M, Haberkorn U, Mier W. Protein Pept. Lett. 2007;14:273. doi: 10.2174/092986607780090874. [DOI] [PubMed] [Google Scholar]
- 568.Lee S, Xie J, Chen X. Biochemistry. 2010;49:1364. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Wang H, Chen K, Cai W, Li Z, He L, Kashefi A, Chen X. Mol. Cancer Ther. 2008;7:1044. doi: 10.1158/1535-7163.MCT-07-2084. [DOI] [PubMed] [Google Scholar]
- 570.Liu S. Mol. Pharmaceutics. 2006;3:472. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 571.Dijkgraaf I, Beer AJ, Wester HJ. Front. Biosci. 2009;14:887. doi: 10.2741/3284. [DOI] [PubMed] [Google Scholar]
- 572.Kenny L, Coombes R, Oulie I, Contractor K, Miller M, Spinks T, Mcparkland B, Cohen P, Hui A, Palmieri C, Osman S, Glaser M, Turton D, Al-Nahhas A, Aboagye E. J. Nucl. Med. 2008;49:879. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 573.Zhao D, Jin X, Li F, Liang J, Lin Y. J. Nucl. Med. 2012;53:1872. doi: 10.2967/jnumed.112.107821. [DOI] [PubMed] [Google Scholar]
- 574.Zhu Z, Miao W, Li Q, Dai H, Ma Q, Wang F, Yang A, Jia B, Jing X, Liu S, Shi J, Liu Z, Zhao Z, Wang F, Li F. J. Nucl.Med. 2012;53:716. doi: 10.2967/jnumed.111.098988. [DOI] [PubMed] [Google Scholar]
- 575.Aloj L, Morelli G. Curr. Pharm. Des. 2004;10:3009. doi: 10.2174/1381612043383511. [DOI] [PubMed] [Google Scholar]
- 576.Vegt E, De Jong M, Wetzels J, Masereeuw R, Melis M, Oyen W, Gotthardt M, Boerman O. J. Nucl. Med. 2010;51:1049. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
- 577.Gotthardt M, Eerd-Vismale J, Oyen W, De Jong M, Zhang H, Rolleman E, Maecke H, Behe M, Boerman O. J. Nucl.Med. 2007:596. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 578.Gagnon M, Hausner S, Marik J, Abbey C, Marshall J, Sutcliffe J. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17904. doi: 10.1073/pnas.0906925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Laurent S, Forge D, Port M, Roch A, Robic C, Elst L, Muller R. Chem. Rev. 2008;108:2064. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 580.Corot C, Robert P, Idee JM, Port M. Adv. Drug DeliveryRev. 2006;58:1471. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 581.Huang G, Zhang C, Li S, Khemtong C, Yang SG, Tian R, Brown KC, Gao J. J. Mater. Chem. 2009;19:6367. doi: 10.1039/b902358e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Falciani C, Lozzi L, Pini A, Bracci L. Chem. Biol. 2005;12:417. doi: 10.1016/j.chembiol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 583.Bracci L, Falciani C, Lelli B, Lozzi L, Runci Y, Pini A, De Montis MG, Tagliamonte A, Neri P. J. Biol. Chem. 2003;278:46590. doi: 10.1074/jbc.M308615200. [DOI] [PubMed] [Google Scholar]
- 584.Falciani C, Lozzi L, Pini A, Corti F, Fabbrini M, Bernini A, Lelli B, Niccolai N, Bracci L. Chem. Biol. Drug Des. 2007;69:216. doi: 10.1111/j.1747-0285.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 585.Pini A, Falciani C, Bracci L. Curr. Protein Pept. Sci. 2008;9:468. doi: 10.2174/138920308785915227. [DOI] [PubMed] [Google Scholar]
- 586.Bastings MMC, Helms BA, Van Baal I, Hackeng TM, Merkx M, Meijer EW. J. Am. Chem. Soc. 2011;133:6636. doi: 10.1021/ja110700x. [DOI] [PubMed] [Google Scholar]
- 587.Helms BA, Reulen SWA, Nijhuis S, De Graaf-Heuvelmans PTHM, Merkx M, Meijer EW. J. Am. Chem. Soc. 2009;131:11683. doi: 10.1021/ja902285m. [DOI] [PubMed] [Google Scholar]
- 588.Mammen M, Choi SK, Whitesides GM. Angew. Chem. Int. Ed. 1998;37:2755. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 589.Kim Y, Lillo AM, Steiniger SCJ, Liu Y, Ballatore C, Anichini A, Mortarini R, Kaufmann GF, Zhou B, Felding-Habermann B, Janda KD. Biochemistry. 2006;45:9434. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 590.Reynolds F, Panneer N, Tutino CM, Michael W, Skrabal WR, Moskaluk C, Kelly KA. PLoS One. 2011;6:1. doi: 10.1371/journal.pone.0022471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Joyce J, Laakkonen P, Bernasconi M, Bergers G, Ruoslahti E, Hanahan D. Cancer Cell. 2003;4:393. doi: 10.1016/s1535-6108(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 592. See ref 73. [Google Scholar]
- 593.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, Cardo-Vila M, Giordano RJ, Jaalouk DE, Ozawa MG, Moya CA, Souza GR, Staquicini FI, Kunyiasu A, Scudiero DA, Holbeck SL, Sausville EA, Arap W, Pasqualini R. Cancer Res. 2006;66:34. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 594.Kapoor P, Singh H, Gautam A, Chaudhary K, Kumar R, Raghava GPS. PLoS One. 2012;7:e35187. doi: 10.1371/journal.pone.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Ru B, Huang J, Dai P, Li S, Xia Z, Ding H, Lin H, Guo F, Wang X. Molecules. 2010;15:8279. doi: 10.3390/molecules15118279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Shtatland T, Guettler D, Kossodo M, Pivovarov M, Weissleder R. BMC Bioinf. 2007;8:280. doi: 10.1186/1471-2105-8-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Duchrow T, Shtatland T, Guettler D, Pivovarov M, Kramer S, Weissleder R. BMC Bioinf. 2009;10:317. doi: 10.1186/1471-2105-10-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Jain RK. Adv. Drug Delivery Rev. 2012;64:353. doi: 10.1016/j.addr.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Reardon D, Nabors L, Stupp R, Mikkelsen T. Expert Opin.Invest. Drugs. 2008;17:1225. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Minchinton AI, Tannock IF. Nat. Rev. Cancer. 2006;6:583. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 601.Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJP, Weiner LM, Marks JD, Adams GP. Cancer Res. 2011;71:2250. doi: 10.1158/0008-5472.CAN-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Rudnick SI, Adams GP. Cancer Biother. Radiopharm. 2009;24:155. doi: 10.1089/cbr.2009.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Ruoslahti E. Adv. Mater. 2012;24:3747. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Bae YH, Park K. J. Controlled Release. 2011;153:198. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Jain RK. Adv. Drug Delivery Rev. 2001;46:149. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 606.Thurber G, Schmidt M, Wittrup K. Adv. Drug Delivery Rev. 2008;60:1421. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Thurber G, Schmidt M, Wittrup K. Trends Pharmacol. Sci. 2008;29:57. doi: 10.1016/j.tips.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen T, Corti A, Ponzoni M. Cancer Res. 2003;63:7400. [PubMed] [Google Scholar]
- 609.Chang DK, Lin CT, Wu CH, Wu HC. PLoS ONE. 2009;4:e4171. doi: 10.1371/journal.pone.0004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Kirpotin D, Drummond D, Shao Y, Shalaby R, Hong K, Nielsen U, Marks J, Benz CC, Park JW. Cancer Res. 2006;66:6732. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 611.Mamot C, Drummond D, Noble C, Kallab V, Guo Z, Hong K, Kirpotin D, Park JW. Cancer Res. 2005;65:11631. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 612.Glaser V. Genetic Engineering and Biotechnology News. Vol. 29. New Rochelle, NY: GEN Publishing; 2009. [Google Scholar]
- 613.Ladner R, Sato A, Gorzelany J, De Souza M. DrugDiscovery Today. 2004;9:525. doi: 10.1016/S1359-6446(04)03104-6. [DOI] [PubMed] [Google Scholar]
- 614.Nestor J. In: Comprehensive Medicinal Chemistry II. Taylor J, Triggle D, editors. Vol.2. Oxford, U.K.: Elsevier Ltd.; 2007. p. 573. [Google Scholar]
- 615.Otvos L. In: Peptide-Based Drug Design. Otvos L, editor. Vol. 494. New York: Humana Press; 2008. p. 1. [DOI] [PubMed] [Google Scholar]
- 616.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. DrugDiscovery Today. 2010;15:40. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 617.Albericio F, Kruger HG. Future Med. Chem. 2012;4:1527. doi: 10.4155/fmc.12.94. [DOI] [PubMed] [Google Scholar]
- 618.Ahrens VM, Bellmann-Sickert K, Beck-Sickinger AG. Future Med. Chem. 2012;4:1567. doi: 10.4155/fmc.12.76. [DOI] [PubMed] [Google Scholar]
- 619.Kratz F. J. Controlled Release. 2008;132:171. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 620.Dennis MS, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, Damico LA. J. Biol. Chem. 2002;277:35035. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 621.Mcgregor D. Curr. Opin. Pharmacol. 2008;8:616. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 622.Lu Y, Yang J, Sega E. AAPS J. 2006;8:E466. doi: 10.1208/aapsj080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Malik D, Baboota S, Ahuja A, Hasan S, Ali J. Curr. DrugDelivery. 2007;4:141. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 624.Antosova Z, Mackova M, Kral V, Macek T. TrendsBiotechnol. 2009;27:628. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 625.Bray BL. Nat. Rev. Drug Discovery. 2003;2:587. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 626.Cines D, Yasothan U, Kirkpatrick P. Nat. Rev. DrugDiscovery. 2008;7:887. doi: 10.1038/nrd2741. [DOI] [PubMed] [Google Scholar]
- 627.Frampton J, Lyseng-Williamson K. Drugs. 2009;69:307. doi: 10.2165/00003495-200969030-00006. [DOI] [PubMed] [Google Scholar]
- 628.Bussel J, Kuter D, George J, Mcmillan R, Aledort L, Conklin G, Lichtin A, Lyons R, Nieva J, Wasser J, Wiznitzer I, Kelly R, Chen CF, Nichol J. N. Engl. J. Med. 2006;355:1672. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 629.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, Davis AM, Tansik RL, Mattheakis LC, Boytos CM, Schatz PJ, Baccanari DP, Wrighton NC, Barrett RW, Dower WJ. Science. 1997;276:1696. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 630.Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R. Cancer Cell. 2004;6:507. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 631.Hebst R, Hong D, Chap L, Kurzrock R, Jackson E, Silverman J, Rasmussen E, Sun YN, Zhong D, Hwang Y, Evelhoch J, Oliner J, Le N, Rosen L. J. Clin. Oncol. 2009;27:3557. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 632.Karlan BY, Oza AM, Richardson GE, Provencher DM, Hansen VL, Buck M, Chambers SK, Ghatage P, Pippitt CH, Brown JV, Covens A, Nagarkar RV, Davy M, Leath CA, Nguyen H, Stepan DE, Weinreich DM, Tassoudji M, Sun Y-N, Vergote IB. J. Clin. Oncol. 2012;30:362. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 633.Wrighton NC, Farrell F, Cchang R, Kashyap A, Barbone F, Mulcahy L, Hjohnson D, Barrett RW, Jolliffe L, Dower WJ. Science. 1996;273:458. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 634.Wrighton NC, Balasubramanian P, Barbone F, Kashyap A, Farrell F, Jolliffe L, Barrett RW, Dower WJ. Nat. Biotechnol. 1997;15:1261. doi: 10.1038/nbt1197-1261. [DOI] [PubMed] [Google Scholar]
- 635.Gregorc V, Santoro A, Bennicelli E, Punt C, Citterio G, Timmer-Bonte J, Caligaris Cappio F, Lambiase A, Bordignon C, Van Herpen C. Br. J. Cancer. 2009;101:219. doi: 10.1038/sj.bjc.6605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Bieker R, Kessler T, Schwoppe C, Padro T, Persigehi T, Bremer C, Dreischaluck J, Kolkmeyer A, Heindel W, Mesters R, Berdel W. Blood. 2009;113:5019. doi: 10.1182/blood-2008-04-150318. [DOI] [PubMed] [Google Scholar]
- 637.Kim KH, Dmitriev I, O’malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, Zinn KR, Curiel DT, Alvarez RD. Clin. Cancer Res. 2012;18:3440. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]