Abstract
This work illustrates a two-step strategy for the fabrication of polymer/drug nanoparticles. Utilizing solvent/non-solvent precipitation and gaseous basification, composite nanoparticles with 0–100% drug loadings were fabricated. Drug release kinetics were dictated by nanoparticle composition allowing for future tuning for therapeutic applications.
Keywords: nanoparticles, polymers, anesthetics, pain management
Pain management is currently one of the greatest challenges facing the medical community. A recent report estimates that over 100 million Americans suffer from chronic pain costing the nation $560-$635 billion annually.[1] A variety of medications are available to patients dealing with pain such as acetaminophen, non-steroidal anti-inflammatory drugs, corticosteroids, anti-convulsants, and opioids, but none of these have been found to induce sustained analgesia and most are associated with long lasting side-effects if utilized too frequently. Local anesthetics have been shown to provide analgesia through local neural blockade and have received considerable scientific[2] and clinical interest[3, 4] as an alternative to traditional pain-relief medications. A significant limitation associated with their use is a limited duration of action (1–12 hours)[5] which is ideal for short-term pain relief applications, but ineffective for the management of moderate-term (3–6 days) and long-term (7–30 days) pain. In this report, we developed a facile two-step process for the fabrication of polyanhydride/anesthetic base nanoparticles that possess 0–100% drug loadings. The composite nanoparticles were able to provide more extended drug release (64–150 hours) than from pure anesthetic base nanoparticles (40 hours). The extended release kinetics directly correlated to increasing polymer content which allows for the tuning of drug release to correspond to therapeutic levels of interest. The process outlined herein for the creation of polymer/drug nanoparticles has the potential to be utilized as a platform technology for a wide range of clinical applications.
The use of biomaterials to achieve controlled release of local anesthetics has the potential to yield safe, long-acting pain relief systems. Previous research has focused on a variety of biomaterials-based carriers including dispersions,[6] disks,[7] fibers,[8] vesicles,[3, 9, 10] microparticles,[11, 12] and nanoparticles[13, 14] comprised of lipids[3, 6, 9, 10, 12] and polymers like polysaccharides,[8, 13] polyesters,[11, 14] and polyanhydrides.[7] These delivery vehicles have been shown to provide sustained release of local anesthetics, but current fabrication methodologies require the drug be entrapped within the biomaterials carrier. Entrapment limits the maximum drug loading capacity to ~ 1% for liposomes[10] and ~ 20% for polymeric particles,[15] since higher drug loadings can disrupt the delivery vehicle, yield undesirable release kinetics, and lead to low drug encapsulation efficiencies. Developing new fabrication techniques capable of allowing for higher drug loadings has the potential to yield biomaterial carriers with highly tunable drug release kinetics.
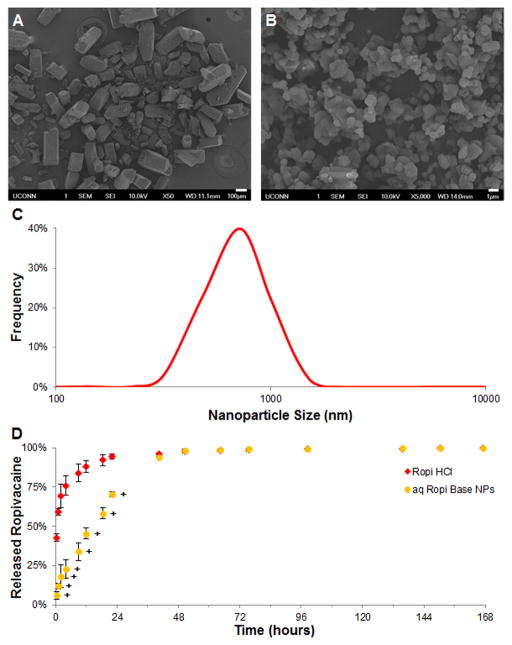
Ropivacaine hydrochloride (Ropi HCl), a local amino amide anesthetic, has been found to be capable of providing neural blockade for up to 12 hours[16] with lower cardiotoxicity and neurotoxicity than other local anesthetics[17] making it an attractive candidate for extended pain relief applications. Ropi HCl crystals are rough-edged, rectangular bricks 100–500 μm in each dimension (Figure 1A) and are readily soluble in water. The addition of ammonium hydroxide solution to a Ropi HCl solution dissociates the hydrochloride from the ropivacaine base (Ropi Base) causing the precipitation of sharp-edged, non-spherical nanoparticles (aq Ropi Base NPs, Figure 1B) with an average size of 778 ± 266 nm (Figure 1C). The in vitro release of ropivacaine (Figure 1D) from aq Ropi Base NPs was observed to be significantly slower (~90% in 40 hours) compared to that of neat Ropi HCl (~90% in 12 hours). Ropi Base is known to be ~500 times less water soluble than Ropi HCl[18] which is responsible for the extended release. This one-step aqueous, alkaline precipitation technique was utilized to create nanoparticles that when delivered in a chitosan thermogel were able to provide anesthetic effects in vivo for up to 48 hours.[19]
Figure 1.
Aqueous, alkaline precipitation of anesthetic nanoparticles. A-B) Representative scanning electron micrographs of Ropi HCl (A, scale bar = 100 μm) and aq Ropi Base NPs (B, scale bar = 1 μm). Ropi HCl was found to be comprised of large, brick-like particles whereas aqueous precipitation yielded smooth, circular Ropi Base NPs. C) A size distribution analysis of aq Ropi Base NPs showed a near normal distribution of particle size (778 ± 266 nm). D) The release of ropivacaine from aq Ropi Base NPs was significantly prolonged compared to the release of ropivacaine from Ropi HCl (N = 3; + = p < 0.05 over Ropi HCl).
While promising for short-term anesthetic delivery applications like post-operative pain management,[19] ropivacaine release from aq Ropi Base NPs can only be sustained for 1–2 days and cannot be modulated using the one-step technique. In order to control ropivacaine release kinetics for moderate-term and long-term applications, new techniques capable of fabricating polymer/anesthetic nanoparticles were investigated. One commonly utilized technique for the fabrication of polyanhydride nanoparticles is solute precipitation using solvent/non-solvent miscible pairs.[20–24] A solvent/non-solvent system comprised of methylene chloride/pentane was found capable of precipitating polyanhydride poly(sebacic anhydride) (pSA) and Ropi HCl allowing for the generation of non-aqueous composite nanoparticles (non-aq pSA/Ropi HCl NPs) ranging from 0–100% drug loading (Figure 2A – 2C). Non-aq Ropi HCl NPs possessed rough, angular morphologies (Figure 2A) similar to aq Ropi Base NPs (Figure 1B), but were slightly smaller in size (Figure 2D, 506 ± 218 nm). On the other hand, non-aq 20/80 pSA/Ropi HCl NPs had more spherical morphologies (Figure 2B) but similar size (Figure 2D, 481 ± 149 nm) to non-aq Ropi HCl NPs. Non-aq pSA NPs were found to be small (Figure 2D, 279 ± 87 nm) and spherical (Figure 2C), which is similar to previously published results for polyanhydride nanoparticles.[21, 22] The in vitro release of ropivacaine (Figure 2E) from non-aq Ropi HCl NPs was found to be rapid (~90% in 12 hours) and very similar to neat Ropi HCl. Since non-aqueous nanoparticle fabrication does not alter the chemical structure of ropivacaine like alkaline aqueous nanoparticle fabrication, the high water solubility of Ropi HCl dictates its fast release from the nanoparticles. Even the inclusion of slowly degrading pSA (20/80 pSA/Ropi HCl NPs) was unable to provide controlled release over non-aq Ropi HCl NPs or neat Ropi HCl.
Figure 2.
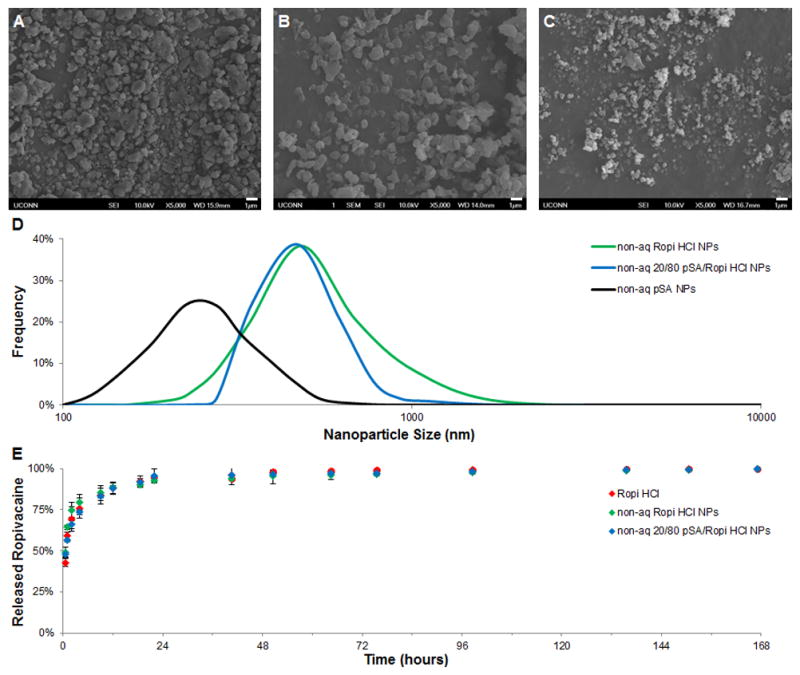
Non-aqueous precipitation of polymer/anesthetic nanoparticles. A-C) Representative scanning electron micrographs of non-aq Ropi HCl (A, scale bar = 1 μm), 20/80 pSA/Ropi HCl (B, scale bar = 1 μm), and pSA (C, scale bar = 1 μm) show that the non-aqueous precipitation technique yields nanoparticles. D) Size distribution analyses of the non-aqueously precipitated nanoparticles showed similar, near normal distributions of particle sizes regardless of chemistry. Non-aq Ropi HCl NPs, non-aq 20/80 pSA/Ropi HCl NPs, and non-aq pSA NPs had average sizes of 506 ± 218 nm, 481 ± 149 nm, and 279 ± 87 nm, respectively. E) The release of ropivacaine from non-aq Ropi HCl NPs and 20/80 pSA/Ropi HCl NPs was rapid and very similar to that of Ropi HCl (N = 3; + = p < 0.05 over Ropi HCl, non-aq Ropi HCl NPs and non-aq 20/80 pSA/Ropi HCl NPs).
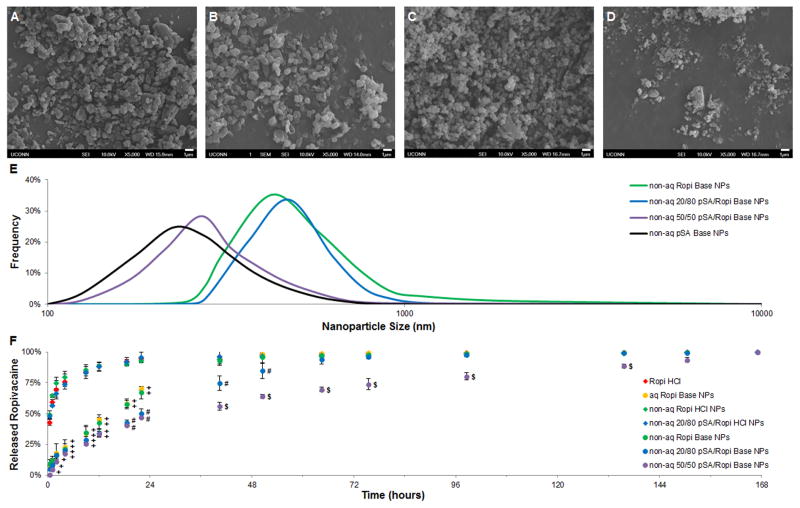
To extend drug release kinetics, a technique for basifying the Ropi HCl within the nanoparticles had to be developed. Submerging the pSA/Ropi HCl NPs in a basic solution was the simplest solution, but because polyanhydride degradation is base catalyzed,[25] this process would rapidly degrade the pSA and negate its ability to control drug release. It was hypothesized that exposing the pSA/Ropi HCl NPs to a basic gas (ammonia) would convert the Ropi HCl within nanoparticles into Ropi Base without significantly degrading the pSA or altering nanoparticle structure. Gaseous basification was used to fabricate non-aq Ropi Base NPs (Figure 3A), non-aq 20/80 pSA/Ropi Base NPs (Figure 3B), non-aq 50/50 pSA/Ropi Base NPs (Figure 3C), and non-aq pSA Base NPs (Figure 3D). Basified nanoparticles were observed to possess similar morphologies to their non-basified counterparts (see Figure 2) with nanoparticle sphericity correlating to pSA content. Nanoparticle mean size and size distribution (Figure 3E) were also not altered by the gaseous basification process. Ropivacaine release from non-aq Ropi Base NPs (Figure 3F) was found to be very similar (~70% in 24 hours) to that of aq Ropi Base NPs, providing strong evidence that the gaseous basification process successfully converted Ropi HCl within the nanoparticles into Ropi Base. Non-aq 20/80 pSA/Ropi Base NPs slightly extended ropivacaine release (~90% in 64 hours), whereas non-aq 50/50 pSA/Ropi Base NPs significantly extended ropivacaine release (~90% in 150 hours). With the lower water solubility of Ropi Base, the polyanhydride component of the nanoparticles was able to mediate extended drug release kinetics.
Figure 3.
Gaseous basification of polymer/anesthetic nanoparticles extends drug release kinetics. A-D) Representative scanning electron micrographs of gaseously basified non-aq Ropi Base NPs (A, scale bar = 1 μm), 20/80 pSA/Ropi Base NPs (B, scale bar = 1 μm), 50/50 pSA/Ropi Base NPs (C, scale bar = 1 μm), and pSA NPs (D, scale bar = 1 μm). E) Size distribution analyses of the gaseously basified, non-aq NPs showed similar, near normal distributions of particle sizes regardless of chemistry. Non-aq Ropi Base NPs, non-aq 20/80 pSA/Ropi HCl NPs, non-aq 50/50 pSA/Ropi HCl NPs, non-aq pSA NPs had average sizes of 510 ± 222 nm, 447 ± 157 nm, 306 ± 105 nm, and 280 ± 95 nm, respectively. F) Gaseous basification modulated ropivacaine release for all formulations with greater pSA content correlated to slower drug release (N = 3; + = p < 0.05 over Ropi HCl, non-aq Ropi HCl NPs, and non-aq 20/80 pSA/Ropi HCl NPs; # = p < 0.05 over Ropi HCl, non-aq Ropi HCl NPs, non-aq 20/80 pSA/Ropi HCl NPs, aq Ropi Base NPs, and non-aq Ropi Base NPs; $ = p < 0.05 over Ropi HCl, non-aq Ropi HCl NPs, non-aq 20/80 pSA/Ropi HCl NPs, aq Ropi Base NPs, non-aq Ropi Base NPs, and non-aq 20/80 pSA/Ropi Base NPs).
In summary, a facile two-step fabrication process was developed to enable the fabrication of polyanhydride/anesthetic nanoparticles with controllable drug release kinetics. A solvent/non-solvent miscible pair capable of precipitating both polyanhydride and ropivacaine hydrochloride nanoparticles independently was identified and utilized to create composite polynahydride/ropivacaine hydrochloride nanoparticles. Additionally, a gaseous basification technique was developed to convert ropivacaine hydrochloride within nanoparticles into ropivacaine base without altering particle structure or size as well as maintain polymer integrity. These fundamental results provide a promising platform for the creation of not only polyanhydride/anesthetic base nanoparticles, but a variety of polymer/drug nanoparticles with extended and controllable drug release kinetics.
Experimental Section
Materials
Ropivacaine hydrochloride (Ropi HCl) was a generous gift from AstraZeneca (London, United Kingdom). Sebacic acid (SA), anhydrous petroleum ether, and sodium hydroxide were purchased from Sigma-Aldrich (Saint Louis, MO). Acetic anhydride, chloroform, dry petroleum ether, methylene chloroform, and ammonium hydroxide solution (14 N) were procured from Fisher Scientific (Pittsburgh, PA). Pentane and ammonium hydroxide solution (30 wt %) were acquired from Acros Organics (Fair Lawn, NJ). Spectra/Por® dialysis membrane tubing (MWCO: 6,000–8,000 Da) was purchased from Spectrum Laboratories (Rancho Dominguez, CA). Deionized, distilled water (ddH2O) was generated by a Millipore Milli-Q integral water purification system (Billerica, MA).
One-step Aqueous, Alkaline Nanoparticle Precipitation
Similar to a recently reported method,[19] 14 N ammonium hydroxide solution was added to a 4% w/v Ropi HCl aqueous solution at a 10x molar equivalent to induce alkaline precipitation of ropivacaine base nanoparticles (aq Ropi Base NPs). The aq Ropi Base NPs were filtered, washed with ddH2O, lyophilized overnight, and stored under desiccant until further use. Scanning electron microscopy (SEM) using a JEOL 6320F Field Emission Scanning Electron Microscope (Tokyo, Japan) was used to evaluate the morphology of Ropi HCl and aq Ropi Base NPs. Size distribution analysis of aq Ropi Base NPs was carried out using the image analysis software ImageJ (NIH, Bethesda, MD).
Poly(sebacic anhydride) Synthesis
Poly(sebacic anhydride) (pSA) was synthesized by a two-step polymerization process similar to previously described methods.[23, 26] SA was first refluxed in an excess of acetic anhydride to synthesize acetylated SA prepolymer (preSA). PreSA was polymerized into pSA by melt polycondensation at 180 °C under high vacuum (< 0.3 mm Hg) for 90 minutes. Proton (1H) NMR was used to determine polymer purity and number average molecular weight using polymer end group analysis.[27] 1H NMR (400 MHz, CDCl3): δ = 1.32 (s, 8H), 1.65 (t, 4H), 2.44 (m, 4H); Mn ~ 8,400 g/mol. These results were consistent with previously reported values for pSA.[23, 26]
Non-Aqueous Nanoparticle Precipitation
Similar to previously published techniques for polyanhydride nanoparticle fabrication,[20, 21] a solvent/non-solvent system was utilized to induce rapid non-aqueous (non-aq) precipitation of pSA NPs, Ropi HCl NPs, and composites of pSA/Ropi HCl NPs at 20/80 and 50/50 wt/wt ratios. Briefly, pSA and/or Ropi HCl were dissolved in methylene chloride (3% w/v) and rapidly poured into an unstirred bath of pentane at a 50:1 ratio of non-solvent to solvent causing immediate solute precipitation. The precipitant was filtered and either left on the filter paper in the Buchner funnel to be gaseously basified or lyophilized overnight and stored under desiccant until further use. SEM and ImageJ were used to evaluate morphology and size distribution, respectively.
Gaseous Basification
A novel gaseous basification technique was developed to convert non-aq Ropi HCl NPs into ropivacaine base nanoparticles (non-aq Ropi Base). Ammonia gas was generated by adding 12 g of sodium hydroxide into 50 mL of ammonium hydroxide solution (30 wt %) in a 1000 mL Erlenmeyer flask.[28] After the production of ammonia gas was verified by pH paper, a Buchner funnel with the filtered nanoparticles was placed on top of the Erlenmeyer flask forcing ammonia gas to flow over the nanoparticles for 5 minutes. The gaseously basified nanoparticles were washed with pentane, dried on the filter paper, lyophilized overnight, and stored under desiccant until further use. Gaseous basification was also utilized for the conversion of non-aq pSA/Ropi HCl NPs into non-aq pSA/Ropi Base NPs. As before, morphology and size distribution were assessed using SEM and ImageJ, respectively.
In Vitro Ropivacaine Release
Samples (10 mg of Ropi HCl, aq Ropi Base NPs, non-aq Ropi HCl NPs, 20/80 pSA/Ropi HCl NPs, non-aq Ropi Base NPs, 20/80 pSA/Ropi Base NPs, or 50/50 pSA/Ropi Base NPs) were dispersed in 1 mL of phosphate buffered saline (PBS), injected into dialysis bags, and sealed with dialysis bag clips. Dialysis bags were incubated in a 250 mL PBS bath (> 5X saturation concentration to ensure sink conditions) at 37°C. One mL samples were taken from the PBS bath at specific time points over a seven day period and replaced with equal volumes of PBS. Samples were analyzed for ropivacaine content by reverse-phase high-performance liquid chromatography (HPLC, Agilent 1100 Series, Agilent Technologies, Wilmington, DE) using a 50% PBS/50% acetonitrile (with 0.1% trifluoroacetic acid) mobile phase at a flow rate of 1 mL/min over a 5 μm reverse-phase column (Zorbax Eclipse SDB-C18, Agilent Technologies, Wilmington, DE). Ropivacaine elution was detected by an Agilent 1100 diode array detector at 262 nm. Samples taken from a PBS bath exposed to an equivalent amount of pSA and pSA Base were used to identify any contaminating effects from the polymer itself. A standard curve of ropivacaine in PBS was generated over the range of 0.2 - 20 μg/mL and used to convert absorbance to concentration. A cumulative release profile was generated by normalizing the data against the total amount of encapsulated ropivacaine and reported as fractional drug release.
Statistical Analysis
All results are reported as mean ± standard deviation. JMP® software (SAS Institute, Cary, NC) was used to make comparisons between groups using an ANOVA followed by Tukey’s HSD test to determine pairwise statistically significant differences (p < 0.05).
Acknowledgments
The reported research was supported by USAMRMC Grant W81XWH-07-1-0425 and the Raymond and Bevery Sackler Foundation. Dr. Laurencin is a recipient of the National Science Foundation Presidential Faculty Fellow Award, and the National Science Foundation Presidential Award for Excellence in Science, Engineering and Math Mentoring. Dr. Narasimhan acknowledges the Vlasta Klima Balloun Professorship.
Contributor Information
Dr. Bret D. Ulery, Institute for Regenerative Engineering, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Orthopaedic Surgery, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA.
Ho-Man Kan, Institute for Regenerative Engineering, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Orthopaedic Surgery, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA.
Bryce A. Williams, Department of Chemical and Biological Engineering, Iowa State University, 2114 Sweeney Hall, Ames, IA 50011, USA.
Prof. Balaji Narasimhan, Department of Chemical and Biological Engineering, Iowa State University, 2114 Sweeney Hall, Ames, IA 50011, USA
Prof. Kevin W.-H. Lo, Institute for Regenerative Engineering, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Division of Endocrinology, Department of Medicine, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA
Prof. Lakshmi S. Nair, Institute for Regenerative Engineering, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Orthopaedic Surgery, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Chemical, Materials and Biomolecular Engineering, University of Connecticut, 191 Auditorium Road, Unit 3222, Storrs, CT 06269, USA
Prof. Cato T. Laurencin, Email: Laurencin@uchc.edu, Institute for Regenerative Engineering, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Orthopaedic Surgery, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. Department of Chemical, Materials and Biomolecular Engineering, University of Connecticut, 191 Auditorium Road, Unit 3222, Storrs, CT 06269, USA
References
- 1.Gaskin DJ, Richard P. Journal of Pain. 2012;13:715. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Araujo DRd, Cereda CMS, Brunetto GB, Pinto LMA, Santana MHA, Paula Ed. Canadian Journal of Anesthesia. 2004;51:566. doi: 10.1007/BF03018399. [DOI] [PubMed] [Google Scholar]; Wang CF, Djalali AG, Gandhi A, Knaack D, Girolami UD, Strichartz G, Gerner P. Anesth Analg. 2009;108:1027. doi: 10.1213/ane.0b013e318193596a. [DOI] [PubMed] [Google Scholar]; Weldon CB, Tsui JH, Shankarappa SA, Nguyen VT, Ma M, Anderson DG, Kohane DS. J Control Release. 2012;161:903. doi: 10.1016/j.jconrel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas E, Onel E, Miller H, Ragupathi M, White PF. Am Surg. 2012;78:574. doi: 10.1177/000313481207800540. [DOI] [PubMed] [Google Scholar]
- 4.Wohlrab J, Finke R, Franke WG, Wohlrab A. Dermatologic Surgery. 2012;38:91. doi: 10.1111/j.1524-4725.2011.02146.x. [DOI] [PubMed] [Google Scholar]; Truitt MS, Murry J, Amos J, Lorenzo M, Mangram A, Dunn E, Moore E. The Journal of Trauma: Injury Infection and Critical Care. 2011;71:1548. doi: 10.1097/TA.0b013e31823c96e0. [DOI] [PubMed] [Google Scholar]
- 5.Ghali AM. Saudi Journal of Anasthesia. 2012;6:22. doi: 10.4103/1658-354X.93050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pawlowski J, Orr K, Kim K-m, Pappas AL, Sukhani R, Jellish WS. Journal of Clinical Anesthesia. 2012;24:109. doi: 10.1016/j.jclinane.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Puglia C, Sarpietro MG, Bonina F, Castelli F, Zammataro M, Chiechio S. Journal of Pharmaceutical Sciences. 2011;100:1892. doi: 10.1002/jps.22416. [DOI] [PubMed] [Google Scholar]; Litonius E, Lokajova J, Yohannes G, Neuvonen PJ, Holopainen JM, Rosenberg PH, Wiedmer SK. Journal of Chromatography A. 2012;1254:125. doi: 10.1016/j.chroma.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Masters DB, Berde CB, Dutta S, Turek T, Langer R. Pharmaceutical Research. 1993;10:1527. doi: 10.1023/a:1018995913972. [DOI] [PubMed] [Google Scholar]; Park ES, Maniar M, Shah JC. Journal of Controlled Release. 1998;52:179. doi: 10.1016/s0168-3659(97)00223-x. [DOI] [PubMed] [Google Scholar]
- 8.Janaswamy S, Gill KL, Campanella OH, Pinal R. Carbohydrate Polymers. 2013;94:209. doi: 10.1016/j.carbpol.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard BM, Ott LR, Haan D, Brubaker AN, Cole PI, Nelson KG, Ross PE, Rebelatto MC, Newton PE. Expert Opinion on Investigational Drugs. 2011;20:1327. doi: 10.1517/13543784.2011.611499. [DOI] [PubMed] [Google Scholar]; Franz-Montan M, Paula Ed, Groppo FC, Silva ALR, Ranali J, Volpato MC. British Journal of Oral and Maxillofacial Surgery. 2012;50:60. doi: 10.1016/j.bjoms.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2009;106:7125. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NGM, Berde CB, Langer R. PAIN. 2003;104:415. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]; Horie RT, Sakamoto T, Nakagawa T, Tabata Y, Okamura N, Tomiyama N, Tachibana M, Ito J. The Laryngoscope. 2010;120:377. doi: 10.1002/lary.20713. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G, Langer R, Kohane DS. J Biomed Mater Res A. 2004;68A:651. doi: 10.1002/jbm.a.20074. [DOI] [PubMed] [Google Scholar]
- 13.Grillo R, Melo NFDd, Araujo DRd, Paula Ed, Rosa AH, Fraceto LF. J Drug Target. 2010;18:688. doi: 10.3109/10611861003649738. [DOI] [PubMed] [Google Scholar]
- 14.Melo NFSD, Araujo DRD, Grillo R, Morales CM, Matos APD, Paula Ed, Rosa AH, Fraceto LF. Journal of Pharmaceutical Sciences. 2012;101:1157. doi: 10.1002/jps.22829. [DOI] [PubMed] [Google Scholar]; Moraes CM, Matos APd, Lima Rd, Rosa AH, Paula Ed, Fraceto LF. Journal of Biological Physics. 2007;33:455. doi: 10.1007/s10867-008-9094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Melo NFd, Araujo DRd, Grillo R, Moraes CM, Matos APd, Paula Ed, Rosa AH, Fraceto LF. Journal of Pharmaceutical Sciences. 2012;101:1157. doi: 10.1002/jps.22829. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Small. 2009;5:1575. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredrickson MJ, Abeysekera A, White R. Reg Anesth Pain Med. 2012;37:495. doi: 10.1097/AAP.0b013e3182580fd0. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya H, Ueno T, Mizogami M. Bioorg Med Chem. 2011;19:3410. doi: 10.1016/j.bmc.2011.04.030. [DOI] [PubMed] [Google Scholar]; Takenami T, Wang G, Nara Y, Fukushima S, Yagishita S, Hiruma H, Kawakami T, Okamoto H. Can J Anaesth. 2012;59:456. doi: 10.1007/s12630-012-9685-9. [DOI] [PubMed] [Google Scholar]
- 18.Thomas JM, Schug SA. Clin Pharmacokinet. 1999;36:67. doi: 10.2165/00003088-199936010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Foley PL, Ulery BD, Kan HM, Burks M, Cui Z, Wu Q, Nair LS, Laurencin CT. Biomaterials. 2013;34:2539. doi: 10.1016/j.biomaterials.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Carino GP, Jacob JS, Mathiowitz E. Journal of Controlled Release. 2000;65:261. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 21.Ulery BD, Phanse Y, Sinha A, Wannemuehler MJ, Narasimhan B, Bellaire BH. Pharmaceutical Research. 2009;26:683. doi: 10.1007/s11095-008-9760-7. [DOI] [PubMed] [Google Scholar]
- 22.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. PLoS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kipper MJ, Shen E, Determan A, Narasimhan B. Biomaterials. 2002;23:4405. doi: 10.1016/s0142-9612(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 24.Determan AS, Graham JR, Pfeiffer KA, Narasimhan B. Journal of Microencapsulation. 2006;23:832. doi: 10.1080/02652040601033841. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhan B, Kipper MJ. Advances in Chemical Engineering. 2004;29:169. [Google Scholar]
- 26.Shen E, Kipper MJ, Dziadul B, Lim MK, Narasimhan B. Journal of Controlled Release. 2002;82:115. doi: 10.1016/s0168-3659(02)00125-6. [DOI] [PubMed] [Google Scholar]
- 27.Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Journal of Biomedical Materials Research Part A. 2006;76A:102. doi: 10.1002/jbm.a.30510. [DOI] [PubMed] [Google Scholar]
- 28.Thomas NC. Journal of Chemical Education. 1990;67:431. [Google Scholar]