Abstract
Background
Appreciable local recurrence rates observed in patients with margin-negative, transoral laser microsurgery (TLM)-treated oral cavity squamous cell carcinoma (SCC) necessitate identification of new prognosticators for local control and survival. A histopathologic index (Brandwein–Gensler score [BGS]) and intrinsic/iatrogenic/chronic conditions causing immune compromise are investigated.
Methods
From a prospectively assembled database of TLM-treated oral cavity SCC, specimens for 60 patients with a minimum of 2-years follow-up could undergo BGS assignment. Local control, disease-specific survival (DSS), and overall survival (OS) were study endpoints.
Results
“Low-BGS” was recorded in 28 patients (47%) and “high-BGS” in 32 patients (53%), whereas immune compromise was observed in 18%. In multivariate analyses, immune compromise was the only predictor for local control. T classification and immune compromise were prognostic for DSS and OS. “High-BGS” was prognostic only for OS.
Conclusion
“High-BGS” was associated with recurrences but immune compromise was the most significant predictor of local control and survival in margin-negative, TLM-treated oral cavity SCC. Strategies that maintain/restore tumor-specific immune responses in immune compromised oral cavity SCC hosts need to be developed.
Keywords: immune compromise, Brandwein–Gensler score risk model, oral carcinoma, transoral laser microsurgery (TLM), local control
INTRODUCTION
Oral cavity squamous cell carcinoma (SCC) remains an aggressive disease with substantial mortality. Local control is a critical determinant of survival in oral cavity SCC.1–3 Failure at the primary site is a common mode of recurrence and local recurrence rates in recent studies vary from 10% to 34%.1,2,4,5 Of several factors known to govern local control rates, positive surgical margins continue to be the most consistently reported.4–13 Achieving negative margins during resection correlates with higher local control. This, in turn, translates into increased survival in most studies, thus making margins the only factor of several patient, tumor, and treatment-related variables that can be impacted by surgical technique.
Local recurrences portend a bad outcome despite the excellent surgical margin control achieved with transoral laser microsurgery (TLM). In a recent study on survival and prognostic factors for 95 patients with oral cavity SCC treated primarily by TLM at our institution, we observed that TLM accomplished final margin negativity of 95%.14 Local recurrence was observed in 18 of 95 patients (19%) but only 2 of these occurred in patients with positive margins. Immune compromise was prognostic for local control.8 Local recurrence rates of this degree observed in patients with surgically resected margin-negative oral cavity SCC, necessitate investigation of new prognosticators for local control. In this study, we undertook to examine the role of histologic factors as incorporated in the Brandwein–Gensler score (BGS),15 evaluating its relationship to immune compromise and other factors.
Of the various histologic risk systems for oral cavity SCC reported, the recent multiparameter scoring proposed by Brandwein–Gensler et al15 has been found in preliminary studies of non-TLM surgical resection to predict local recurrence and overall survival (OS) in oral cavity SCC. Based on the risk categories specified in the BGS model, it was proposed as a useful prognosticator for oral cavity primaries in the presence of negative margins. The model was later validated in certain studies, but in all of these studies, conventional, non-TLM surgery was utilized and the margin status was not uniformly negative.16–19
In view of the change in treatment paradigms toward minimally invasive approaches and our findings of appreciable local recurrences despite high level of margin control,14 our objective was to assess the application of BGS risk modeling in TLM-treated patients with oral cavity SCC, while controlling for other factors. Hypotheses generated can be tested in prospective trials.
PATIENTS AND METHODS
Study population
From a prospectively assembled TLM database of patients with head and neck cancer, we identified 95 patients consecutively treated with TLM for histologically confirmed oral cavity SCC at Washington University Medical School of Medicine from May 1995 through July 2010. Data collection included demographic, disease, treatment, and pathology-related variables and follow-up status, as recorded in the TLM database. All information was updated and verified through medical records, contact with patients or family members, and death registries. We recently reported a detailed analysis of the above-mentioned prospectively assembled data to document survival outcomes and prognostic factors in the 95 patients with oral cavity SCC treated with TLM ± neck dissection ± adjuvant therapy.8 From these 95 patients, we now report on the subset of patients (n = 60) to whom a BGS could be assigned. Of this cohort of 60 patients, we report on another “sub-subset” analysis of just the T1 and T2 cases (n = 43). The current study adds new information by focusing on the implications of a histologic risk assessment index, BGS, and immune status in the milieu of traditional prognosticators. The Human Research Protection Office at Washington University Medical School of Medicine approved data collection for the study.
The inclusion criteria for this study were: (1) previously untreated histologically proven oral cavity SCC; (2) patients treated primarily with TLM ± neck dissection ± adjuvant therapy and rendered disease-free at the completion of surgery; and (3) minimum follow-up of 24 months, or to recurrence or death. Patients presenting with a primary oral cavity SCC with a history of previous head and neck cancer were included only if the index tumor was not in the oral cavity and treatment of the index tumor had been surgical, with no radiation administered to the head and neck region. Patients who received any prior surgery, radiation, or chemotherapy for the same primary tumor, and patients with distant metastasis at presentation were excluded.
Brandwein–Gensler histologic risk assessment model
The Brandwein–Gensler histological “risk assessment” model develops a score based on 3 variables, which include the cellular pattern of tumor invasion, lymphocytic infiltrate (LI) at the tumor/host interface, and peri-neural invasion (PNI).15 scores of 0, 1, or 3 are assigned to different categories of the 3 variables, which are included in the BGS model. Five patterns of invasion have been defined to denote the manner in which cancer infiltrates tissues at the tumor/host interface and the highest score for pattern of invasion at the tumor interface (worst pattern of invasion) was recorded; scores were 0 for types 1 to 3, 1 for type 4, and 3 for type 5 pattern. Three patterns of LI at the tumor/host interface are included in the scoring system; continuous bands (score 0); large patches (score 1); and little or none (score 3). PNI was classified as absent (score 0), PNI involving small nerves with diameters <1 mm (score 1), or PNI involving large nerves with diameters ≥1 mm (score 3). Nerves are measured in cross-section only. The sum of the assigned scores for all 3 variables are used to compute the final BGS score in which 0 is “low,” 1 or 2 is “intermediate,” and 3 through 9 is “high” risk.
For validation of this risk model in our series, all available histological slides were retrospectively reviewed and scored by our study pathologists. The cases were randomly divided into thirds and each third was reviewed and scored by 1 of the 3 head and neck pathologists (S.K.E., J.S.L., R.D.C.). This was done in order to provide a “real practice” review that recapitulated exactly what occurs already at our institution on active clinical cases. Of note, the pathologists did not receive any special training in BGS interpretation or application. The pathologists were also blinded to patient’s previously reported histological data and to clinical features and outcomes. Cases were excluded based on the criteria set forth in the articles describing and examining BGS.15,18 Cases with only microinvasive SCC were excluded, defined as SCC with invasion limited to the immediate adjacent submucosa, and 2 mm or less in depth. Cases of spindle cell and verrucous carcinoma were also excluded because of their known variation in clinical behavior relative to conventional SCC. Cases missing either all slides or missing the primary tumor slides were excluded.
Margin assessment
Techniques of TLM resection of the primary tumor have been previously described.14 To ensure completeness of tumor resection, the margins on the excised labeled tumor specimens were inked intraoperatively by the surgeon, oriented with marker stitches, and sent for immediate frozen section analysis. For example, in a lateral tongue tumor, the resection would be accomplished in 3 segments (anterior, middle, and posterior, requiring 2 transtumoral cuts). Ink is then applied immediately after removal of each segment from the mouth to precisely indicate the location of the intended margin of clearance. Immediate frozen section analysis with evaluation of the inked areas is then performed. As an alternative, separate frozen section sampling from the surgical resection bed was utilized in a proportion of cases, adding several millimeters of clearance to obtain the ultimate margin. Complete tumor resection was verified when the frozen sections from the inked resection specimens, or the resection bed, were negative. Real-time intraoperative communication between the operating surgeon and the pathologist regarding specimen orientation and ink placement was routinely practiced. For patients undergoing marginal mandibulectomy, bone marrow from the ends of the resection margin was curetted and sent for frozen section analysis. No further bony margins were taken if the results were negative. Margins were revised, if required, in accordance with the permanent histological evaluation of the entire bony specimen and marrow frozen sections.
Margins were not categorized as “close”: no “close” margin status was assigned because the distance of tumor to the true final margins is not necessarily the distance to the inked surface of the main resection pieces since additional resection pieces were often taken as the frozen section pathology results became available. For the study, the final margin assessment after the first TLM resection was recorded in 2 groups, based on the pathology reports: (1) Positive: tumor cells present at the margin in the final pathology report from any specimen; and (2) Negative: tumor cells not present at the margin in the final pathology report from any specimen. Re-resection was performed in all patients with reported positive margins. The presence of tumor in re-resected specimens and the status of final margins after re-resection were recorded for the study.
Immune compromise
History of intrinsic, iatrogenic, or chronic conditions that are documented in the literature20–24 to cause immune compromise (eg, autoimmune disease, intake of immunosuppressant drugs, lymphoreticular disorders, history of chemoradiation, immunodeficiencies, and chronic debilitating illness) were recorded retrospectively from the medical records.
Other pathologic variables
Primary tumor depth (in millimeters), and tumor grade (well/moderately/poorly differentiated) were recorded from the original pathology reports. Lymphatic and vascular space invasion were reassessed and established as present or absent by the study pathologists’ review.
Statistical analysis
The primary endpoints for this study were local control, disease-specific survival (DSS), and OS. Local recurrence was defined as biopsy proven tumor in the immediate vicinity (ie, within 2 cm) of the original primary. Local control was defined as the time between surgery and development of local recurrence. DSS was measured from the time of surgery to the date of death from oral cancer or the direct effects of its treatment. OS was measured from the time of surgery to the date of death because of any cause. Data were analyzed using SAS 9.2 software (SAS Institute, Cary, NC). Heterogeneity between any 2 groups was investigated using chi-square or Fisher’s exact test for categorical data. All statistical tests used were 2-sided. Survival probability was estimated by Kaplan–Meier analysis and survival curves were compared by log-rank statistic. For all analyses, statistical significance was indicated at a p value of < .05. Univariate and multivariate Cox proportional hazard models were used to assess the impact of various prognostic variables and hazard ratios (HRs) 95% confidence intervals (CIs) were calculated. The proportional hazard assumption was assessed using (1) estimated minus log (minus log) survival curves over different categories of variables being investigated; (2) goodness-of-fit tests based on the Schoenfeld residuals; and (3) time-dependent covariates. In case the proportional hazard assumption was violated, an extended Cox model was applied with the use of Heaviside functions that allow a different hazard ratio for a time-dependent variable before and after an empirically selected cutoff time point. Separate Cox analyses were done for T1 to T2 oral cavity SCC to identify prognosticators specific to this subgroup.
RESULTS
Patient characteristics
A total of 95 patients with oral cavity SCC consecutively treated by TLM met entry criteria. Archived specimens from the primary tumor resection were procured in order to re-review them and assign BGS risk categories. Scoring could only be completed for 60 of 95 cases (63%) and these cases comprised the final study cohort. The causes for exclusions of 35 cases were: missing or incomplete slides for tumor/host interface evaluation (n = 24); microinvasive carcinoma only (n = 5), verrucous carcinoma (n = 4), and spindle cell carcinoma (n = 2). There were 38 men and 22 women with a median age of 60 years (minimum-maximum, 23–85 years) for the overall cohort, and 63 years (minimum-maximum, 35–78 years) for men and 57 years (minimum-maximum, 23–85 years) for women. Mean follow-up for all survivors was 61 months, with a median of 45.5 months (minimum-maximum, 24–165 months). In this cohort of 60 patients, 41 (68%) were alive, of whom 39 (65%) were disease-free and 2 (3%) were alive with disease. A total of 19 patients (32%) died; 12 (20%) from oral cavity SCC-related causes and 7 patients (12%) from non–oral cavity SCC-related causes. The causes for these 7 non–oral cancer-related deaths were: cardiopulmonary (n = 4); pneumonia (n = 1); second primary in head and neck (n = 1); and small cell lung carcinoma (n = 1). Tumor, treatment, and pathologic data are displayed in Table 1.
TABLE 1.
Frequency distribution of local recurrence and any disease recurrence (local, regional, or distant) according to tumor, treatment, pathologic, and systemic variables.
| Variables | Overall cohort, n = 60 No. of patients (%) |
Local recurrence, n = 11 No. of patients (%)* |
Any disease recurrence, n = 25 No. of patients (%)* |
|---|---|---|---|
| Tumor variables | |||
| Site | |||
| Tongue | 38 (63) | 8 (21) | 17 (45) |
| Floor of mouth | 9 (15) | 1 (11) | 5 (56) |
| Retromolar trigone | 7 (12) | 2 (28) | 2 (29) |
| Lower alveolar ridge | 4 (7) | 0 | 1 (25) |
| Hard palate | 2 (3) | 0 | 0 |
| T classification | |||
| T1 | 27 (45) | 2 (7) | 9 (33) |
| T2 | 16 (27) | 4 (25) | 8 (50) |
| T3 | 6 (10) | 2 (33) | 2 (33) |
| T4 | 11 (18) | 3 (27) | 6 (55) |
| N classification | |||
| N0 | 39 (65) | 5 (13) | 12 (31) |
| N1 | 9 (15) | 2 (22) | 5 (56) |
| N2 | 12 (20) | 4 (33) | 8 (67) |
| AJCC stage | |||
| I | 22 (37) | 1 (5) | 6 (27) |
| II | 7 (11) | 1 (14) | 3 (43) |
| III | 10 (17) | 2 (20) | 5 (50) |
| IV | 21 (35) | 7 (33) | 11 (52) |
| Treatment variables | |||
| Neck dissection | |||
| Not done | 23 (38) | 1 (4) | 7 (30) |
| Done | 37 (62) | 10 (27) | 18 (49) |
| Adjuvant therapy | |||
| None | 41 (68) | 7 (17) | 15 (37) |
| Radiotherapy | 10 (17) | 2 (20) | 5 (50) |
| Chemoradiotherapy | 9 (15) | 2 (22) | 5 (56) |
| Pathologic variables | |||
| WPOI at interface | |||
| Type 1 or 2 or 3 | 13 (22) | 0 | 1 (3) |
| Type 4 | 26 (43) | 5 (19) | 11 (42) |
| Type 5 | 21 (35) | 6 (29) | 13 (62) |
| Perineural invasion | |||
| Absent | 36 (60) | 5 (14) | 12 (33) |
| Small nerves <1 mm | 17 (28) | 3 (18) | 8 (47) |
| Large nerves ≥1 mm | 7 (12) | 3 (43) | 5 (71) |
| LI at interface | |||
| Little or none | 14 (32) | 4 (29) | 7 (50) |
| Large patches | 27 (45) | 3 (11) | 9 (33) |
| Continuous band | 19 (23) | 4 (21) | 9 (47) |
| LVSI | |||
| Absent | 52 (87) | 10 (19) | 22 (42) |
| Present | 8 (13) | 1 (13) | 3 (36) |
| Tumor grade | |||
| Well differentiated | 11 (18) | 1 (9) | 1 (9) |
| Moderately differentiated | 36 (60) | 5 (14) | 16 (44) |
| Poorly differentiated | 13 (22) | 5 (38) | 8 (61) |
| Margins after first resection | |||
| Negative | 56 (93) | 11 (20) | 23 (41) |
| Positive** | 4 (7) | 0 | 2 (50) |
| Tumor depth | |||
| <4 mm | 18 (30) | 0 | 6 (33) |
| ≥4 mm | 25 (42) | 5 (20) | 10 (40) |
| Unknown | 17 (28) | 6 (35) | 9 (53) |
| BGS risk category | |||
| Low | 4 (7) | 0 | 0 |
| Intermediate | 24 (40) | 2 (17) | 8 (33) |
| High | 32 (53) | 9 (28) | 17 (53) |
| Systemic variables | |||
| Age | |||
| Median (minimum-maximum) | 60 (23–85) | 56 (42–77) | 56 (23–78) |
| Sex | |||
| Female | 22 (37) | 5 (23) | 11 (50) |
| Male | 38 (63) | 6 (16) | 14 (37) |
| Comorbidity (ACE-27) | |||
| None to mild (0–1) | 46 (77) | 7 (15) | 17 (37) |
| Moderate to severe (2–3) | 14 (33) | 4 (29) | 8 (57) |
| Immune compromise | |||
| Absent | 49 (82) | 6 (12) | 17 (35) |
| Present | 11 (18) | 5 (45) | 8 (73) |
Abbreviations: AJCC, American Joint Committee on Cancer; WPOI, worst pattern of invasion; LI, lymphocytic infiltrate at tumor/host interface; LVSI, lymphovascular space invasion; BGS, Brandwein–Gensler score; ACE-27, Adult Comorbidity Evaluation-27 in which comorbidity is graded as none (grade 0), mild (grade 1), moderate (grade 2), and severe (grade 3).
The denominator used for calculation of percentage is the number from the column “overall cohort.”
All were negative after re-resection.
Brandwein–Gensler risk model
Of the 60 evaluable patients for BGS, 4 (7%) were classified as “low” risk (score 0), 24 (40%) as “intermediate” risk (scores 1–2,) and 32 (53%) as “high” risk (scores 3–9). Due to the small numbers and absence of disease-related events in the “low” risk category (and because most of the reported data support such aggregation in this system15), this group was combined with the “intermediate” risk category. The “low” and “intermediate” categories were then labeled together as “low-BGS” for comparative analyses with the “high” risk group labeled as “high-BGS.” “High-BGS” significantly correlated with higher local recurrences and distant metastases. The distribution of recurrences and disease-related survival across the two BGS categories are shown in Table 2.
TABLE 2.
Univariate correlation of Brandwein–Gensler score and disease outcomes.
| BGS risk categories | Total | Local recurrence
|
Regional recurrence
|
Distant metastasis
|
Any disease recurrence
|
Deaths of disease
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (% of total) | No | Yes (% of total) | No | Yes (% of total) | No | Yes (% of total) | No | Yes (% of total) | No | ||
| Low* | 28 | 2 (7) | 26 | 5 (18) | 23 | 1 (4) | 27 | 8 (29) | 20 | 0 | 28 |
| High | 32 | 9 (28) | 23 | 9 (28) | 23 | 8 (25) | 24 | 17 (53) | 15 | 12 (38) | 20 |
| Total | 60 | 11 (18) | 49 | 14 (23) | 46 | 9 (15) | 51 | 25 (42) | 35 | 12 (20) | 48 |
| p value | .048† | .379 | .029† | .054 | < .001† | ||||||
Abbreviation: BGS, Brandwein–Gensler score.
Low BGS risk comprised low (score 0) and intermediate (score 1–2) BGS risk groups.
Statistically significant p value on chi-square or Fisher’s exact test.
Immune compromise
Immune compromise was recorded in 11 patients (18%). The causes were autoimmune diseases (n = 4; 2 patients each with systemic lupus erythematosus and lichen planus,20 all 4 patients were administered immuno-suppressant drugs), Hodgkin lymphoma (n = 2; of which 1 patient had whole body irradiation), immunoglobulin M and A deficiency (n = 1), history of chemoradiation for breast cancer22 (n = 1), acquired immunodeficiency syndrome (n = 1), and chronic debilitating illness (n = 2; 1 patient with poorly controlled diabetes23 and 1 patient with diabetes and cirrhosis).24 The presence of immune compromise correlated significantly with disease recurrences (p = .039). Five of the 11 patients (45%) with immune compromise had recurrences at the primary site compared to 6 of 49 patients (12%) with no immune compromise (p = .01). Although not statistically significant, presence of immune compromise showed a trend toward correlation with regional recurrence (p = .05) and distant metastasis (p = .05). Six of 11 patients (55%) with immune compromise died of disease compared to only 6 of 49 patients (12%) without it (p = .005).
Margins
After first resection, “negative” margins were recorded in 56 patients (93%) and “positive” in 4 patients (7%). All 4 patients reported to have positive surgical margins underwent re-resection. Of these 4 patients, microscopic SCC was detected only in 1 patient and was resected to a negative margin; this patient is alive and disease-free. Of the remaining 3 patients with no SCC on re-resection, 1 is alive and disease-free 6 years after wedge excision for lung metastasis, 1 died of aggressive regional recurrence but was free of local disease, and the third died of cardiopulmonary compromise. Thus, none of the patients had final truly positive margin after re-resection. However, for the purpose of statistical analyses of margins, the 4 of 60 patients (7%) who were reported to have positive margins after their first resection were considered “positive.” These analyses did not demonstrate any negative impact of positive margins on local control or any survival outcome (Table 3).
TABLE 3.
Univariate Cox regression analysis for local control, disease-specific survival, and overall survival with hazard ratio (95% confidence intervals) for the entire study cohort.
| Variables | Local control
|
DSS
|
OS
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 0.98 (0.04–1.03) | .63* | 0.97 (0.93–1.02) | .37* | 1.00 (0.96–1.04) | .87 |
| Site, tongue vs other sites | 1.5 (0.4–5.68) | .54 | 1.79(0.48–6.62) | .38 | 1.03 (0.40–2.640 | .94 |
| Smoking, ever vs never | 0.48 (0.14–1.58) | .23* | 0.39 (0.12–1.2) | .10* | 0.56 (0.21–1.4) | .24* |
| Comorbidity, ACE-27 2–3 vs 0–1 | 2.23 (0.65–7.65) | .22 | 2.92 (0.92–9.24) | .068 | 3.38 (1.32–8.61) | .011† |
| Immune compromise, present vs absent | 4.65 (1.40–15.36) | .012† | 6.15 (1.94–19.48) | .002† | 4.23 (1.62–11.08) | .003† |
| T classification, increasing | 1.57 (0.97–2.55) | .066 | 1.88 (1.17–3.01) | .008† | 1.83 (1.25–2.68) | .002† |
| T classification, T3–T4 vs T1–T2 | 2.54 (0.76–8.41) | .127 | 2.94 (0.94–9.19) | .064 | 3.69 (1.48–9.2) | .005† |
| Nodal disease, present vs absent | 2.62 (0.79–8.65) | .115 | 4.84 (1.41–16.61) | .012† | 3.13 (1.23–7.92) | .016† |
| AJCC stage, III–IV vs I–II | 5.13 (1.1–23.9) | .037† | 13.51 (1.71–106.82) | .014† | 7.38 (2.1–25.9) | .002† |
| Margins after first resection, positive vs negative | 0.04 (0.00–813) | .53* | 0.04 (0.00–454) | .50* | 0.56 (0.07–4.25) | .57* |
| Adjuvant treatment, any vs none | 1.37 (0.40–4.69) | .61 | 3.57 (1.10–11.54) | .033* | 2.4 (0.91–6.31) | .07 |
| Tumor depth, ≥4 mm vs ≤4 | 5.32 (0.03–760.28) | .284 | 5.15 (0.61–41.13) | .13 | 3.89 (0.82–18.27) | .086 |
| LVSI, yes vs no | 0.64 (0.08–5.11) | .68* | 1.48 (0.32–6.78) | .61 | 2.27 (0.79–6.51) | .12 |
| Grade, poor vs moderate vs well differentiated | 2.73 (1.01–7.37) | .047† | 2.54 (0.99–6.56) | .053 | 2.48 (1.14–5.38) | .021† |
| BGS, high vs low | 5.08 (1.08–23.78) | .039† | Could not be evaluated‡ | 26.2 (3.45–199.1) | .002† | |
| WPOI 5 vs 1–4 | 2.93 (0.87–9.8) | .08 | 6.54 (2.43–17.59) | .000† | 6.54 (2.43–17.6) | .000† |
| LI pattern 3 vs 1–2 | 2.09 (0.61–7.16) | .24 | 3.72 (1.42–9.72) | .007† | 3.72 (1.42–9.72) | .007† |
| PNI ≥1 mm vs absent and <1 mm | 4.21 (1.05–16.97) | .04† | 11.79 (3.83–36.29) | .000† | 11.8 (3.83–36.29) | .000† |
Abbreviations: DSS, disease-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; LVSI, lymphocytic infiltrate at tumor/host interface; BGS, Brandwein–Gensler score; WPOI, worst pattern of invasion; LI, lymphovascular invasion; PNI, perineural invasion.
ACE-27, Adult Comorbidity Evaluation-27 where comorbidity is graded as none (grade 0), mild (grade 1), moderate (grade 2), and severe (grade 3).
Variables with negative regression coefficient.
Variables with significant HR.
DSS could not be evaluated in Cox analyses because there was no event in the “low-BGS” category.
Patterns of recurrence
Disease recurred in a total of 25 patients (42%). Eight patients recurred at more than 1 site for a total of 11 local recurrences, 14 regional recurrences and 9 distant metastases. The site of recurrence in the 25 patients was distributed as 6 local, 7 regional, 4 distant, 3 locoregional, 1 local and distant, 3 regional and distant, and 1 local, regional, and distant. The distribution of recurrences by various tumor and pathological variables is presented in Table 1. The 11 local recurrences are grouped together in Table 1 because, in all these patients, the disease recurred first at the local site alone or in combination with recurrence at another site. The pattern of recurrence was local alone in 6 patients, local followed by regional and distant in 1 patient, local followed by distant in 1 patient, and simultaneous locoregional in 3 patients.
Correlation between variables
In patients with immune compromise, 82% had “high-BGS” (p = .048). Presence of immune compromise correlated with nodal metastasis (p = .001). No correlation was seen with high T classification (p = .712) or administration of adjuvant therapy (p = .071). “High-BGS” risk category correlated with T classification (p = .043), nodal metastasis (p < .001), and administration of adjuvant therapy (p = .023) (Figure 1). No correlation of immune compromise or BGS with margin status, age, tumor site, comorbidity, or smoking status was observed. The correlation and confounding effect of the variables was adjusted for in the multivariate models.
FIGURE 1.
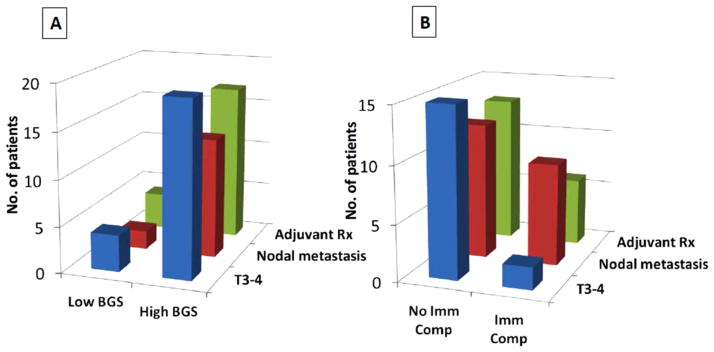
Comparison of frequency of high T classification, nodal metastasis, adjuvant therapy in: (A) patients with low and high Brandwein–Gensler score (BGS) risk groups, and (B) patients with and without immune compromise (Imm Comp). Rx, therapy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Univariate and multivariate Cox regression analyses for entire cohort (n=60)
Several models were tested for the multivariate analyses using variables found significant in univariate analysis. The number of independent variables tested in the multivariate models did not exceed 3 because of the limited number of events. The reported HRs from the multivariate analyses were based on estimation precision (95% CIs) and statistical significance as indicated by the p values.
Local control
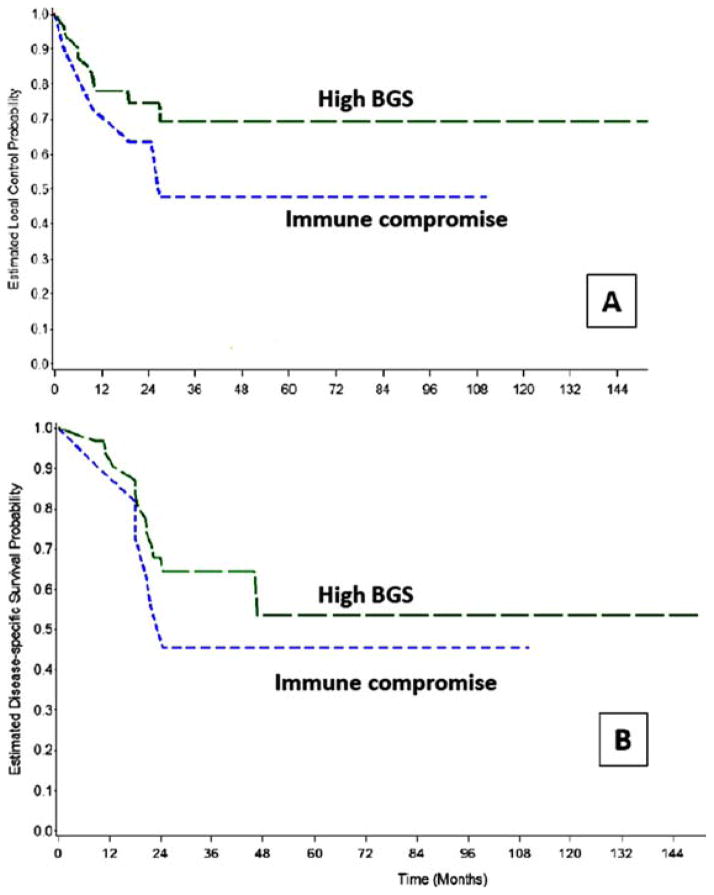
On univariate analysis, immune compromise, “high-BGS,” American Joint Committee on Cancer (AJCC) stages III to IV, and increasing grade were found to be prognostic for reduced local control (Figures 2 and 3, and Table 3). On multivariate analysis, immune compromise was the only factor that correlated with reduced local control (HR = 3.8; 95% CI = 1.04–14.26; p = .043).
FIGURE 2.
Comparison of (A) local control and (B) disease-specific survival Kaplan Meier estimates for presence of immune compromise and high Brandwein–Gensler score (BGS). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 3.
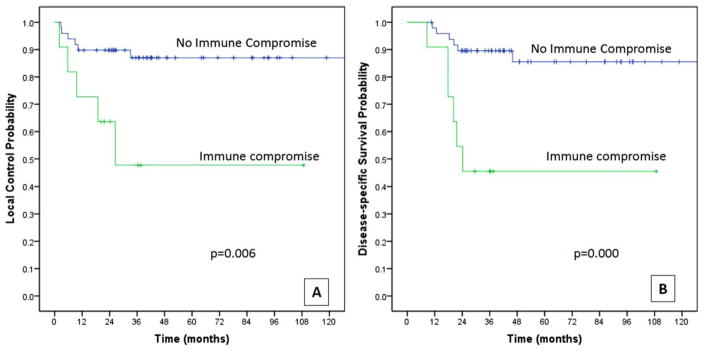
(A) Local control and (B) disease-specific survival estimates by presence or absence of immune compromise. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Disease-specific survival
On univariate analysis, immune compromise, increasing T classification, nodal metastasis, AJCC stages III to IV, absence of any adjuvant therapy and “high-BGS” were found to be prognostic for reduced DSS (Figures 2 and 3, and Table 3). HR could not be estimated for BGS because there were no deaths from disease in the “low-BGS” group. For similar reasons, BGS groups could not fit in the multivariate model for DSS. Although there were 8 recurrences (2 local, 5 regional, and 1 distant in lung) in the “low-BGS” group, no deaths occurred as each was salvaged surgically. Other factors prognostic for DSS in multivariate analysis were immune compromise (HR = 9.17; 95% CI = 2.09–40.21; p = .003) and T3 to T4 versus T1 to T2 primaries (HR = 5.42; 95% CI = 1.43–20.6; p = .013).
Overall survival
On univariate analysis, immune compromise, “high-BGS,” moderate or severe comorbidity (Adult Comorbidity Evaluation 2–3) versus mild or none (Adult Comorbidity Evaluation 0–1), T3 to T4 versus T1 to T2 primaries, nodal disease, AJCC stages III to IV, and increasing tumor grade were prognostic (Table 3). On multivariate analysis, the factors associated with reduced OS were immune compromise (HR = 5.16; 95% CI = 1.25–21.28; p = .02), “high-BGS” (HR = 30.9; 95% CI = 3.43–278.8; p = .002), and T3 to T4 versus T1 to T2 primaries (HR = 3.59; 95% CI = 1.27–10.12; p = .04).
Cox regression analyses using separate models were also performed for investigating the impact of the highest risk categories of the 3 BGS variables. Univariate results are reported in Table 3. In multivariate analyses, none of the 3 BGS variables was prognostic for local control. Significant correlation with reduced DSS was seen with PNI ≥1 mm versus absent PNI and PNI <1 mm (HR = 5.76; 95% CI = 1.23–26.96; p = .026), controlling for immune compromise, adjuvant treatment, T classification, and nodal metastasis. Both PNI ≥1 mm (HR = 3.85; 95% CI = 1.08–13.68; p = .036) and worst pattern of invasion type 5 versus types 1 to 4 (HR = 7.2; 95% CI = 1.92–27.02; p = .03) were predictive of reduced OS in multivariate models.
Subgroup Cox multivariate regression analysis of T1 and T2 primaries (n=43)
Immune compromise was the only factor prognostic for local control (HR = 10.7; 95% CI = 1.49–76.79; p = .018). For DSS, immune compromise (HR = 27.9; 95% CI = 2.46–316.8; p = .007) was prognostic in models adjusted for adjuvant therapy and tumor grade. Due to the lack of any events for DSS in the “low-BGS” group, no useful multivariate model could be generated with BGS as a variable. For OS, immune compromise (HR = 12.2; 95% CI = 1.02–146.07; p = .048) was prognostic in models adjusting for BGS, nodal metastasis, grade, and comorbidity. “High-BGS” was significantly prognostic for OS but the 95% CIs for the HR were very wide, similar to the CIs for immune compromise because of the small sample size. The relationship between BGS, immune compromise, and the frequency of local recurrences in T1 and T2 primaries is shown in Figure 4.
FIGURE 4.
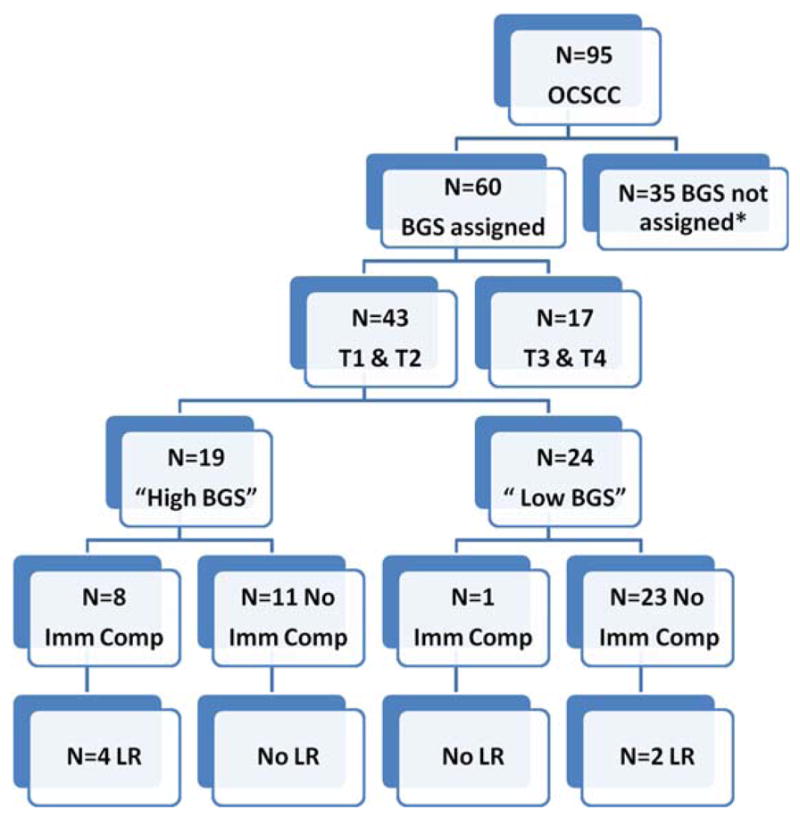
Relationship between local recurrence, Brandwein–Gensler score (BGS), and immune compromise (Imm Comp) in T1 to T2 primaries. *Please see Results for causes of nonassignment of BGS. OCSCC, oral cavity squamous cell carcinoma; LR, local recurrence. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Analysis of adjuvant therapy
Adjuvant therapy was administered in 19 patients (32%), radiotherapy in 10 patients (17%), and chemoradiotherapy in 9 patients (15%). Administration of adjuvant therapy was not a predictor for local control or any of the disease outcomes (Table 3). For patterns of recurrence by adjuvant therapy, see Table 1. Furthermore, the effect of adjuvant therapy was assessed separately in “high-BGS” patients, both in the overall cohort and the early (T1 and T2) primaries. In patients with “high-BGS” in the overall cohort (n = 32 of 60 patients), absence of any adjuvant therapy did not correlate significantly with reduced local control (HR = 6.3; 95% CI = 0.39–101; p = .192). Both the 2-year and 5-year local control of patients receiving adjuvant therapy were 80% compared to 69% at 2 years and 59% at 5 years in patients with no adjuvant therapy (log rank, p value = .442; Figure 5). In the group of T1 to T2 primaries with “high-BGS” (n = 19 of 43 patients), the 2-year local control for patients receiving adjuvant therapy was 82% compared to 83% in patients with no adjuvant therapy, whereas the 5-year estimates were 82% in the adjuvant therapy group and 62% in the no adjuvant group (log rank, p value = .734; Figure 5). The numbers are small to make definitive conclusions about the role of adjuvant therapy in “high-BGS.”
FIGURE 5.
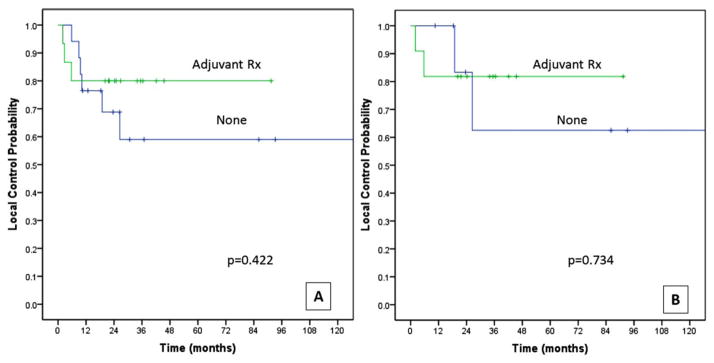
Local control estimates by administration of Adjuvant therapy for (A) High Brandwein–Gensler score (BGS) patients in the entire study cohort (n = 32), and (B) high BGS patients in a cohort with T1 to T2 primaries (n = 19). Rx, therapy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The retrospective assignment of the BGS could be done for 60 of the original 95 patients. However, there were no significant differences in any of the main study variables between the cohort of 60 with BGS and the group of 35 for whom BGS was not available (age, p = .953; sex, p = .908; primary subsite, p = .995; T classification, p = .559; nodal positivity, p = .089; AJCC stage, p = .171; tumor depth, p = .929; comorbidity, p = .958; immune compromise, p = .611; and adjuvant therapy, p = .12). Furthermore, there was no difference in the disease outcomes (local recurrence, p = .884; any disease recurrence, p = .873) or survival status (p = .171). There was a significant difference between the 2 groups in the mean follow-up duration, attributed to the long study span (1995–2010). Complete histology slides could not be retrieved for the 24 patients enrolled in the early years of the study. Mean follow-up for the study cohort was 62 months (minimum-maximum, 24–165 months) compared to 94 months (minimum-maximum, 45–204 months) in the group without a BGS.
DISCUSSION
In our margin-negative, TLM-treated patients with oral cavity SCC, the current analysis reveals that immune compromise and “high-risk” BGS (univariate) are variables that significantly correlate with local control and disease-specific survival. Overall, local recurrence developed in 18% (n = 11 of 60) of our patients with negative histological margins, but in the “high-BGS” group it was 28% (n = 9 of 32), and in the immune compromised group it reached 45% (n = 5 of 11). Death from disease occurred in 20% (n = 12 of 60) of the overall cohort, but in 38% (n = 12 of 32) of the high BGS group, and 55% (n = 6 of 11) of the immune compromised group.
Local failure rates varying from 10% to 40% have been reported across various non-TLM surgical series in patients with histologically free margins.15 Several biological factors and genetic aberrations have been cited as possible explanations for this observation. Numerous molecular assays and grading systems have been proposed to identify patients who are likely to benefit from additional therapy, albeit with negative margins. However, oral cavity SCC has been associated with a myriad of biomolecular abnormalities and no specific marker has been shown to uniformly and reliably predict tumor behavior.25 Cost and time issues further limit the utility of molecular assays. However, histopathology-based scoring systems are applicable to contemporary clinical use. One of these systems, proposed by Brandwein–Gensler et al,13 is of current interest, although its generalizability and utility in TLM-treated cases is yet to be established.
Prognostic significance and application of Brandwein–Gensler score in transoral laser microsurgery
Our study assesses the utility of BGS for the first time in a setting of oral cavity SCC treated by TLM. When BGS was first proposed for risk classification in patients with negative margins, the authors failed to find any prognostic significance of positive or close margins for local recurrence. A possible reason cited was the ability to achieve wider resection margins, owing to the advances in free flap reconstruction. The validation studies, that followed, differed in their interpretation of margin significance. The first validation study18 was done on a mix of oral cavity, oropharyngeal, and laryngeal carcinomas from 3 institutions. This study found close margins to be important for OS, but only in univariate analysis;18 the observation was attributed to inherent variations in surgical technique within and between centers. In the other 2 studies by Vered et al16,17 on a population of 50 patients with tongue SCC, positive margins were prognostic for locoregional recurrence in univariate analyses. By contrast, in our study, all patients were treated homogeneously using a resection principle that focuses on achieving negative margins, with maximal sparing of normal tissues. We verified, with re-resection when necessary, that final margins were indeed negative in all patients from this study, thus physically minimizing or even eliminating true-positive margins as a variable.
Our study serves as the first independent validation for the feasibility of BGS application in a tightly controlled group of TLM-treated patients with oral cavity SCC. It also provided correlation of BGS with DSS at the univariate level, an outcome that is more informative for accurate prognostication than OS, the primary outcome in all previous studies. In addition to local recurrence, our study also evaluates the association of BGS with distant metastasis.
Eighty-two percent of the local recurrences and 89% of the distant metastasis occurred in the “high-BGS” group, the frequencies being significantly different from the “low-BGS” risk group (Table 3). “High-BGS” risk category was associated with poorer local control, DSS, and OS estimates in univariate analysis. When evaluated in multivariate analysis, immune compromise emerged as the only strong predictor of local control, whereas “high-BGS” was not prognostic for local control. Negative prognostication with “high-BGS” was retained in the multivariate analysis for OS, consistent with previous studies,15,16,18 albeit with wide 95% CI for the HR. Deaths from disease occurred exclusively in the “high-BGS” risk category, compared to none in the “low-BGS” risk category. For this reason, the influence of DSS could not be evaluated with Cox regression analysis (see Results), although the univariate analysis indicates a strong association of disease-related deaths with “high-BGS.” It is not possible to make definitive statements regarding the predictive power of BGS, because it correlated with other prognostic variables, such as T classification and N classification, of which high T classification was prognostic in multivariate analysis for reduced DSS.
Prognostic significance of immune compromise
Host immune compromise emerged as a consistent prognosticator for local control and survival outcomes. All pattern disease recurrence occurred significantly more often in patients with immune compromise than in patients without it (73% vs 35%, respectively; p = .039). Immune compromise also correlated significantly with higher frequency of local recurrences and deaths from disease. Similar findings were observed in our preliminary study of 95 patients.14 Host immune compromise was also associated with “high-BGS,” however, unlike “high-BGS,” it did not correlate with advanced T classification (see Results and Figure 1) and remained independently prognostic for local control and DSS in multivariate analysis.
Systemic immunity is considered to provide better immune surveillance compared to the LI at the tumor/ host interface.26,27 Composition of the infiltrate in the tumor microenvironment has been shown to contain lymphocytes that have absent or weak cytolytic activity, and to demonstrate a “widespread functional paralysis” toward tumor cells.26,27 In contrast, blood-borne leukocytes have a greater cytotoxic potential toward tumor cells.26 The hypothesis exists in the literature that depressed immune function, in general, predates carcinogenesis not only for head and neck squamous cell carcinoma but also for other solid tumors of the lung, esophagus, and cervix.28–31 It is therefore intuitive to consider that immune compromise, secondary to innate or iatrogenic immunosuppressive conditions, as documented in our patients,20,22–24 only worsens preexisting anergy. Even in the absence of any known immune deficiency, patients with head and neck cancer have demonstrated impaired cell-mediated immune responses32. This lowered host resistance from immune compromise clearly facilitates disease recurrence.
In addition to conditions widely known to directly impact the immune system, our immune compromised cohort also included chronic debilitating illnesses like diabetes and cirrhosis. Diabetes mellitus has been demonstrated to cause not only depressed immune functions23 but also increase the risk of and worsen the long-term outcomes in oral cavity SCC,33,34 breast,35 colorectal,36 and endometrial37 carcinomas. Impairment of immune function and an increased risk of carcinogenesis has also been suggested for patients with cirrhosis both in hepatic and extrahepatic carcinomas.24,38 Both patients with these 2 chronic illnesses in our study died of disease, 1 because of locoregional recurrence and 1 because of locoregional recurrence and distant metastasis. Therefore, despite adding a degree of heterogeneity, the above cited data and the observed events justify inclusion of such cases in our immune compromised group.
Based on our findings, development of treatment strategies directed at promotion of antitumor immunorestorative mechanisms needs investigation. Research on immunotherapy for head and neck cancer is in a seminal phase. Immunotherapy for minimal residual disease or reduction of distant metastases after standard therapy of head and neck squamous cell carcinoma is currently under evaluation.39 Immune compromise has long been correlated with increased risk of malignancy, including oral cancer.31,40 Both the current study and our previous report14 on TLM-treated oral cavity SCC underscore the need for developing strategies that restore tumor-specific immune responses, possibly using immunotherapy as adjuvant treatment in immunocompromised hosts. Immune compromise in head and neck cancer has also been linked to nutritional status,41 in particular zinc deficiency,30,42 which should be monitored and deficits rectified.
Prognostic significance of margins
Positive margins were recorded in 4 of 60 patients after first TLM resection; however, all 4 patient’s tumor beds were re-resected and the residual tumor was identified in only 1 of these 4 patients. Thus, effectively, complete histological clearance had been achieved in 59 of 60 patients with the first procedure and eventually in all 60 patients with the re-resection. It is, therefore, important to note that the aforementioned predictors, immune compromise and BGS, were identified in patients using strict margin management and re-resection principles, which are intrinsic to the TLM technique. Negative margins remain critical for local control as demonstrated by multiple series.7–12,15 TLM is a technique that can achieve the same in the oral cavity and other sites of the aerodigestive tract with minimal normal tissue disruption.14,43
Using the multibloc TLM approach for oral cancer resection,14 negative margins are verified with intraoperative frozen sections. Furthermore, supplemental, normal-appearing tissue is sampled from the surgical defect, both deep and mucosal, for the final surgical margin assessment, which adds another level of margin clearance.44 The main advantage of TLM over en bloc transoral excision is precision and confidence of margin management, the key element in any curative-intent cancer resection. The transtumoral resection technique of TLM is particularly efficacious in intraoperative depth assessment of the tumors, in contrast to traditional en bloc transoral resection in which there is a higher risk of cutting through or too close to the tumor. The laser affords a bloodless cut and improved precision. The operating microscope provides high magnification and illumination of surface and deep tissue, which allows confident clearance of margins and detection of unexpected disease extensions (eg, along the neurovascular structures).
Risk prognostication of early primaries
Primary site recurrences occurred in 14% of the T1 and T2 primaries, compared to almost 30% (n = 5 of 17) of the T3s and T4s. This is consistent with local failure rates of 10% to 34% reported in the literature for early stage oral cancers.2,3,5 Treatment is usually intensified for advanced tumors, which is why we performed subset analyses for early tumors, to identify prognostic variables and the value of BGS modeling. “High-BGS” was associated with reduced OS but not local control. Presence of immune compromise was again the strongest predictor for this group in the Cox analyses. Two local recurrences, however, occurred in patients with no immune compromise and in the “low-BGS” group (Figure 4); other variables that went uninvestigated may be responsible. Although the sample for this subset analysis was small, our data indicates that BGS may facilitate a method for risk assessment of small TLM-treated oral cavity primaries in the absence of other prognosticators, such as immune compromise and nodal metastasis.
Limitations
Limitations of retrospectively performed, histopathologic scoring and analysis may argue against application of findings from this study. However, it is the first to explore immune compromise as a prognosticator and the utility of the BGS risk assessment method for oral cavity SCC treated to negative margins with TLM, a minimally invasive approach. Patients with intrinsic, iatrogenic, or chronic conditions causing immunodeficiency were considered immune compromised for our cohort. Laboratory assays for T lymphocyte counts, blood monocyte function, or skin tests for anergy, providing additional evidence for immune compromise, may have been more definitive and could be considered for future prospective studies. However, all the categories included in our study have been literature documented as compromising optimum function of the immune system. For BGS risk assessment, the small sample size was an impediment to statistical validity, especially evident for the subset analyses of the T1 and T2 primaries, and the significance of adjuvant therapy in the “high-risk” group. However, of all the reports that have assessed the BGS model, our study has the longest follow-up, consists of a cohort treated with a uniform surgical technique, and was restricted to patients with negative margins. Further validation of the BGS model in larger cohorts is desirable before its application as an adjuvant therapy determinant for TLM-treated patients with oral cancer. Finally, there may have been other variables, such as molecular aberrations, which were influential, but beyond the scope of our investigation.
CONCLUSIONS
Our study demonstrates that in a setting of TLM for oral cavity SCC resection with optimum margin control, systemic immune compromise is a strong independent prognosticator of local control and survival outcomes. More intense follow-up for patients with a known history of immune compromise should be implemented with correction of the underlying cause of suppressed immunity, if feasible. Inclusion of an immunologist in the multidisciplinary care team, improvement of nutritional status, and, eventually, immunotherapy may be important additions to the existing management of such patients. At least at the univariate level, high BGS also correlated with greater frequency of local recurrences and deaths from disease. They were also associated with significantly higher distant metastatic rates, suggesting the need for close systemic surveillance. On balance, however, a patient’s compromised immune status seems to be the more critical factor in outcomes after margin negative, TLM resection of oral cavity carcinoma, and requires careful assessment and a remedy, if feasible.
Acknowledgments
Support for statistical analysis (Ningying Wu) came from the Siteman Comprehensive Cancer Center Support Grant (NIH Grant # P30CA091842).
Footnotes
This work was presented at the American Head and Neck Society annual meeting, Orlando, Florida, April 10, 2013.
References
- 1.González–García R, Naval–Gías L, Román–Romero L, Sastre–Pérez J, Rodríguez–Campo FJ. Local recurrences and second primary tumors from squamous cell carcinoma of the oral cavity: a retrospective analytic study of 500 patients. Head Neck. 2009;31:1168–1180. doi: 10.1002/hed.21088. [DOI] [PubMed] [Google Scholar]
- 2.Iseli TA, Lin MJ, Tsui A, Guiney A, Wiesenfeld D, Iseli CE. Are wider surgical margins needed for early oral tongue cancer? J Laryngol Otol. 2012;126:289–294. doi: 10.1017/S002221511100332X. [DOI] [PubMed] [Google Scholar]
- 3.Rennemo E, Zätterström U, Boysen M. Outcome of local failures after oral cancer—recurrence vs. second primary. J Oral Pathol Med. 2010;39:657–661. doi: 10.1111/j.1600-0714.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 4.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32:30–34. doi: 10.1054/ijom.2002.0313. [DOI] [PubMed] [Google Scholar]
- 5.Sessions DG, Spector GJ, Lenox J, Haughey BH, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112:616–625. doi: 10.1097/00005537-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kurita H, Nakanishi Y, Nishizawa R, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010;46:814–817. doi: 10.1016/j.oraloncology.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Byers RM, Bland KI, Borlase B, Luna M. The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978;136:525–528. doi: 10.1016/0002-9610(78)90275-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen TY, Emrich LJ, Driscoll DL. The clinical significance of pathological findings in surgically resected margins of the primary tumor in head and neck carcinoma. Int J Radiat Oncol Biol Phys. 1987;13:833–837. doi: 10.1016/0360-3016(87)90095-2. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Guillamondegui O, Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21:408–413. doi: 10.1002/(sici)1097-0347(199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Jones AS. Prognosis in mouth cancer: tumour factors. Eur J Cancer B Oral Oncol. 1994;30B:8–15. doi: 10.1016/0964-1955(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 11.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg. 1990;160:410–414. doi: 10.1016/s0002-9610(05)80555-0. [DOI] [PubMed] [Google Scholar]
- 12.Slootweg PJ, Hordijk GJ, Schade Y, van Es RJ, Koole R. Treatment failure and margin status in head and neck cancer. A critical view on the potential value of molecular pathology. Oral Oncol. 2002;38:500–503. doi: 10.1016/s1368-8375(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 13.Rogers SN, Brown JS, Woolgar JA, et al. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Sinha P, Hackman T, Nussenbaum B, Wu N, Lewis JS, Jr, Haughey BH. Transoral laser microsurgery for oral squamous cell carcinoma: oncologic outcomes and prognostic factors. Head Neck. 2013 doi: 10.1002/hed.23293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandwein–Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 16.Vered M, Dayan D, Dobriyan A, et al. Oral tongue squamous cell carcinoma: recurrent disease is associated with histopathologic risk score and young age. J Cancer Res Clin Oncol. 2010;136:1039–1048. doi: 10.1007/s00432-009-0749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vered M, Dobriyan A, Dayan D, et al. Tumor-host histopathologic variables, stromal myofibroblasts and risk score, are significantly associated with recurrent disease in tongue cancer. Cancer Sci. 2010;101:274–280. doi: 10.1111/j.1349-7006.2009.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandwein–Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Bai S, Carroll W, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7:211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charazinska–Carewicz K, Ganowicz E, Krol M, Gorska R. Assessment of the peripheral immunocompetent cells in patients with reticular and atrophic-erosive lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:202–205. doi: 10.1016/j.tripleo.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Altundag O, Gullu I, Altundag K, et al. Induction chemotherapy with cisplatin and 5-fluorouracil followed by chemoradiotherapy or radiotherapy alone in the treatment of locoregionally advanced resectable cancers of the larynx and hypopharynx: results of single-center study of 45 patients. Head Neck. 2005;27:15–21. doi: 10.1002/hed.20107. [DOI] [PubMed] [Google Scholar]
- 22.Prestwich RJ, Errington F, Hatfield P, et al. The immune system--is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20:101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 24.Young GP, Dudley FJ, Van Der Weyden MB. Suppressive effect of alcoholic liver disease sera on lymphocyte transformation. Gut. 1979;20:833–839. doi: 10.1136/gut.20.10.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braakhuis BJ, Bloemena E, Leemans CR, Brakenhoff RH. Molecular analysis of surgical margins in head and neck cancer: more than a marginal issue. Oral Oncol. 2010;46:485–491. doi: 10.1016/j.oraloncology.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Hoşal AS, Unal OF, Ayhan A. Possible prognostic value of histopathologic parameters in patients with carcinoma of the oral tongue. Eur Arch Otorhinolaryngol. 1998;255:216–219. doi: 10.1007/s004050050046. [DOI] [PubMed] [Google Scholar]
- 27.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 28.Wolf GT, Lovett EJ, III, Peterson KA, Beauchamp ML, Baker SR. Lymphokine production and lymphocyte subpopulations in patients with head and neck squamous carcinoma. Arch Otolaryngol. 1984;110:731–735. doi: 10.1001/archotol.1984.00800370033008. [DOI] [PubMed] [Google Scholar]
- 29.Hadden JW, Endicott J, Baekey P, Skipper P, Hadden EM. Interleukins and contrasuppression induce immune regression of head and neck cancer. Arch Otolaryngol Head Neck Surg. 1994;120:395–403. doi: 10.1001/archotol.1994.01880280023004. [DOI] [PubMed] [Google Scholar]
- 30.Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol. 2003;3:1061–1071. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 31.Penn I. Depressed immunity and the development of cancer. Clin Exp Immunol. 1981;46:459–474. [PMC free article] [PubMed] [Google Scholar]
- 32.Pumhirun P, Wasuwat P. Anergy testing in patients with head and neck cancer. Asian Pac J Allergy Immunol. 2003;21:189–192. [PubMed] [Google Scholar]
- 33.Wu CH, Wu TY, Li CC, Lui MT, Chang KW, Kao SY. Impact of diabetes mellitus on the prognosis of patients with oral squamous cell carcinoma: a retrospective cohort study. Ann Surg Oncol. 2010;17:2175–2183. doi: 10.1245/s10434-010-0996-1. [DOI] [PubMed] [Google Scholar]
- 34.Goutzanis L, Vairaktaris E, Yapijakis C, et al. Diabetes may increase risk for oral cancer through the insulin receptor substrate-1 and focal adhesion kinase pathway. Oral Oncol. 2007;43:165–173. doi: 10.1016/j.oraloncology.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 36.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 37.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 39.Davidson HC, Leibowitz MS, Lopez–Albaitero A, Ferris RL. Immunotherapy for head and neck cancer. Oral Oncol. 2009;45:747–751. doi: 10.1016/j.oraloncology.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas DW, Seddon SV, Shepherd JP. Systemic immunosuppression and oral malignancy: a report of a case and review of the literature. Br J Oral Maxillofac Surg. 1993;31:391–393. doi: 10.1016/0266-4356(93)90197-5. [DOI] [PubMed] [Google Scholar]
- 41.Brookes GB, Clifford P. Nutritional status and general immune competence in patients with head and neck cancer. J R Soc Med. 1981;74:132–139. doi: 10.1177/014107688107400209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes G, West A, Good RA. Nutrition, immunity, and cancer--a review. Part III: effect of diet on the diseases of aging. Clin Bull. 1979;9:91–106. [PubMed] [Google Scholar]
- 43.Hinni ML, Zarka MA, Hoxworth JM. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope. 2013;123:1190–1198. doi: 10.1002/lary.23900. [DOI] [PubMed] [Google Scholar]
- 44.Hinni ML, Ferlito A, Brandwein–Gensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35:1362–1370. doi: 10.1002/hed.23110. [DOI] [PubMed] [Google Scholar]