In vitro transcribed mRNA is an immunogenic and programmable molecule[1] that embodies key advantages as an antigen-encoding gene for cell-based immunotherapy. For example, the use of mRNA obviates prior knowledge of patient’s Human Leukocyte Antigen (HLA) type, a pre-requisite for class I peptide epitope-based approaches. mRNA is also expressed more efficiently than DNA because it does not need to cross the nuclear envelope. To date a number of methods aimed at using mRNA to stimulate immune responses have been studied. They include transfection of antigen-presenting cells (APCs) ex vivo with mRNAs encoding defined antigens[1, 2] or with total mRNA repertoire from tumor cells,[3] as well as direct injection of mRNA in vivo.[4–6] Dendritic cells (DCs) are the most common APCs that have been loaded with antigen-encoding mRNA, as well as antigens in a variety of formats. A major disadvantage of using transfected DCs as a vaccine is that the process of harvesting, culturing and loading DCs is time- and resource-intensive. It requires that patients undergo at least one 4-hour leukapheresis procedure, followed by separation of the peripheral blood mononuclear cells (PBMCs), from which the monocytes are isolated and cultured for a week in a defined medium with cytokines. The resulting DCs are typically matured before or after being loaded with mRNA and frozen for storage. Aliquots are subsequently thawed prior to administration to patients. In efforts to circumvent these somewhat cumbersome procedures, many groups have investigated direct injection of antigen-encoding mRNA.[4–6] While this has the advantage of simplicity, there are still manufacturing steps that require time, specialized resources and often proprietary formulations. This is particularly true in the case of mRNA, which has distinct advantages as a source of antigen but must be protected in vivo from nucleases. Recently, a study demonstrated that DC vaccination is significantly less effective in antigen-presenting cell (APC)-deficient mice[7] compared to wild-type mice. The authors concluded that ex vivo transferred DCs function primarily as vehicles for transferring antigens to endogenous APCs, which are responsible for the subsequent activation of T cells.[7] This raises the possibility of using alternative cell types for mRNA cell based vaccination. In the search of such an alternative, we find that the blood is an attractive cell source because it is biocompatible, quickly available in large quantities and contains a variety of immune cells. Notably, erythrocytes loaded with protein tumor antigens have been extensively studied as vaccine carriers.[8–13] In addition, peripheral blood antigen-presenting cells loaded with tumor antigens also proved to be an effective tumor vaccine, e.g. Provenge[14] which is FDA approved in 2011. In both approaches, however, it is necessary to subject blood cells freshly derived from the body to manipulation[8–13] and cell culture[15] before arriving at the final vaccine preparation. This increases complexity and cost of treatment, dampening the prospect of broad application of cell-based vaccines.[16, 17] We hypothesize that cell-based vaccination can be achieved with a more simplified and direct approach by loading mRNA directly into whole blood cells immediately after isolation from the body. We take advantage of the fact that blood is made up of a heterogeneous cell mixture that includes not only erythrocytes, but also leukocytes and reticulocytes. Notably, reticulocytes still retain the ability to translate mRNA into proteins.[18] Hence, by loading mRNA into autologous whole blood cells, mRNA may be delivered to endogenous host APCs via erythrocytes (naturally enriched in RNase-inhibitor[19]) in form of untranslated mRNA. Additionally, leukocytes and reticulocytes may deliver both untranslated mRNA as well as protein resulting from translation of the loaded mRNA. In this report we show that blood harvested from mice can be immediately loaded with mRNA and used as a vaccine to induce B and T cell responses, as well as anti-tumor immune responses. This is a relatively simple protocol that does not involve cell culture and can generate the cellular therapy product in about an hour.
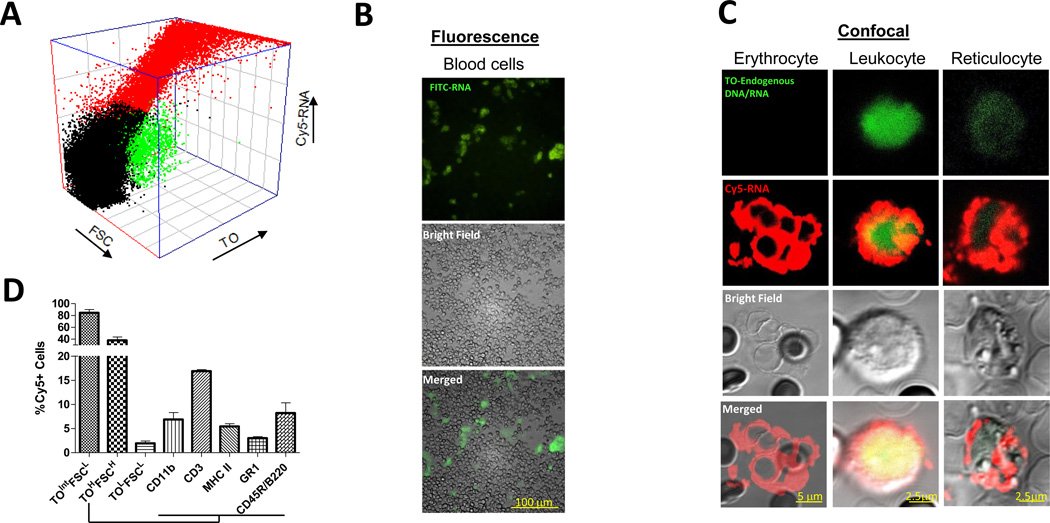
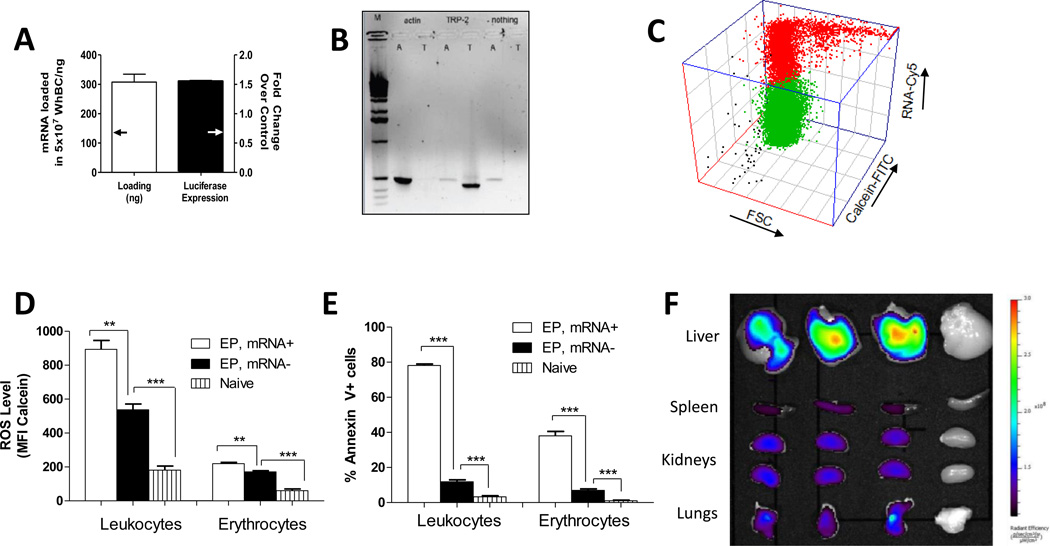
We show that mRNA was loaded by electroporation (Figure 1A) into erythrocytes (TOLowFSCLow, TO: thiazole orange, FSC: forward scatter), reticulocytes (TOIntermediateFSCLow) and leukocytes (TOHighFSCHigh). The finding was further confirmed by both fluorescence and confocal microscopy (Figure 1B and Figure 1C). Using flow cytometry, we further determined, within the leukocyte population, that mRNA was loaded into MHC class II+ antigen-presenting cells, CD3+ T cells, CD11b+ monocytes, GR1+ granulocytes and CD45R+/B220+ murine plasmacytoid DCs (Figure 1D). Quantification of the fluorescently-labeled mRNA showed that about 300ng of RNA was loaded into 5×107 blood cells (Figure 2A), and amplification by RT-PCR of full length mRNA from mRNA-loaded blood cells (Figure 2B) indicated that untranslated mRNA remained stable within the cytoplasm of whole blood cells.[19] Luciferase expression detected in luciferase mRNA-loaded blood cells (Figure 2A) confirmed the bioactivity of the mRNA. Thus, intact and functional mRNA could be loaded into whole blood cells by electroporation.
Figure 1.
Whole blood cells can be loaded with mRNA.
A) Ungated 3D plot of Cy5-labeled GFP mRNA (Cy5-RNA) loaded into reticulocytes (TOintermediateFSClow), leukocytes (TOhighFSChigh), erythrocytes (TOlowFSClow) based on size (FSC) and presence of endogenous DNA/RNA (Thiazole Orange, TO)
B) Fluorescence image of mRNA-loaded whole blood cells. GREEN: FITC-labeled GFP mRNA.
C) Confocal images of mRNA encapsulated in erythrocytes, leukocytes and reticulocytes. RED: Cy5-labeled GFP mRNA loaded by electroporation, GREEN: Thiazole stain of cellular DNA/RNA
D) Loading efficiency of Cy5-labeled GFP mRNA into reticulocytes (TOintermediateFSClow), leukocytes (TOhighFSChigh), erythrocytes (TOlowFSClow), monocytes (CD11b), T cells (CD3), antigen presenting cells (MHC II), granulocytes (GR1) and murine plasmacytoid DCs (CD45R/B220).
Figure 2.
Characterization of electroinserted mRNA, biological properties and biodistribution of mRNA-loaded whole blood cells.
A) Mass of Cy5-labeled luciferase mRNA loaded in 107 whole blood cells (left axis) and luciferase expression per 107 whole blood cells normalized to 107 non-electroporated cells
B) RT-PCR analysis of RNA recovered from whole blood cells loaded with actin mRNA (“A”), TRP-2 mRNA (“T”) or nothing, respectively. Lane M is a 1 Kb DNA ladder (Invitrogen).
C) Ungated 3D plot of cell viability based on conversion of calcein-AM to calcein in whole blood cells loaded with Cy5-labeled GFP mRNA.
D) ROS levels in whole blood cells based on mean fluorescence intensity (MFI) of intracellular calcein[30]
E) Surface phosphatidylserine analysis by annexin V staining
F) IVIS image of biodistribution of whole blood cells loaded with Cy5-labeled GFP mRNA administered intravenously via tail vein 2 hours post administration. This experiment was repeated 2 times with n=3 and one experiment is depicted. (c)–(e) were assayed 2 hours post-electroporation.
** p<0.01, *** p<0.001 (One-way anova/bonferroni multiple comparison test)
Next we characterized the biological properties of whole blood cells two hours post-electroporation. This time point was chosen because mRNA-loaded whole blood cells were typically administered into all mice by the second hour post-electroporation. We observed that mRNA-loaded blood cells remained viable (Figure 2C) based on the conversion of non-fluorescent calcein-AM to fluorescent calcein by intracellular esterases. We also found elevated levels of reactive oxygen species (ROS) in electroporated blood cells based on higher mean fluorescence intensity contributed by oxidized calcein-AM. Only those that were loaded with mRNA possessed higher levels of ROS (Figure 2D). ROS are pro-inflammatory and thereby a potentially favorable property for whole blood cell vaccines.[20] Using annexin V staining, we observed that cells loaded with mRNA externalized phosphatidylserine (PS) compared to unloaded blood cells (Figure 2E, Figure S1). Surface presentation of PS could be caused by scrambling of cell membranes lipids facilitated by pore formation during electroporation,[21] or it could indicate that the blood cells were apoptotic. To ascertain whether PS externalization was physically mediated, we tracked PS externalization immediately after electroporation and found that cells were stained positive for annexin V immediately after electroporation (Figure S2). Surface PS also appeared to be irreversible, increasing slightly during recovery but dropping back to levels seen immediately after electroporation by 24 h (Figure S2). We then analyzed mRNA-loaded whole blood cells for apoptosis based on caspase-3 activities[22–24] and found that they were comparable to naÔve cells (Figure S3). This indicated that surface PS presentation was not caspase-mediated and suggested that PS presentation might not be biologically mediated. Surface PS commonly known as “eat-me” signals displayed selectively on live mRNA-loaded cells presumably target them to the mononuclear-phagocyte system for uptake. To summarize, we show that electroporation of whole blood cells results in mRNA electroinsertion and priming for antigen uptake in vivo.
Next we determined the biodistribution of mRNA-loaded whole blood cells following intravenous administration and found that RNA-loaded whole blood cells were distributed to multiple organs, including the APC-rich liver and spleen (Figure 2F). Additionally, mRNA-loaded blood cells, despite being relatively low in abundance (Figure 1A), efficiently co-localized with APCs in vitro (Figure S4). This was consistent with prior reports on live erythrocytes that had phosphatidylserine artificially inserted in the cell membrane.[25] Hence we confirmed that mRNA-loaded whole blood cells were distributed to the liver and spleen, where they could be targeted to antigen presenting cells.
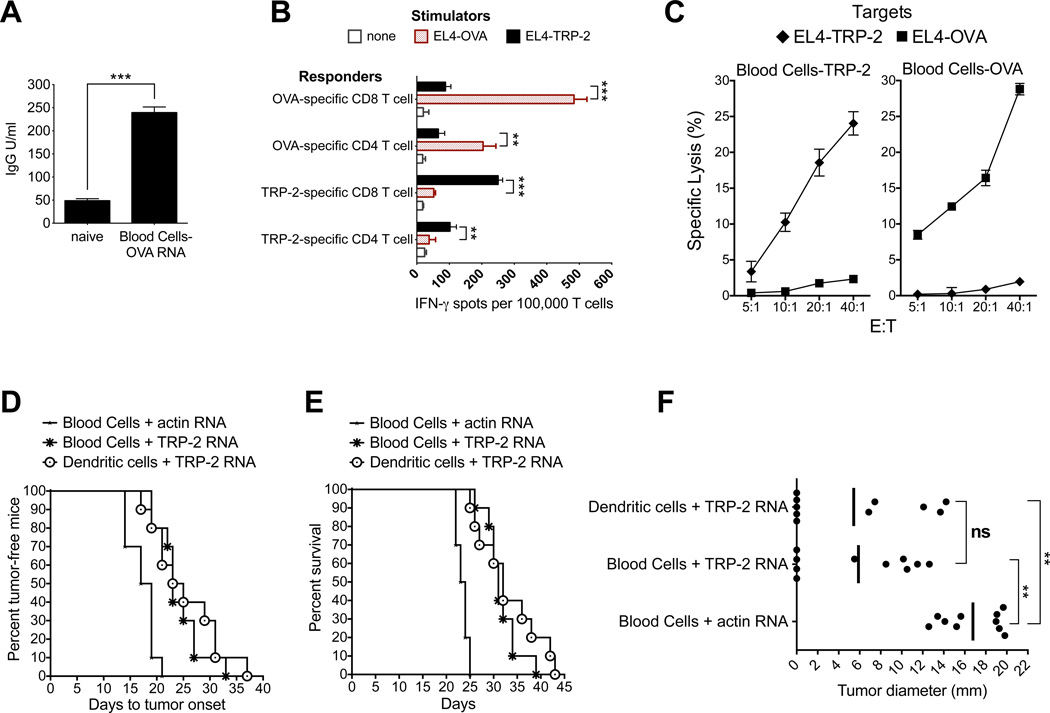
Based on the data presented in Figure 1 and 2, we hypothesized that whole blood cells loaded with mRNA encoding antigen protein could induce antigen-specific immune responses. We first monitored induction of B cell responses by measuring the presence of antigen-specific serum IgG in mice immunized twice with ovalbumin (OVA) mRNA-loaded blood cells. As shown in Figure 3A, OVA-specific serum IgG could be detected in immunized mice. To monitor induction of T cell responses, two groups of mice were immunized once with blood cells loaded with mRNA encoding either OVA or the murine melanoma antigen, tyrosinase-related protein 2 (TRP-2). Upon re-stimulation of splenic lymphocytes in vitro, we analyzed T cells for antigen-specific function by measuring IFN-γ secretion upon antigen re-encounter or lysis of target cells expressing antigen. Antigen-specific IFN-γ secretion by OVA- or TRP-2-specific CD8+ and CD4+ T cells is shown in Figure 3B. T cells also showed antigen-specific reactivity by selectively lysing target cells that expressed the corresponding antigen (Figure 3C). Hence, we conclude that mRNA-loaded blood cells injected immediately after electroinsertion and without further manipulation can induce both humoral and cellular immune response.
Figure 3.
Immunization with mRNA-loaded whole blood cells induces immune responses in vivo and is comparable to dendritic cell (DC) vaccination in the B16 melanoma immunotherapy model.
A) Induction of OVA-specific antibody responses. Serum collected from naÔve mice was used as the negative control. Statistical analysis was done using a student’s t test. ** p<0.01, *** p<0.001
B) Induction of antigen-specific IFN-γ secreting T cells in mice immunized with mRNA-loaded whole blood cells. Isolated CD4+ and CD8+ T cells were stimulated with EL4 cells previously transfected with either TRP-2 or OVA mRNA. After overnight incubation an IFN-γ ELISpot was performed. Statistical analysis was done using a student’s t test. ** p<0.01, *** p<0.001
C) Induction of antigen-specific cytotoxic T cells in mice immunized with mRNA-loaded whole blood cells. A europium-release CTL assay was performed 5-days post-stimulation. EL4 thymoma cells electroporated with antigen-encoding mRNA (TRP-2 or OVA) were used as targets to measure antigen-specific lysis.
D) Delay in tumor onset in mice immunized with mRNA-loaded whole blood cells. Time to tumor onset was recorded based on the detection of palpable tumors (4–5 mm diameter). Log-rank analysis (Mantel-Cox test) was used for statistical analysis: Blood cells+actin mRNA vs Blood cells+TRP-2 mRNA, p=0.0001; Blood cells+actin mRNA vs DCs+TRP-2 mRNA, p=0.0007; Blood cells+TRP-2 mRNA vs DCs+TRP-2 mRNA, p=0.52. Median time to tumor onset is as follows: Blood Cells+actin mRNA=18, Blood Cells+TRP-2 mRNA=23, DCs+TRP-2 mRNA=24.
E) Enhanced survival in mice immunized with mRNA-loaded whole blood cells. Survival was recorded based on tumor growth to 20 mm in diameter at which point mice were sacrificed. Log-rank analysis (Mantel-Cox test) was used for statistical analysis: Blood cells+actin mRNA vs Blood cells+TRP-2 mRNA, p=0.0001; Blood cells+actin mRNA vs DCs+TRP-2 mRNA, p=0.0001; Blood cells+TRP-2 mRNA vs DCs+TRP-2 mRNA, p=0.33. Median time to tumor onset is as follows: Blood Cells+actin mRNA=23.5, Blood Cells+TRP-2 mRNA=31, DCs+TRP-2 mRNA=32.
F) Induction of anti-tumor immunity is comparable in mice immunized with mRNA-loaded whole blood cells and DCs transfected with mRNA. Figure depicts tumor diameter in individual mice and average tumor diameter on day 22. The overall significance of the study as determined by Kruskal-Wallis (ANOVA) test is p=0.0002. The comparison between groups was done using the non-parametric Mann-Whitney test: Blood cells+actin mRNA vs Blood cells+TRP-2 mRNA, p<0.0001; Blood cells+actin mRNA vs DCs+TRP-2 mRNA, p=0.0002; Blood cells+TRP-2 mRNA vs DCs+TRP-2 mRNA, p=0.97.
We next evaluated the therapeutic efficacy of mRNA-loaded blood cells in the B16 melanoma immunotherapy model. Mice were immunized one time 2 days post-tumor implantation with mRNA-loaded whole blood cells and as a positive control, TRP-2 mRNA-loaded DCs (Figure 3D, 3E and 3F). Immunization with melanoma antigen TRP-2 mRNA-loaded whole blood cells delayed tumor onset (Figure 3D) and enhanced survival (Figure 3E) as compared to whole blood cells loaded with mouse actin mRNA. Moreover, there was no difference in tumor onset (p=0.52, Figure 3D), survival (p=0.33, Figure 3E) and average tumor diameter (p=0.97, Figure 3F) in mice immunized with TRP-2 mRNA-loaded whole blood cells and TRP-2 mRNA-loaded DCs. These results demonstrate that immunization with TRP-2 mRNA-loaded whole blood cells leads to an antitumor response that is comparable to TRP-2 mRNA-loaded DCs.
This brief report provides proof-of-concept of a rapid and affordable cellular immunotherapy. Our approach is based on the hypothesis that immunization with mRNA-loaded whole blood cells will lead to an anti-tumor immune response. As this vaccine is designed to be an autologous cell product, it is unlikely to be toxic or be affected by blood types of individuals. We have also not observed other visible side effects from this vaccine formulation in our animal experiments.
Our formulation is distinctly different from red blood cell vaccines.[8–13] Firstly, buffy coat cells (leukocytes) are not removed by Ficoll-Paque separation.[9] As a result, mRNA is also loaded into leukocytes, which leads to the translation of the mRNA into protein (Figure 2A). Secondly, mRNA is loaded into whole blood cells by electroporation instead of hypotonic loading. The latter is not suitable because it requires prolonged incubation of labile mRNA with cells, which requires an RNAse-free environment. Thirdly, our formulation is rapidly distributed to the liver and spleen compared to most red blood cell vaccine which requires additional pretreatment to achieve opsinization[26]. This rapid distribution could have been a consequence of cluster formation (Figure 1B and 1C) as well as the externalization of phosphatidylserine (PS) “eat-me” signals on the cell surface (Figure 2E).
As important, immunization with mRNA-loaded whole blood cells leads to induction of immune responses. Notably, the dose applied in this study contains only about 150 nanograms of mRNA (Figure 2A). Although nanogram quantities of mRNA encapsulated in nanoparticles using lipid-based gene carriers have been effective for erythropoietin delivery,[27] tumor vaccination may require microgram quantities as previously reported for intravenously administered mRNA-nanoparticle vaccine.[28] It is also reasonable to assume that synthetic gene carriers will be more efficient than erythrocytes in transfecting APCs. As such, there is a very low chance for host APCs to be directly transfected by mRNA encapsulated inside erythrocytes. Our results suggest that transfected leukocytes may be involved in the induction of immune response, especially since a variety of them are loaded with tumor antigen encoding mRNA. This speculation is consistent with predominant role of cell-based vaccines for transfering antigens to host APCs.[7] Future studies will address the mechanism of immune response induction, which will allow us to further understand and optimize this vaccine formulation.
Experimental Section
mRNA encapsulation into whole blood cells
Whole blood was obtained from 4–6 week old C57Bl/6 female mice by cardiac puncture using citrate as a stabilizer, diluted in PBS and filtered through a 70µm mesh. Whole blood cells were washed a second time with complete Opti-MEM by centrifugation at 1200 rpm for 5 min. Supernatant was discarded and the cell density of the pellet was determined using a hemocytometer.
Electroporation media (EP media) was composed of 5% sucrose, Hepes (5mM), NaCl (75mM), MgCl2 (1mM) and CaCl2 (2mM). Complete Opti-MEM is composed of Opti-MEM (Invitrogen) supplemented with reduced gluthathione (4mM), beta-mercaptoethanol (βME, 0.011mM) and MgCl2 (2mM). For a typical preparation, blood cells were diluted into electroporation media (500µl) to obtain a final cell density of approximately 1 to 5 × 108/ml. Cell suspension (200µl) was transferred to a cuvette (0.2mm width, VWR) and mixed with mRNA (20µg). Cells were electroporated at room temperature using a BTX Square Wave electroporator at 300–325V for 2.0 millisecond and immediately transferred to pre-warmed complete Opti-MEM (2ml, 10× volume) and maintained at 37°C for at least 10 minutes. Recovered cells were analyzed using flow cytometry (BD FACSCaliber) for loading efficiency.
Immunotherapy model
2.5×107 mRNA-loaded (melanoma antigen murine tyrosinase-related protein2 [TRP-2] or chicken ovalbumin [OVA] mRNA) blood cells were prepared (described in supporting information) and injected intravenously into each mouse. To monitor the induction of antibody responses, mice were immunized with blood cells transfected with OVA mRNA two times with an eight-day interval. Seven days later, serum levels of anti-OVA IgG were measured with a commercial kit. To determine the induction of antigen-specific T cell responses, splenocytes were harvested 10 days post-immunization and depleted of red blood cells. Non-adherent splenic lymphocytes were cultured with irradiated stimulator cells (F10.9 melanoma cells or F10.9-OVA cells) at a responder: stimulator ratio of 10:1. T cells were harvested after a 5 day culture and used in a CTL (cytotoxic T lymphocyte) assay or separated into CD4 and CD8 T lymphocytes for an IFN-γ ELISpot (enzyme-linked immunosorbent spot). To evaluate the efficacy of mRNA-loaded whole blood cells in an immunotherapy model, 2.5×104 B16-F10.9 cells were injected subcutaneously into the flanks of C57Bl/6 mice (N=10/group). 2 days later, 2.5×107 mRNA-transfected blood cells were injected intravenously into mice. Alternately, mice were immunized with 2×105 mRNA-transfected DCs intraperitoneally[29]. Time to tumor onset was recorded based on the detection of palpable tumors on a daily basis. For overall survival, mice were sacrificed once tumor diameter reached 20mm and date of sacrifice recorded. Tumor diameter was measured with vernier calipers based on the longest side of the tumor.
Supplementary Material
Acknowledgements
This study was supported by Duke Department of Surgery (SN). We gratefully acknowledge the following other sources of financial support; Department of Defense W81XWH-12-1-0260 (SN, KL), NIH AI096305 (KL) and the National University of Singapore (NUS-OGS, KP). The authors would like to thank David Snyder for help with murine studies and Dr. James Frederiksen for reading the manuscript.
Footnotes
Addtional materials and methods are provided in supporting information.
Contributor Information
Kyle K.L. Phua, Department of Chemical & Biomolecular Engineering, National University of Singapore, Singapore 117576 Department of Biomedical Engineering, Duke University Durham, NC 27708, USA.
David Boczkowski, Department of Surgery, Duke University Medical Center Durham, NC 7710, USA.
Jens Dannull, Department of Surgery, Duke University Medical Center Durham, NC 27710, USA.
Scott Pruitt, Experimental Medicine, Merck Research Laboratories Rahway, NJ 07065, USA.
Kam W. Leong, Department of Biomedical Engineering, Duke University Durham, NC 27708, USA
Smita K. Nair, Email: smita.nair@duke.edu, Department of Surgery, Duke University Medical Center Durham, NC 27710, USA.
Cited References
- 1.Boczkowski D, Nair SK, Snyder D, Gilboa E. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 3.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 4.Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, Tureci O, Sahin U. Cancer Res. 2010;70:9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- 5.Van Lint S, Goyvaerts C, Maenhout S, Goethals L, Disy A, Benteyn D, Pen J, Bonehill A, Heirman C, Breckpot K, Thielemans K. Cancer Res. 2012;72:1661–1671. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 6.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I, Rammensee HG, Holderried TA, Kanz L, Pascolo S, Brossart P. Mol Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. PLoS One. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo V, Cipponi A, Raccosta L, Rainelli C, Fontana R, Maggioni D, Lunghi F, Mukenge S, Ciceri F, Bregni M, Bordignon C, Traversari C. J Clin Invest. 2007;117:3087–3096. doi: 10.1172/JCI30605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray AM, Pearson IF, Fairbanks LD, Chalmers RA, Bain MD, Bax BE. Vaccine. 2006;24:6129–6139. doi: 10.1016/j.vaccine.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Dollins CM, Boczkowski D, Sullenger BA, Nair S. Immunology. 2008;125:229–240. doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamidi M, Zarei N, Zarrin AH, Mohammadi-Samani S. Int J Pharm. 2007;338:70–78. doi: 10.1016/j.ijpharm.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Chiarantini L, Matteucci D, Pistello M, Mancini U, Mazzetti P, Massi C, Giannecchini S, Lonetti I, Magnani M, Bendinelli M. Clinical and Diagnostic Laboratory Immunology. 1998;5:235–241. doi: 10.1128/cdli.5.2.235-241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banz A, Cremel M, Mouvant A, Guerin N, Horand F, Godfrin Y. Journal of Immunotherapy. 2012;35:409–417. doi: 10.1097/CJI.0b013e3182594352. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL. Cancer Immunol Immunother. 2013;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH, Vuk-Pavlovic S. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 16.Chambers JD, Neumann PJ. N Engl J Med. 2011;364:1687–1689. doi: 10.1056/NEJMp1103057. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson B, Wilking N. Hum Vaccin Immunother. 2012;8:1360–1363. doi: 10.4161/hv.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowley PT, Morris JA. J Biol Chem. 1967;242:1533–1540. [PubMed] [Google Scholar]
- 19.Moenner M, Vosoghi M, Ryazantsev S, Glitz DG. Blood Cells Mol Dis. 1998;24:149–164. doi: 10.1006/bcmd.1998.0182. [DOI] [PubMed] [Google Scholar]
- 20.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. J Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekle E, Wolfe MD, Oubrahim H, Chock PB. Biochem Biophys Res Commun. 2008;376:256–260. doi: 10.1016/j.bbrc.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg CP, Engels IH, Rothbart A, Lauber K, Renz A, Schlosser SF, Schulze-Osthoff K, Wesselborg S. Cell Death Differ. 2001;8:1197–1206. doi: 10.1038/sj.cdd.4400905. [DOI] [PubMed] [Google Scholar]
- 23.Lui JC, Wong JW, Suen YK, Kwok TT, Fung KP, Kong SK. Arch Toxicol. 2007;81:859–865. doi: 10.1007/s00204-007-0214-5. [DOI] [PubMed] [Google Scholar]
- 24.Siniscalco D, Sapone A, Giordano C, Cirillo A, de Novellis V, de Magistris L, Rossi F, Fasano A, Maione S, Antonucci N. Journal of Autism and Developmental Disorders. 2012;42:1403–1410. doi: 10.1007/s10803-011-1373-z. [DOI] [PubMed] [Google Scholar]
- 25.Schroit AJ, Madsen JW, Tanaka Y. J Biol Chem. 1985;260:5131–5138. [PubMed] [Google Scholar]
- 26.Kim SH, Kim EJ, Hou JH, Kim JM, Choi HG, Shim CK, Oh YK. Biomaterials. 2009;30:959–967. doi: 10.1016/j.biomaterials.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Kariko K, Muramatsu H, Keller JM, Weissman D. Mol Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffres PA, Midoux P. Nanomedicine-Nanotechnology Biology and Medicine. 2011;7:445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Porgador A, Snyder D, Gilboa E. J Immunol. 1996;156:2918–2926. [PubMed] [Google Scholar]
- 30.Uggeri J, Gatti R, Belletti S, Scandroglio R, Corradini R, Rotoli BM, Orlandini G. Histochemistry and Cell Biology. 2004;122:499–505. doi: 10.1007/s00418-004-0712-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.